Fabrication of anodized superhydrophobic 5083 aluminum alloy surface for marine anti-corrosion and anti-biofouling*
ZHANG Jie , WANG Jia ZHANG Binbin , ZENG Yuxiang DUAN Jizhou HOU Baorong
1 CAS Key Laboratory of Marine Environmental Corrosion and Biofouling, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
2 Open Studio for Marine Corrosion and Protection, Pilot National Laboratory for Marine Science and Technology, Qingdao 266237, China
3 University of Chinese Academy of Sciences, Beijing 100049, China
4 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
Abstract Marine corrosion and biofouling seriously affect the service life of marine structural materials, resulting in performance failure, enormous economic loss, and even catastrophic safety accidents. It is worthwhile and desirable to develop high-efficiency strategy for anti-corrosion and anti-biofouling. In this paper, superhydrophobic 5083 aluminum alloy (AA5083) surface with micro-nano hierarchical morphology was fabricated through anodization followed by 1H,1H,2H,2H-perfluorooctyltriethoxysilane (POTS) modifi cation. The surface morphologies, roughness, and chemical compositions were revealed by scanning electron microscopy, atomic force microscopy, and X-ray diffraction. The self-cleaning ability, corrosion resistance and algae adhesion suppression ability of the fabricated surfaces were investigated, indicating an excellent water-proofi ng, anti-corrosion and anti-biofouling performance. We believe the superhydrophobic creation of metallic materials is expected to have potential applications in marine corrosion and antibiofouling fi elds.
Keyword: 5083 aluminum alloy; anodization; superhydrophobic; corrosion resistance; anti-biofouling
1 INTRODUCTION
Corrosion and biofoulings are natural occurring phenomenon with costly and detrimental impact on most of marine-related metallic materials (Hou et al., 2017). Seawater is a strong electrolyte with strong chemical corrosiveness. The dissolved oxygen and corrosive chloride ions in the seawater can spread and wet the metallic substrate surface, causing pitting corrosion, crevice corrosion etc. and accelerating the corrosion of marine structural components (Song et al., 2019). Besides, the life activities and secretions of marine organisms and microorganisms can lead to biological adhesion and severe localized corrosion on the metallic surface, affecting the mechanical properties, and threatening the service life and safety of the materials (Permeh et al., 2019; Zhang et al., 2019a). Therefore, how to construct efficient anticorrosion and anti-biofouling protective layer is of great signifi cance and urgent to expand the application scope and prolong the service life of the metallic materials in marine environment.
5xxx series aluminum alloys are low-density materials with high strength, good plasticity and excellent processability, which have been widely used in aerospace, automobile manufacturing and shipbuilding industry (Bhattacharjee et al., 2017; Tazari and Siadati, 2017). Among 5xxx series aluminum alloys, 5083 aluminum alloy (AA5083) is the most commonly used one in the fi eld of marine applications (Bhattacharjee et al., 2017). In general, the naturally formed oxide fi lm of 5083 aluminum alloy surface protects the aluminum matrix (Ramalingam and Ramasamy, 2017). However, the oxide fi lm is loose and porous. When exposed to marine corrosive environment, chloride ions are easy to penetrate into the naturally formed oxide fi lm through the structural gaps, leading to severe localized corrosion (Canepa et al., 2018). In addition, the surface energy of the oxide fi lm is relatively high which provides abundant attachment positions for typical marine microorganisms, causing serious biological attachment (Moazzam et al., 2016). Therefore, it is desirable to construct high-efficiency anti-corrosion and anti-biofouling layer on 5083 aluminum alloy substrates.
In recent years, biomimetic superhydrophobic materials exhibit broad application prospects in the fi elds of oil-water separation (Xi et al., 2020; Yin et al., 2020), self-cleaning (Katiyar et al., 2020; Vazirinasab et al., 2020), anti-icing (Pan et al., 2019; Xu et al., 2019a), anti-corrosion (Mahalakshmi et al., 2011; Li et al., 2019; Tong et al., 2019), and anti-fouling (Lv and Liu, 2019; Wang et al., 2020), etc. Many researchers have prepared superhydrophobic materials on titanium (Vanithakumari et al., 2014; Mirzadeh et al., 2019), carbon steel (Tian et al., 2019), copper (Shi et al., 2019), aluminum (Tong et al., 2020) and other metallic substrates for corrosion protection. For example, Xu et al. (2019b) prepared a superhydrophobic nickel coating by jet electrodeposition on a copper substrate, demonstrating excellent corrosion resistance in 3.5 wt.% NaCl solution. Tong et al. (2020) developed a superhydrophobic coating on aluminum alloy with long-term water repellency and corrosion resistance using the non-fluorinated self-assembly deposition of Trimethylethoxysilane- (TMES) modifi ed SiO2. However, to the best of our knowledge, only limited research works have been reported for the development of superhydrophobic surface on 5083 aluminum alloy substrate. Thus, in this paper, we report a facile strategy to prepare a superhydrophobic surface on a 5083 aluminum alloy substrate by anodization and 1H,1H,2H,2H-Perfluorooctyltriethoxysilane (POTS) hydrophobic molecules assembly. Detailed studies about the chemical composition and surface morphology were carried out successively. In addition, wetting property, self-cleaning ability, corrosionresisting behavior, and anti-adhesion ability were investigated to show its application prospect in the fi eld of marine anticorrosion and antifouling.
2 MATERIAL AND METHOD
2.1 Material and reagent
5083 aluminum alloy specimens (Size: 30 mm×20 mm×0.5 mm, Composition: 4.0%–4.9% Mg, 0.4%–1.0% Mn, 0.4% Si, 0.25% Zn, 0.15% Ti, 0.1% Cu, 0.1%–0.4% Fe, 0.05%–0.25% Cr) were purchased from Shandong Shengxin Technology Co., Ltd. The 1H,1H,2H,2H-perfluorooctyltriethoxysilane (POTS, 97%) was purchased from J&K Scientifi c Co. Ltd, China. Other chemical reagents, including ethanol absolute, glycerol and phosphoric acid, etc. were purchased from Sinopharm Chemical Reagent Co., Ltd. All the reagents were of analytical grade and used as received without any purifi cation.
2.2 Preparation of superhydrophobic 5083 aluminum alloy
5083 aluminum alloy sheets were mechanically polished using silicon carbide papers. The samples were then alternately cleaned in anhydrous ethanol and deionized water under ultrasonication for 10 min and dried in a vacuum oven. The electrochemical polishing process was carried out by direct current (DC) power with 5083 aluminum alloy substrate as anode and platinum sheet as cathode at 50°C. The polishing electrolyte is consisted of glycerol (10 vol.%) and phosphoric acid (90 vol.%). The time of the electrochemical polishing process was 90 seconds. After mechanical and electrochemical polishing, the anodization process was performed using DC power supply. The current density was 1.5–2 A/dm2. 5083 aluminum alloy substrate and platinum sheet were used as anode and cathode respectively. 0.3 mol/L oxalic acid aqueous solution was used as the electrolyte of the anodization process. The anodization reaction time was 1 h. The reaction temperature was 25°C. After anodization treatment, the samples were rinsed with anhydrous alcohol repeatedly and dried with a blower. Then the samples were immersed in 1 vol.% POTS/ethanol solution for 30 min at room temperature and dried in an oven at 120°C for 30 min. The schematic illustration of the fabrication of anodized superhydrophobic 5083 aluminum alloy is shown in Fig.1.
2.3 Characterization techniques
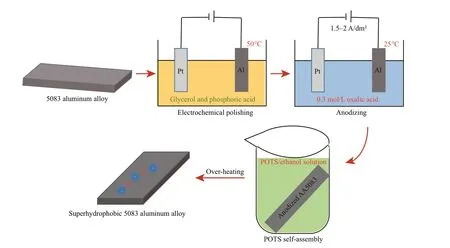
Fig.1 Schematic illustration of the fabrication of anodized superhydrophobic 5083 aluminum alloy (AA5083)
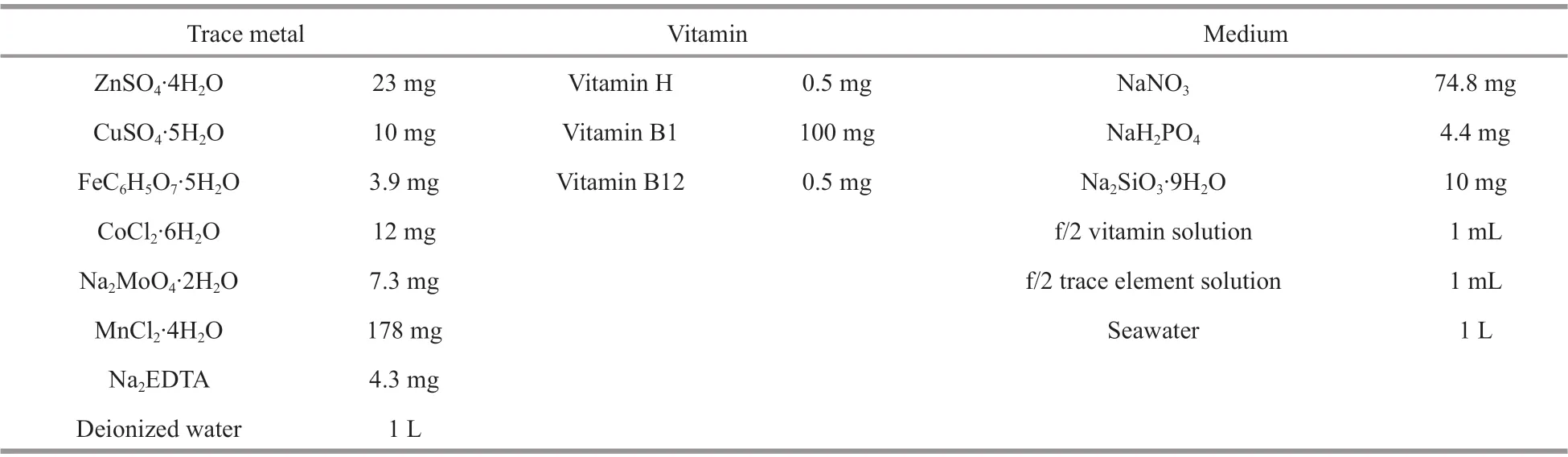
Table 1 Prescription for f/2 culture medium
Surface morphology and roughness of superhydrophobic 5083 aluminum alloy surface were measured with field emission scanning electron microscope (FE-SEM, Japan Hitachi S4300N) and atomic force microscope (AFM, Germany Bruker Multimode 8). The X-ray diffraction (XRD, Rigaku, Japan) was used to record the surface chemical compositions of the prepared sample. Water contact angles of the samples were measured by Dataphysics OCA 25 (Germany) with 3-μL deionized water droplets at a constant room temperature.
2.4 Electrochemical test
Ametek Parstat 4000+ electrochemical workstation was used to test open circuit potential (OCP) and electrochemical impedance spectroscopy (EIS) of bare 5083 aluminum alloy and superhydrophobic 5083 aluminum alloy. All tests were conducted in 3.5 wt.% NaCl solution. Superhydrophobic 5083 aluminum alloy, Pt electrode and saturated calomel electrode (SCE) were used as working electrode, counter electrode and reference electrode respectively. After the OCP stabilized, EIS measurement was carried out in frequency range of 100 kHz–10 MHz. ZSimpWin was used to fi t impedance data and obtain equivalent circuit and corresponding value.
2.5 Navicular algae cultivation and anti-fouling experiment
The samples were placed in the navicular algae culture solution, which consisted of navicular algae seed and f/2 culture medium in a volume ratio of 1∶3. Table 1 shows the prescription of f/2 culture medium. GXZ-280D intelligent light incubator was used for culturing navicular algae at a temperature of 23°C with light intensity of 3 000 lx. Then fluorescence microscopy was used to observe the adhesion of navicular algae on bare 5083 aluminum alloy surface and superhydrophobic 5083 aluminum alloy surface at a certain time.
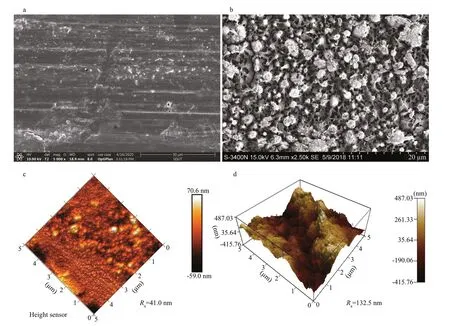
Fig.2 SEM images (a, b) and 3D atomic force microscopy (AFM) images (c, d) of pristine 5083 aluminum alloy (a, c) and as-prepared superhydrophobic 5083 aluminum alloy (b, d) surfaces
3 RESULT AND DISCUSSION
3.1 Surface morphology
The surface topographies of pristine 5083 aluminum alloy and as-prepared superhydrophobic 5083 aluminum alloy were characterized using SEM. The micro-nano structure of pristine 5083 aluminum alloy and the superhydrophobic 5083 aluminum alloy surface was shown in Fig.2a & b. The micro-nano structure of the superhydrophobic 5083 aluminum alloy surface present a sponge-like structure. It can be observed from SEM observation that uniform micronano pores are formed on the surface of 5083 aluminum alloy after anodization. The diameter of the micro-nano pores is about 1–2 μm. The micro-nano hierarchical structure benefi ts the formation of air cushion between the structure gaps, contributing to the Cassie-Baxter contact of anti-wetting superhydrophobicity. The surface roughness of pristine 5083 aluminum alloy and as-fabricated superhydrophobic 5083 aluminum alloy were characterized by AFM. Figure 2c & d show the 3D AFM images of pristine 5083 aluminum alloy and superhydrophobic 5083 aluminum alloy surfaces, respectively. The scanning area of the AFM measurement is 5 μm×5 μm. Generally, average roughness ( Ra), root mean square roughness ( Rq) were used to present surface roughness. In this case, for pristine 5083 aluminum alloy the values of Raand Rqwere 41.0 nm and 48.3 nm, respectively. While for the as-prepared superhydrophobic 5083 aluminum alloy surface, the Raand Rqvalues were apparently increased to 132.5 nm and 156.8 nm, respectively. Anodization improves the surface roughness of 5083 aluminum alloy, which is favorable for the formation of superhydrophobic surfaces.
3.2 Chemical components
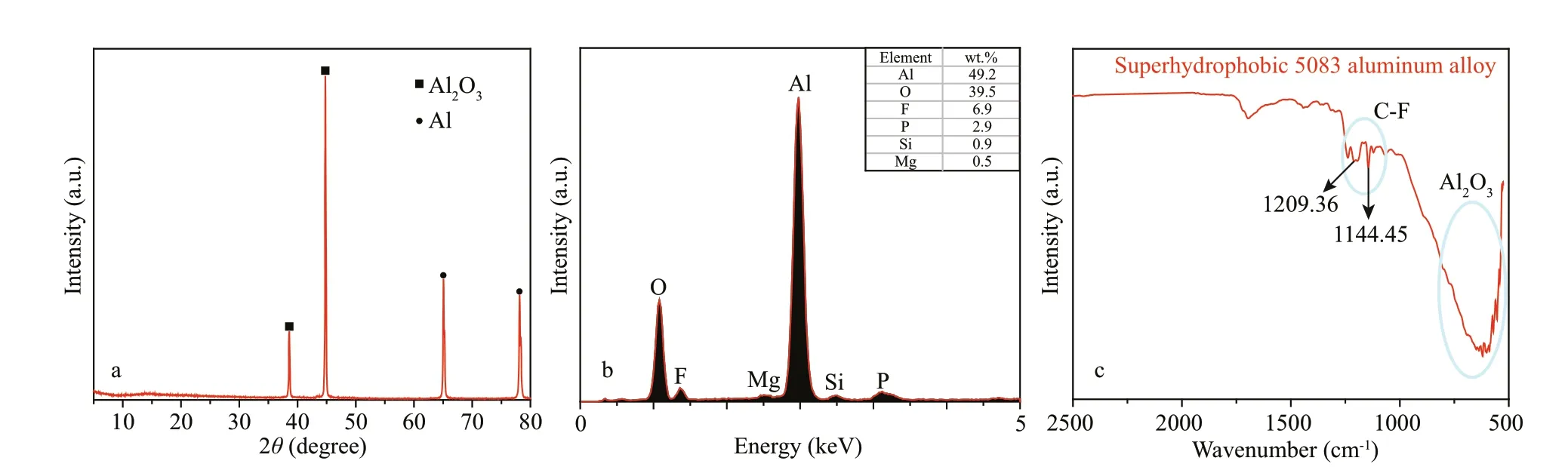
Fig.3 XRD pattern (a) of anodized 5083 aluminum alloy, and EDS pattern (b), and FTIR spectra (c) of superhydrophobic 5083 aluminum alloy
The XRD spectrum shown in Fig.3a demonstrated the main component of the superhydrophobic 5083 aluminum alloy. Oval icon represents aluminum (Al, JCPDS fi le No. 04-0787). Rectangular icon represents aluminum oxide (Al2O3, JCPDS fi le No. 23-1009). The XRD results show that stable aluminum oxide formed on the surface of aluminum matrix after anodization. Figure 3b reveals the EDS pattern of superhydrophobic 5083 aluminum alloy. The EDS shows that the surface of superhydrophobic 5083 aluminum alloy is mainly composed of Al and O elements, further proving the existence of aluminum oxide. In addition, the existence of element F was observed, which could be assigned to POTS molecules. In addition, FTIR analyses also suggest the existence of Al2O3and POTS on the surface of superhydrophobic 5083 aluminum alloy (Fig.3c). The FTIR absorption peak located at 500–1 000 cm-1is attributed to the Al-O bond (Chen et al., 2014; Wang et al., 2014). The peaks at 1 144.45 cm-1and 1 209.36 cm-1are assigned to C-F bond (Gao et al., 2018), which are derived from POTS molecules. The FTIR results confi rm that the POTS successfully incorporated on the surface anodized 5083 aluminum alloy. POTS has a lower surface than water. After POTS was successfully assembled on the surface of the micro-nano pores of anodized alumina, the sample was superhydrophobic.
3.3 Wettability and self-cleaning ability
Water flow sputtering and contact angle measurement were used to verify the superhydrophobicity of the as-prepared surface, as shown in Fig.4a & b. Figure 4a shows that the water flow can bounce off the superhydrophobic surface and leave no trace on the surface. Figure 4b shows that the static contact angle of the superhydrophobic surface is 159°±1°, which indicates an excellent antiwetting and water repellent properties. The selfcleaning ability is important for real-world applications. In this work, sand particles were applied as surface contaminations to test the self-cleaning ability of the as-prepared superhydrophobic 5083 aluminum alloy. First, the sand particles were evenly distributed on the surface of the superhydrophobic sample. The tilt angle of the superhydrophobic sample was about 8°. Then, water droplets were squeezed out by a dropper and naturally tumble from the top of the sample. Figure 4c displays the pictures of the selfcleaning process. After water drops tumbling down the superhydrophobic sample, the sand particles on the surface was completely taken away. After selfcleaning test, the sample remains good superhydrophobic properties. The videos of dewdrop ejection and graphite self-cleaning of the anodized sample and the superhydrophobic sample are also shown. It can be seen from the videos that the anodized samples do not have excellent superhydrophobic properties and self-cleaning properties, which indicates that the preparation of the superhydrophobic sample cannot be separated from the material modifi cation with low surface energy.
3.4 Anti-corrosion performance
In order to characterize the corrosion resistance of superhydrophobic 5083 aluminum alloy, the open circuit potential (OCP) and electrochemical impedance spectroscopy (EIS) were carried out in a 3.5 wt.% NaCl aqueous solution. Figure 5 reveals the EIS plots and fi tting curve of bare 5083 aluminum alloy (black line) and superhydrophobic 5083 aluminum alloy (red line). Figure 5a & b displays the Nyquist plots of superhydrophobic 5083 aluminum alloy and bare 5083 aluminum alloy, respectively. It can be clearly observed that the diameter of the Nyquist plots of superhydrophobic 5083 aluminum alloy is much larger than that of bare 5083 aluminum alloy. The Nyquist plot of superhydrophobic 5083 aluminum alloy has two overlapped capacitive loops while bare 5083 aluminum alloy has a single capacitive loop. Similarly, we can draw same conclusion from Bode plots (Fig.5c). There are two electrode processes at superhydrophobic 5083 aluminum alloy surface, which occurred in fi lm/metal interface at the low frequency range and superhydrophobic fi lm/electrolyte interface at the high frequency range. For bare 5083 aluminum alloy, there is only one time constant at Bode plots, which produced by charge transfer resistance and electric double layer. Figure 5d displays the Bode plots of log| Z| vs. log F and fi tting lines of the bare 5083 aluminum alloy and superhydrophobic 5083 aluminum alloy. The impedance modulus value of the superhydrophobic 5083 aluminum alloy is fi ve orders higher than that of bare 5083 aluminum alloy, indicating a high-efficiency corrosion resistance.

Fig.4 Reflection process of water flow (a), contact angle (b), and self-cleaning ability (c) of the as-prepared superhydrophobic 5083 aluminum alloy
Figure 6a & b displays the equivalent circuit of bare 5083 aluminum alloy and superhydrophobic 5083 aluminum alloy specimens, which were used to analyze EIS results. Rs, Rctand Rfilmrepresent solution resistance, charge transfer resistance, and superhydrophobic fi lm resistance respectively. Qdland Qfilm, represent the double-layer and constant phase elements (CPE) modelling capacitance of the superhydrophobic 5083 aluminum alloy fi lm, respectively.

Table 2 The electrochemical parameters of simulated bare 5083 aluminum alloy and superhydrophobic surfaces in 3.5 wt.% NaCl aqueous solution
The electrochemical fi tting parameters of bare 5083 aluminum alloy and superhydrophobic 5083 aluminum alloy are shown in Table 2. Rctis usually used to characterize the corrosion resistance of the fabricated samples. From Table 2, it is clearly that the Rctof the bare 5083 aluminum alloy sample is 3.80×103Ω·cm2, while the Rctof the as-fabricated superhydrophobic 5083 aluminum alloy is 1.01×108Ω·cm2. The superhydrophobic 5083 aluminum alloy possesses higher corrosion resistance. In addition, the Qdlvalues of bare 5083 aluminum alloy and superhydrophobic 5083 aluminum alloy are 2.77×10-5Ω-1·cm-2·snand 1.27×10-8Ω-1·cm-2·sn, respectively, which demonstrates that charge transfer process is difficult to happen. The corrosion inhibition efficiency ( η) of superhydrophobic 5083 aluminum alloy is calculated using the following formula (Zhang et al., 2019b):
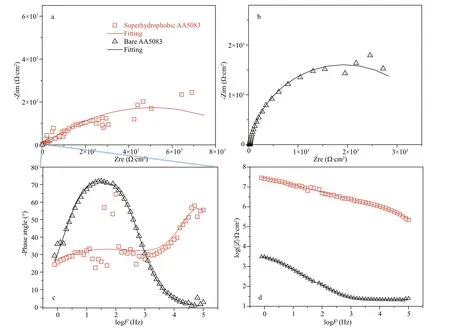
Fig.5 The EIS results and fitted plots of as-prepared superhydrophobic AA5083 (a) and bare AA5083 (b), bode plots of log| Z| vs. logF (d), and phase angle vs. logF for bare AA5083 and superhydrophobic AA5083 (c)
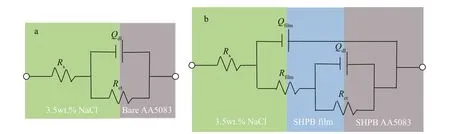
Fig.6 Equivalent circuits of bare 5083 aluminum alloy (bare AA5083) (a) and superhydrophobic 5083 aluminum alloy (SHPB AA5083) (b)
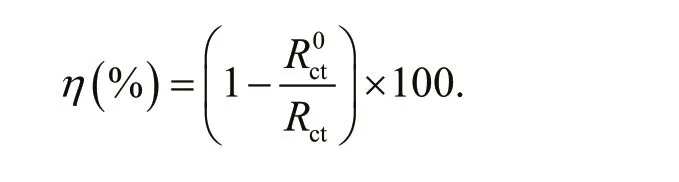
R0ctand Rctrepresent the charge transfer resistance of bare 5083 aluminum alloy and superhydrophobic 5083 aluminum alloy respectively. The corrosion inhibition efficiency ( η) of the superhydrophobic 5083 aluminum alloy surface was calculated, which is approximately 99.99%. The rough hierarchical surface of superhydrophobic material possess cavity structures, entrapping large amount of air and forming a continuous layer of air cushion. When the superhydrophobic 5083 aluminum alloy samples are exposed to aggressive corrosive medium, the air cushion can stably exist between corrosive medium and surface of sample, isolating metallic substrate from corrosive medium.

Fig.7 Fluorescent micrographs of bare 5083 aluminum alloy (Bare AA5083) and superhydrophobic 5083 aluminum alloy (SHPB AA5083) specimens after 3 days (a), 7 days (b), and 14 days (c) anti-adhesion property of navicular algae immersion experiments
3.5 Anti-adhesion property of navicular algae
The life activities of marine organisms and microorganisms will also accelerate the corrosion and failure of marine structures. In this experiment, navicular algae, a typical marine alga, was used to study the anti-adhesion ability of superhydrophobic 5083 aluminum alloy surfaces. Figure 7 shows the fluorescent micrographs pictures of bare 5083 aluminum alloy and superhydrophobic 5083 aluminum alloy surfaces after immersion in the navicular algae culture solution for 3, 7, and 14 days, respectively.
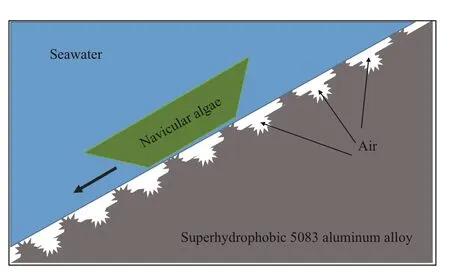
Fig.8 Schematic diagram of the anti-adhesion of navicular algae on the surface of superhydrophobic 5083 aluminum alloy
Figure 7a shows that navicular algae have been widely distributed in bare 5083 aluminum alloy surface after 3 days immersion, while there are no navicular algae on superhydrophobic 5083 aluminum alloy surface. With the extension of immersion time to 7 days, it can be found that navicular algae on the bare 5083 aluminum alloy surface was slightly increased. A few strains of navicular algae (marked with green circles) could be observed on the superhydrophobic 5083 aluminum alloy surface (Fig.7b). In addition, Fig.7c presents the fluorescent micrographs after immersion for 14 days. The navicular algae on the bare 5083 aluminum alloy surface increased further. The navicular algae (marked with green circles) on the surface of superhydrophobic 5083 aluminum alloy also increased slightly. On Day 14, the navicular algae adhering to superhydrophobic 5083 aluminum alloy surface became more than that of Day 7. Compared with bare 5083 aluminum alloy surface, the superhydrophobic 5083 aluminum alloy surface could greatly decrease the adhesion and proliferation of navicular algae, verifying the signifi cant role of superhydrophobicity in antibiofouling. The material intrinsic characteristics and surface roughness of the superhydrophobic samples could make the bubbles inclusion in the gap and conform to the Cassie model, so as to reduce the direct contact between the fouling organisms and the sample surface (as shown in Fig.8). The extremely low surface tension and typical Cassie contact of the surface contributes to the easily sliding of the navicular algae and the signifi cant reduction of the navicular algae coverage.
4 CONCLUSION
We prepared superhydrophobic 5083 aluminum alloy by anodization and POTS self-assembly method. The superhydrophobic 5083 aluminum alloy showed excellent superhydrophobic properties at a contact angle of 159° and good self-cleaning capacity for sand particles. In addition, superhydrophobic 5083 aluminum alloy had perfect resistance to seawater corrosion. The charge transfer resistance of superhydrophobic 5083 aluminum alloy was fi ve orders of magnitude higher than that of bare 5083 aluminum alloy. What is more noteworthy is that the superhydrophobic 5083 aluminum alloy surface could greatly decrease the adhesion and proliferation of navicular algae. Therefore, biomimetic superhydrophobic methods are expected to solve the problems of marine corrosion and biofouling of aluminum alloys in marine applications.
5 DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during this study are available from the corresponding author on reasonable request.
 Journal of Oceanology and Limnology2020年4期
Journal of Oceanology and Limnology2020年4期
- Journal of Oceanology and Limnology的其它文章
- Review on observational studies of western tropical Pacifi c Ocean circulation and climate*
- Research progress of TiO 2 photocathodic protection to metals in marine environment*
- Smart anticorrosion coating based on stimuli-responsive micro/nanocontainer: a review*
- Status of genetic studies and breeding of Saccharina japonica in China*
- To be the best in marine sciences*
- A review of progress in coupled ocean-atmosphere model developments for ENSO studies in China*
