Therapeutic properties of the new phytochemical osmotin for preventing atherosclerosis
Takuya Watanabe
Department of Internal Medicine, Ushioda General Hospital/Clinic, Yokohama 230-0001, Japan.
Abstract
Keywords: Osmotin, atherosclerosis, inflammation, diabetes, obesity, phytochemical, vascular cells, animal models
INTRODUCTION
Atherosclerosis is clinically manifested as coronary artery disease, stroke, and peripheral arterial disease, which are major causes of mortality and morbidity worldwide[1]. Atherosclerosis is a chronic inflammatory vascular disease induced by endothelial injury followed by atheromatous plaque formation, leading to thickening and loss of elasticity in the arterial wall of medium- and large-sized arteries, including the coronary, cerebral, and carotid arteries and the aorta[2]. Risk factors of atherosclerosis include dyslipidemia, diabetes, obesity, and hypertension. The pathophysiology of atheromatous plaque development involves endothelial cell (EC) inflammation and proliferation, monocyte adhesion to ECs and infiltration into the under-endothelial space, inflammatory cytokine release from monocyte-derived macrophages, oxidized low-density lipoprotein (Ox-LDL)-induced macrophage foam cell formation, vascular smooth muscle cell (VSMC) migration and proliferation, and extracellular matrix (ECM) production by VSMCs[3]. The progression and rupture of atheromatous plaques in coronary arteries lead to myocardial ischemia and infarction. Timely reperfusion is critical for the salvage of ischemic myocardium. After coronary angioplasty, restoration of blood flow to the damaged myocardium triggers further ischemic myocardial damage. This paradoxical phenomenon is known as ischemia-reperfusion injury, which is a serious clinical problem in the treatment of patients with coronary artery disease. Several studies have investigated preventive effects of plants on atherogenesis[4-9]. Osmotin, a plant-derived natural protein, is receiving the most attention as a therapeutic target for atherosclerosis and myocardial ischemia-reperfusion injury[10,11].
Osmotin was first isolated from tobacco (Nicotiana tabacum) cells by Singhet al.[12]. Later, osmotin was also found in other plant species, including tomato, potato, oat, pepper, and grape[13]. Osmotin is a plant peptide hormone, also called phytochemical, which belongs to the fifth class of the group of pathogenesisrelated proteins[14]. The osmotin geneAP24is known to be activated by environmental and phytohormone signals[15]. Osmotin plays an important role in the protection against osmotic and oxidative stresses caused by higher salt concentration, cold, and drought[16], and has anti-fungal activity in plants[17]. In plants and yeasts, osmotin exhibits anti-fungal, anti-oxidant, and anti-apoptotic effects through PHO36, which is an adiponectin receptor (AdipoR) homolog[13,18].
Osmotin has recently attracted attention as a homolog of mammalian adiponectin[13,18], which is the most famous adipocytokine (adipokine) with anti-inflammatory, anti-diabetic, and anti-atherogenic properties[19-21]. Osmotin is composed of 246 amino acids including a 21-amino acid signal peptide, which do not share remarkable similarity to human adiponectin (AdipoQ, 244 amino acids) in the amino acid sequence alignment[13]. However, the domain I of osmotin is demonstrated to overlap with the β-barrel domain of AdipoQ by three-dimensional structure analyses[13,18]; thereby, osmotin binds to AdipoR1 and then leads to intracellular signaling[18,22,23]. Therefore, osmotin is regarded as a natural agonist for human AdipoR1[22]. Osmotin exhibits anti-inflammatory and anti-apoptotic effects in mammalian cells through AdipoR1[24,25]. Takahashiet al.[10]recently showed that osmotin exerts atheroprotective effects through human AdipoR1 in human vascular cells. In intracellular signaling pathways, both nuclear factor-κB (NF-κB) and extracellular signal-regulated protein kinase 1/2 (ERK1/2) suppression and AMP-activated protein kinase (AMPK) activation play a pivotal role in the preventive effects of osmotin on inflammation and atherosclerosis[10].
Osmotin as well as adiponectin binds to AdipoR1, leading to the activation of adaptor protein, phosphotyrosine interacting with PH domain and leucine zipper 1 (APPL1) followed by phosphoinositide 3-kinase (PI3K)/Akt, AMPK, peroxisome proliferator-activated receptor-α (PPAR-α), and protein-tyrosine phosphatase 1B (PTP1B)[10]. The PTP1B activation suppresses RAF1 and ERK1/2 phosphorylation[10]. The activation of PI3K/Akt and/or AMPK suppresses apoptosis of vascular cells (ECs, VSMCs, and macrophages) and cardiomyocytes. NF-κB and ERK1/2 suppression and AMPK activation inhibit inflammation, differentiation, migration, and proliferation of the vascular cells; thus, these signalings are therapeutic targets for atherosclerosis[26-28]. In adipocytes, hepatocytes, and skeletal muscle cells, AMPK and PPAR-α activation contributes to improving insulin resistance and lipid metabolism, respectively. AdipoR1 is expressed at high levels in monocytes, macrophages, ECs, VSMCs, cardiomyocytes, adipocytes, hepatocytes, and skeletal muscle cells[29]. The AdipoR agonist AdipoRon, an adiponectin-like synthetic small molecule, is in the spotlight as an oral anti-atherosclerotic drug[30].
Several lines of evidence have recently shown the protective effects of osmotin against atherosclerosis, hyperlipidemia, diabetes, and obesity[10,31-33]. This review article summarizes the therapeutic properties of osmotin for preventing atherosclerosis and myocardial ischemia-reperfusion injury as well as inflammation and neurodegeneration. In addition, the article describes the comparisons of the atheroprotective effects between osmotin and adiponectin or AdipoRon. However, there are no data comparing the exact difference in the potency of these effects among the three agents.
ATHEROPROTECTIVE EFFECT
ln vitro anti-atherosclerotic effects of osmotin
Osmotin has been shown to exert the multiple effects in different cell types including ECs, monocytes/macrophages, VSMCs, adipocytes, and cardiomyocytes[10,11,33]. The anti-atherosclerotic effects of osmotin have been investigated using cultured human vascular cellsin vitro. In this section, the evidence regarding the anti-atherosclerotic effects of osmotin is introduced, as summarized in Figure 1.
Preventive effects of osmotin on vascular inflammation
Osmotin suppresses lipopolysaccharide (LPS)-induced upregulation of monocyte chemoattractant protein 1 (MCP1), tumor necrosis factor-α (TNF-α), intercellular adhesion molecule 1 (ICAM1), vascular cell adhesion molecule 1 (VCAM1), and E-selectin in human umbilical vein endothelial cells (HUVECs)[10]. It suppresses the TNF-α-induced adhesion of human THP1 monocytes to HUVECs[10]. Osmotin shifts toward an anti-inflammatory phenotype (M2) rather than pro-inflammatory phenotype (M1), associated with ERK1/2 and NF-κB downregulation and PPAR-γ upregulation in human THP1 monocyte-derived macrophages[10]. It also suppresses the LPS-induced secretion of interleukin 6 (IL6), pentraxin 3 (PTX3), and TNF-α from human THP1 monocyte-derived macrophages[10]. These findings indicate that osmotin suppresses vascular inflammation and endothelial dysfunction. In addition, it suppresses the proliferation of human EA.hy926 ECs[10], thus preventing intimal medial thickness. Osmotin mimics the suppressive effects of adiponectin on the expression of ICAM1, VCAM1, and E-selectin in ECs, monocyte-EC adhesion, and EC proliferation as well as inflammatory phenotype (M1) and TNF-α expression in macrophages[34-38].
Preventive effects of osmotin on macrophage foam cell formation
Osmotin suppresses Ox-LDL-induced accumulation of cholesterol ester (foam cell formation) by downregulating cluster of differentiation 36 (CD36) and acyl-coenzyme A:cholesterol acyltransferase 1 (ACAT1) as well as upregulating ATP-binding cassette transporter A1 (ABCA1) in human THP1 monocyte-derived macrophages[10]. These effects are consistent with the effects of adiponectin in suppressing Ox-LDL-induced foam cell formation and ACAT1 expression and enhancing ABCA1 expression in human monocyte-derived macrophages[39-41].
Preventive effects of osmotin on the migration and proliferation of VSMCs
Osmotin suppresses angiotensin II-induced migration of human aortic smooth muscle cells (HASMCs)[10]. It also suppresses the proliferation of HASMCs by decreasing the phosphorylation of RAF1, ERK1/2, and NF-κB as well as increasing AMPK expression[10]. Osmotin exerts the same suppressive effects of adiponectin and AdipoRon on the migration and proliferation of VSMCs[42-44].
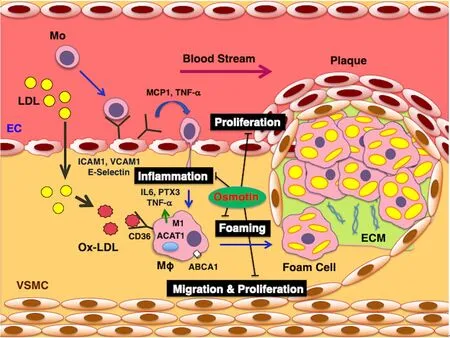
Figure 1. Cellular and molecular mechanisms mediating the preventive effects of osmotin on atherosclerosis. Osmotin suppresses the proliferation of vascular ECs. It suppresses vascular inflammation, characterized as monocyte-EC adhesion, by downregulating MCP1, TNF-α, ICAM1, VCAM1, and E-selectin in ECs, and suppresses inflammatory phenotype (M1) and secretion of IL6, PTX3, and TNF-α in monocyte (Mo)-derived macrophages (Mφ). Osmotin suppresses Ox-LDL-induced foam cell formation by downregulating CD36 and ACAT1 as well as upregulating ABCA1 in Mo-derived macrophages. In VSMCs, osmotin suppresses the migration, proliferation, and production of ECM proteins, such as collagen 1, fibronectin, and matrix metalloproteinase 2. Therefore, osmotin prevents the development and instability of atheromatous plaques. EC: endothelial cells; MCP1: monocyte chemoattractant protein 1; TNF-α: tumor necrosis factor-α; ICAM1: intercellular adhesion molecule 1; VCAM1: vascular cell adhesion molecule 1; IL6: interleukin 6; PTX3: pentraxin 3; Ox-LDL: oxidized low-density lipoprotein; CD36: cluster of differentiation 36; ACAT1: acyl-coenzyme A:cholesterol acyltransferase 1; ABCA1: ATP-binding cassette transporter A1; VSMCs: vascular smooth muscle cells; ECM: extracellular matrix
Modulatory effects of osmotin on ECM production in VSMCs
The intercellular networking that occurs among ECs, VSMCs, and macrophages leads to a fibroproliferative response, in which ECM plays an important role in atheromatous plaques. The ECM is composed of a mixture of vastly different macromolecules including collagens, fibronectin, elastin, and matrix metalloproteinases (MMPs), which are produced by VSMCs in the arterial wall. Osmotin suppresses the production of collagen 1, fibronectin, and MMP2, and increases that of elastin and MMP9 in the HASMCs[10]. The former contributes to preventing the development of atheromatous plaques, while the latter contributes to vascular elasticity and remodeling. However, osmotin has no effect on collagen 3 production in HASMCs[10]. Osmotin mimics the suppressive effects of adiponectin on collagen 1 expression in VSMCs[45].
Suppressive effects of osmotin on the glucose uptake and differentiation of adipocytes
Visceral adipose tissue promotes insulin resistance and metabolic disorders, resulting in the development of atherosclerosis. In particular, perivascular adipose tissue has been recently shown to have a close linkage with atherosclerosis[46]. Perivascular adipocytes residing in the vascular adventitia are recognized as endocrine cells[46]. Cross-talk between perivascular adipocytes and vascular cells in blood vessel wall modulates the formation of atherosclerosis by releasing adipocytokines[47]. Similar to adiponectin[48], osmotin suppresses the differentiation and proliferation of adipocytes by regulating the expression of p21, p27, and cyclin-dependent kinase 2, as well as improves glucose uptake in 3T3-L1 adipocytes[33].
Protective effects of osmotin against ischemia-reperfusion injury in cardiomyocytes
A recent study has shown the protective effects of osmotin against myocardial ischemia-reperfusion injury[11]. Osmotin protects rat cardiac myoblast H9c2 cells against ischemia-reperfusion injury through AdipoR1 by increasing phosphorylation of PI3K/Akt and decreasing that of NF-κB[11]. Osmotin exhibits the same cardioprotective effects of adiponectin and AdipoRon against ischemia-reperfusion injury[49,50]. These findings indicate that osmotin as well as adiponectin and AdipoRon could prevent myocardial damage following coronary events and ischemia-reperfusion injury.
ln vivo anti-atherosclerotic effects of osmotin
Several studies have shown that adiponectin and AdipoRon suppress the development of atherosclerotic lesions in apolipoprotein E-deficient (Apoe-/-) mice, an atherogenic mouse model, on a normal or high-fat diet[51-53]. Recently, the anti-atherosclerotic effects of osmotin have also been investigated using a variety of animal modelsin vivo. Treatment with osmotin suppresses abdominal fat accumulation in C57BL/6 mice fed with a high-fat diet[33]. InApoe-/-mice on a high-cholesterol diet, chronic infusion of osmotin prevents the development of aortic atherosclerotic lesions accompanied by an improved vascular inflammation and plaque instability[10]. In this model, osmotin also improves fasting plasma glucose level, free fatty acid level, and insulin resistance[10]. Similarly, injection of osmotin lowers serum levels of total cholesterol and triglyceride and prevents atherosclerosis in Wistar rats fed with a high-cholesterol diet[31]. Osmotin injection decreases serum levels of glucose, insulin, total cholesterol, and triglyceride in streptozotocininduced diabetic rats fed with a high-fat diet[32]. In addition, it protects against obesity and diabetes-induced nonalcoholic fatty liver disease in leptin-deficient obese (ob/ob) mice and leptin receptor-deficient diabetic (db/db) mice[54]. These effects are attributed to the stimulatory actions of osmotin on AMPK and PPAR-α pathways. Therefore, osmotin is also expected to be useful in the preventive health care in diabetics in future[55].
ANTI-INFLAMMATORY EFFECT
Atherosclerosis is an inflammatory vascular disease. The Canakinumab Anti-inflammatory Thrombosis Outcome Study trial provided direct evidence that inflammation accelerates cardiovascular disease in humans, by showing that a therapeutic antibody targeting IL1β decreased recurrent cardiovascular events[56]. This section introduces the beneficial effects of osmotin on inflammatory diseases other than atherosclerosis. Osmotin suppresses LPS-induced neuroinflammation through AdipoR1 followed by tolllike receptor 4 and NF-κB pathways in BV2 microglial cells[57]. In addition, infusion of osmotin using osmotic pumps attenuates dextran sodium sulfate-induced colitis in mice[58]. These effects are consistent with anti-inflammatory effects of adiponectin and AdipoRon[58-60]. The results fromin vitroandin vivoexperiments indicate that osmotin as well as adiponectin and AdipoRon could prevent inflammatory diseases.
NEUROPROTECTIVE EFFECT
Alzheimer’s disease is the most common form of dementia. The pathogenesis of Alzheimer’s disease involves characteristics such as amyloid-β deposition, tau phosphorylation, and apoptotic neurodegeneration[61]. The risk factors of Alzheimer’s disease are known to be inflammation, lipid metabolism (Apoe), and atherosclerosis[61-63]. A clinical prospective study has shown that atherosclerosis in carotid arteries leads to the progression of Alzheimer’s disease[64]. Osmotin protects against amyloid-β deposition, tau phosphorylation, and apoptotic neurodegeneration through AdipoR1 followed by the AMPK/sirtuin 1/sterol regulatory element-binding protein 2 pathway in neuronal cells[25,65-67]. It also enhances neurite outgrowth and synaptic complexity via AdipoR1/nogo-66 receptor NgR1 signaling[68]. These findings indicate that osmotin is a potential candidate for the treatment of neurological disorders such as Alzheimer’s disease. A preclinical trial study recently reported the usefulness of intravenous administration of osmotin-loaded magnetic nanoparticles in combination with electromagnetic guidance in the treatment of Alzheimer’s disease[69]. These effects of osmotin are compatible with neuroprotective effects of adiponectin and AdipoRon in Alzheimer’s disease[70-73].
ANTI-TUMORIGENESIS EFFECT
Cancer and atherosclerosis have been classified as non-communicable diseases by differing in target cells[74]. However, both diseases have principally identical mechanisms such as cell proliferation induced by inflammation and reactive oxygen species[75]. Adiponectin induces anti-angiogenesis and anti-tumor activity through AdipoR1 via caspase-mediated EC apoptosis[13]. Actually, adiponectin and AdipoRon suppress the proliferation of a variety of human cancer cells[76-78]. Similarly, osmotin induces cell cycle arrest in the G0/G1 phase[33]and suppresses cell proliferation[10]. These findings suggest the possibility that osmotin may suppress tumor growth. However, further studies are needed to clarify this hypothesis.
THERAPEUTIC STRATEGY
Osmotin is a natural plant protein that is ubiquitous in fruits and vegetables[13]. Osmotin is also known to be a phytochemical that is resistant to heat treatment (cooking)[13]. Furthermore, the osmotin protein is relatively stable and may maintain activity even through contact with the human digestive system[13]. Therefore, this phytochemical could be administered by ingestion of fruits and vegetables containing a great amount of osmotin or by oral administration of osmotin and/or its analogs that consist of the amino acid residue including an active center[10]. However, the best drug delivery system (DDS) for osmotin administration is considered to be enveloped in nanocapsules in order to avoid digestive degradation[10,69]. Moreover, continuing preclinical and clinical investigations with osmotin, in particular combined with a nanocapsule system as carrier vehicles into atherosclerotic lesions, are essential for future studies[10]. It is very important for DDS establishment to design the size of the nanocapsule so that it can go through a relatively wide gap between damaged vascular ECs, but not a normal gap between intact ECs in humans. The magnetic nanocapsule-based DDS with external electromagnetic guidance may be more useful to make osmotin cross endothelial gap junction[69]. Future studies are needed to clarify whether oral administration of osmotin-loaded nanocapsules can treat vascular lesions in patients with atherosclerosis. Similarly, osmotin-like proteins derived from many vegetables and fruits are also regarded as adiponectin peptidomimetics and gather attention as novel therapeutic drug candidates for atherosclerosis and related diseases[79].
CONCLUSION
These findings indicate that the novel phytochemical osmotin might be an effective therapeutic agent for atherosclerosis, inflammation, neurodegeneration, and their related diseases and that AdipoR1 might be a crucial therapeutic target for these diseases. Osmotin may open up a new therapeutic window in the treatment of atherosclerosis in cases with hypoadiponectinemia and/or resistance against adiponectin and AdipoRon[10,30,80]. Osmotin also provides benefits to maintain vascular health and prevent vascular disease in healthy individuals.
DECLARATIONS
Acknowledgments
The author thanks Ms. Yui Takahashi for her technical assistance.
Authors’ contributions
The author contributed solely to the article.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported in part by a Grant-in-Aid for Scientific Research (C) (17K08993 to T.W.) from the Japan Society for the Promotion of Science.
Conflicts of interest
The author declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2020.