Thrombolytic Activity of Nattokinase Produced by Bacillus subtilis natto LNUB236
ZHU Junfeng, LIU Shuai, ZHAO Pengyan, ZHAO Jian, LI Jiazeng, WANG Tianqi, WANG Li, LIU Hongsheng,*
(1. School of Life Sciences, Liaoning University, Shenyang 110036, China;2. School of Pharmacy, Liaoning University, Shenyang 110036, China)
Abstract: Objective: The aim of this study is to evaluate the thrombolytic effects of nattokinase (NK) produced by Bacillus subtilis natto LNUB236 in vitro and in vivo. Methods: The thrombolytic effect of NK in vitro was determined by the dissolution rate of blood clots. Meanwhile, the in vivo effect was assessed by using a carrageenan-induced rat tail thrombosis model and a ferric chloride-induced carotid arterial thrombosis model as well as measuring mouse tail bleeding time. The toxicity of NK was measured by acute toxicity test (14 days) and chronic toxicity test (28 and 90 days) in rats. Results: NK significantly increased the dissolution rate of blood clots, while it significantly reduced the length of carrageenan-induced rat tail thrombus and the mass of ferric chloride-induced-carotid thrombus (P < 0.05). In addition, NK significantly prolonged the tail bleeding time of mice. In the acute and chronic toxicity tests, the tissues of the mice in the NK group had no pathological damage, and blood biochemical indexes were in the normal range. Conclusion: The NK produced by Bacillus subtilis natto LNUB236 exerts a thrombolytic activity both in vitro and in vivo, without any toxicity for the body. This study can lay the foundation for the development of functional foods containing NK produced by Bacillus subtilis natto LNUB236.Keywords: nattokinase; thrombolytic activity; toxicity
Natto, a popular traditional food in Japan, is made from soybeans fermented byBacillus subtilis natto. The health benefits of eating natto are well documented and have been attributed to several chemical constituents such as nattokinase (NK) and vitamin K2[1]. NK is a low cost serine protease that is produced byBacilus subtilisduring fermentation of the natto. NK possess a low molecular mass, which facilitates its direct absorption by the oral and alimentary canals[2-3]. A strong fibrinolytic activity was reported when NK was applied directly to the fibrin plates.We have revealed in previous studies that NK possesses a fibrinolytic activity, as well as activates other fibrinolytic enzymes such as tissue plasminogen activator (t-PA),urokinase (UK), thrombin, and kallikrein[4].
Cardiovascular diseases, including high blood pressure and coronary heart disease, are the main cause of death worldwide. Thrombotic events are typical endpoints associated with cardiovascular diseases[5-6]. Indeed, it has been estimated that 2-4 of 1 000 persons may require anticoagulant and antithrombotic therapy each year for the treatment of symptomatic deep-vein thrombosis and pulmonary embolism[7]. Thrombosis is a multistep episode that is associated with endothelial lesion and blood coagulation,along with deposits of fibrin and erythrocytes in static regions, or in regions of low shear stress. It is a pathological condition that is occurs in post-traumatic and post-operative conditions[8-9]. The most effective way to treat clinical thrombotic disease is to use thrombolytic drugs, such as UK,streptokinase (SK) and t-PA. However, these are too expensive and possess a short half-life in the circulation[10-11]. Besides, these agents can only be administered through injection, and they usually cause excessive bleeding and recurrent thrombosis at the site of the residual thrombus[12-13]. As a natural product, NK can be a good alternative protease for oral fibrinolytic therapy because of its stability, strong fibrinolytic activity, and its well-reported long-term safety[14-15].
In previous study, we had identified and extracted NK with high fibrinolytic activity from theBacillus subtilis nattoLNUB236[4]. In this study, we evaluated the thrombolytic effect and the toxicity of NK produced byBacillus subtilis nattoLNUB236.
1 Materials and Methods
1.1 Animals, materials and reagents
Animal experiments were carried out according to theGuidelines for Animal Experimentationset by Liaoning University (Liaoning, China). The study protocol was approved by the Animal Ethics Committee of Liaoning University. Male New Zealand white rabbits (license:SCXK(liao)2018-0002, 2.3-3.0 kg), Sprague-Dawley rats(license: SCXK(liao)2015-0001, 220-280 g) and Kunming mice (license: SCXK(liao)2015-0001, 18-22 g) were purchased from Liaoning Changsheng Biotech (Liaoning, China) and housed under controlled environmental conditions ((25 ± 2) ℃and (55 ± 10)% relative humidity) with a natural light-dark cycle. Animals were given ad libitum access to food and water.
UK was purchased from Nanda Pharmaceuticals(Nanjing, China); Phenyl Sepharose 6 Fast Flow and Sephadex G-75 columns were obtained from GE Healthcare(Piscataway, NJ, USA); Protein standard markers were purchased from Amersham (Buckinghamshire, UK);Bovine fibrinogen and thrombin were obtained from the National Institutes for Food and Drug Control (Beijing,China); Enteric-coated capsules were produced by Qiangji Pharmaceuticals (Guangdong, China);κ-Carrageenan was provided by the Tokyo Chemical Industry (Tokyo, Japan);Enzyme-linked immunosorbent assay test kits for D-dimer and FDPs were purchased from the Shanghai Biological Technology (Shanghai, China). All the other reagents were of analytical grade.
1.2 Instruments and equipments
HZQ-R constant temperature oscillator Harbin Donglian Electronic Technology Development Company Limited; YC-1 Chromatography experiment freezer Beijing Boyi Kang Experimental Instrument Company Limited;Electric constant temperature drying oven Shanghai Longyue Instrument Equipment Company Limited; GR21G high speed refrigerated centrifuge Japan Hitachi Company.
1.3 Methods
1.3.1 Fermentation
The NK-producing strain Bacillus subtilis natto LNUB236 was developed from natto by our research team.Bacillus subtilis natto LNUB236 was grown at 37 ℃ in a 500 mL flask containing 200 mL Luria-Bertani (LB) medium rotating at 170 r/min for 30 h at room temperature.
1.3.2 Purification of NK
NK was purified using hydrophobic chromatography and gel-filtration chromatography. All purification steps were performed at 4 ℃. The crude enzyme solution was separated from the fermentation broth by centrifugation at 8 000 r/min for 20 min at room temperature. The crude enzyme solution was then precipitated using 35%-65% (NH4)2SO4and protein was collected by centrifugation and dissolved in phosphate buffered saline (PBS). The crude enzyme solution was passed in PBS through a Sephadex G-75 column (1.0 cm × 50 cm) and the diluted NK solution was applied to a Phenyl Sepharose 6 Fast Flow column (1.0 cm × 20 cm). Finally, active fractions were pooled and determined by sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE).
1.3.3 Detection of NK
SDS-PAGE was carried out to determine the purity and molecular mass of the purified enzyme. Gels were stained using Coomassie brilliant blue R250. Fibrinolytic activity was determined using fibrin plates. To observe fibrinolytic activity,5 μL of the enzyme solution was added dropwise onto a fibrin plate and incubated for 18 h at 37 ℃. The fibrinolytic activity of NK was estimated by measuring the dimension of the clear zone on the fibrin plate and plotting a calibration curve based on standard solutions of UK.
1.3.4 Thrombolytic effects of NK in vitro
The thrombolytic effect of NK was measured as described previously[16]. Briefly, fresh blood was collected from the ear marginal artery of male New Zealand white rabbits and allowed to clot spontaneously. Then, the formed blood clot was added into different doses of NK in individual tubes. The tubes were incubated in a shaking thermostat for 3 h at 60 r/min at 37 ℃. Physiologic (normal)saline (0.9%) was used as a control. Each condition was performed in triplicates. The supernatant from each triplicate was withdrawn at different time points, and the number of released red blood cells (RBCs) was observed under light microscope. Dissolution of blood clot in each sample was calculated using the equation (1).

Where mais the mass before dissolution/g; mbis the mass after dissolution/g.
1.3.5 Hemolysis of erythrocytes
A suspension of RBCs (2%) was prepared according to the protocol described in the Chinese Pharmacopoeia[17],and different amounts of NK (80, 120, 160, 200, 240 and 280 IU) were added into the suspension. After gentle mixing,the absorbance of the mixture was determined using a spectrophotometer at 545 nm. Hemolysis was determined using the formula (2).
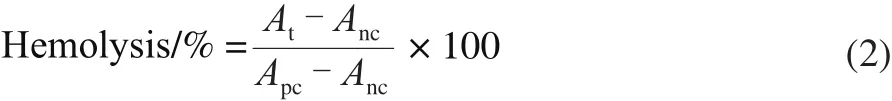
Where Atis the test absorbance; Ancis the negative absorbance; Apcis the positive absorbance. NK was considered not suitable for injection if hemolysis was more than 5%[12].
1.3.6 NK activity in simulated gastric fluid
Oral administration of an aqueous solution of NK can inhibit NK activity directly in simulated gastric fluid(SGF). NK activity in SGF was measured as previously described[18-19]. NK was added to pre-heated SGF (1 L at 37 ℃). Samples were then taken at different times points and spotted onto a fibrin plate. The plate was incubated for 18 h at 37 ℃.NK activity was evaluated by measuring the size of the dissolved area on the fibrin plate in comparison to a standard curve.
1.3.7 Carrageenan-induced rat-tail model
A carrageenan-induced rat-tail model was created according to a previously reported method[20]. A temperature of (15 ± 2) ℃ was used for the establishment of a thrombosis model. Female Sprague-Dawley rats were divided randomly into five groups. Group 1 served as a negative control, in which rats were treated with normal saline (NS). On the other hand, group 2 served as a positive control and rats were treated with UK. Groups 3, 4 and 5 were given 5 000,10 000 and 15 000 IU of NK per kilogram of body mass,respectively. Twelve days after drug administration (p.o.), rats were injected with carrageenan (20 mg/kg, s.c.) in a hind-leg toe, and the length of the tail thrombus was measured after 24 h. Blood from the orbit was collected after measurement of the thrombus, and D-dimer and FDP contents in the plasma were determined using the relevant kit.
1.3.8 Ferric chloride-induced carotid arterial thrombosis
Formation of a carotid arterial thrombus in vivo was investigated as previously described[21]. NK or UK were administered (p.o.) to male Sprague-Dawley rats for 7 days.A carotid arterial thrombus was formed by wrapping a piece of filter paper (1.0 cm × 1.0 cm) that is saturated with 50%ferric chloride around the carotid artery for 15 min. After 40 min, the vessels around both sides of the filter paper were ligated, and the thrombus removed and weighed.
1.3.9 Tail bleeding time in mice
NK enteric-coated capsules were administered orally to rats with experimental induced thrombus is not suitable for mice. Thus, drugs were administered through intraperitoneal injected[22]. Mice were divided randomly into five groups of six and were treated with NS, UK or NK (5 000, 10 000,15 000 IU/d) through intraperitoneal injection for 5 days,respectively. Two hours after the final injection, a sharp incision of 3 mm was made in the tail tip and the tail was immersed immediately in NS at 37 ℃. The time from tail transection to bleeding cessation was defined as the“bleeding time”[23].
1.3.10 Acute and chronic toxicity of NK
Lyophilized powder of NK was filled into special enteric-coated capsules and fed to the rats. The rats were then observed for acute and chronic toxicity[24-25].
1.3.11 Acute toxicity test of NK
Rats were administered with enteric-coated capsules of NK at a dose of 5 000 mg/kg. The control group was orally administered with empty enteric-coated capsules. 10 rats were included in each group, 5 males and 5 females. The toxicity in the rats was observed and recorded in detail for 14 days[26].The body mass was weighed on the first day, the fourth day,the seventh day, the eleventh day, and the fourteenth day of the experiment period. All rats were dissected at the end of the experiment, and observed for visible diseased[27].
1.3.12 Chronic toxicity test of NK
Healthy SD rats of different genders were randomly divided into the following 4 groups: control group, and low,medium and high doses of NK. The low, medium and high doses of NK were 120, 360 and 1 000 mg/kg, respectively. 12 rats were included in each group, half male and half female.The control group was given empty enteric-coated capsules.Each group of rats was administered once a day for 28 or 90 days[28]. The body mass was measured once a week during the experimental period. After stopping the administration,the rats in each group were fasted for 12 h. The rats were then anesthetized with 3% sodium pentobarbital solution,and blood samples were taken to measure hematological,biochemical and coagulation indicators. After the blood was collected, the rats were dissected, and the brain, spleen,thymus and other organs were weighed to calculate organ coefficients. Finally, the main organs of each group were examined for pathologic states[29].
1.4 Statistical analyses
Statistical analysis was performed using PRISM v6.0.Values are represented as ±s.Non-parametric coagulation data was evaluated using the Kruskal-Wallis test.P< 0.05 was considered significant.
2 Results and Analysis
2.1 Purification of NK and its fibrinolytic activity
NK produced byBacillus subtilis nattoLNUB236 was purified using the chromatographic procedure described in the Materials and Methods section, and the protein was subjected to SDS-PAGE and testing on fibrin plates. The purified NK protein showed a single band of about 28 kDa by SDS-PAGE(Fig. 1A), and possessed a strong fibrinolytic activity on fibrin plates (Fig. 1B).
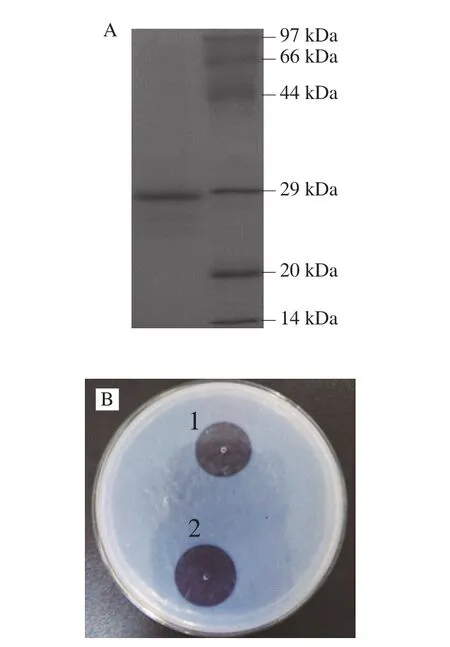
Fig. 1 Purification and fibrinolytic activity of NK
2.2 Thrombolytic activity of NK in vitro
Dissolution of blood clots was detected by adding different doses of NK to artificial rabbit blood clots. NK at 100, 200 and 300 IU/mL lysed the clots at dissolution percentages of 42.58%, 51.87% and 83.12%, respectively.This was significantly higher than the dissolution percentages of UK at the same concentrations (Fig. 2A). UK showed a small change in the dissolution of blood clots compared to that of NK.
Moreover, the erythrocyte count from the NK group was significantly higher than that of the UK and the PBS groups at each time point (Fig. 2B). There was no significant change in the erythrocyte count from the UK and PBS groups which showed very similar counts at each time point. On the other hand, the number of erythrocytes released from the NK group increased significantly with time, and the erythrocytes released showed an intact smooth surface. Interestingly,the enzymatic activity of NK at 300 IU did not result in erythrocyte destruction.
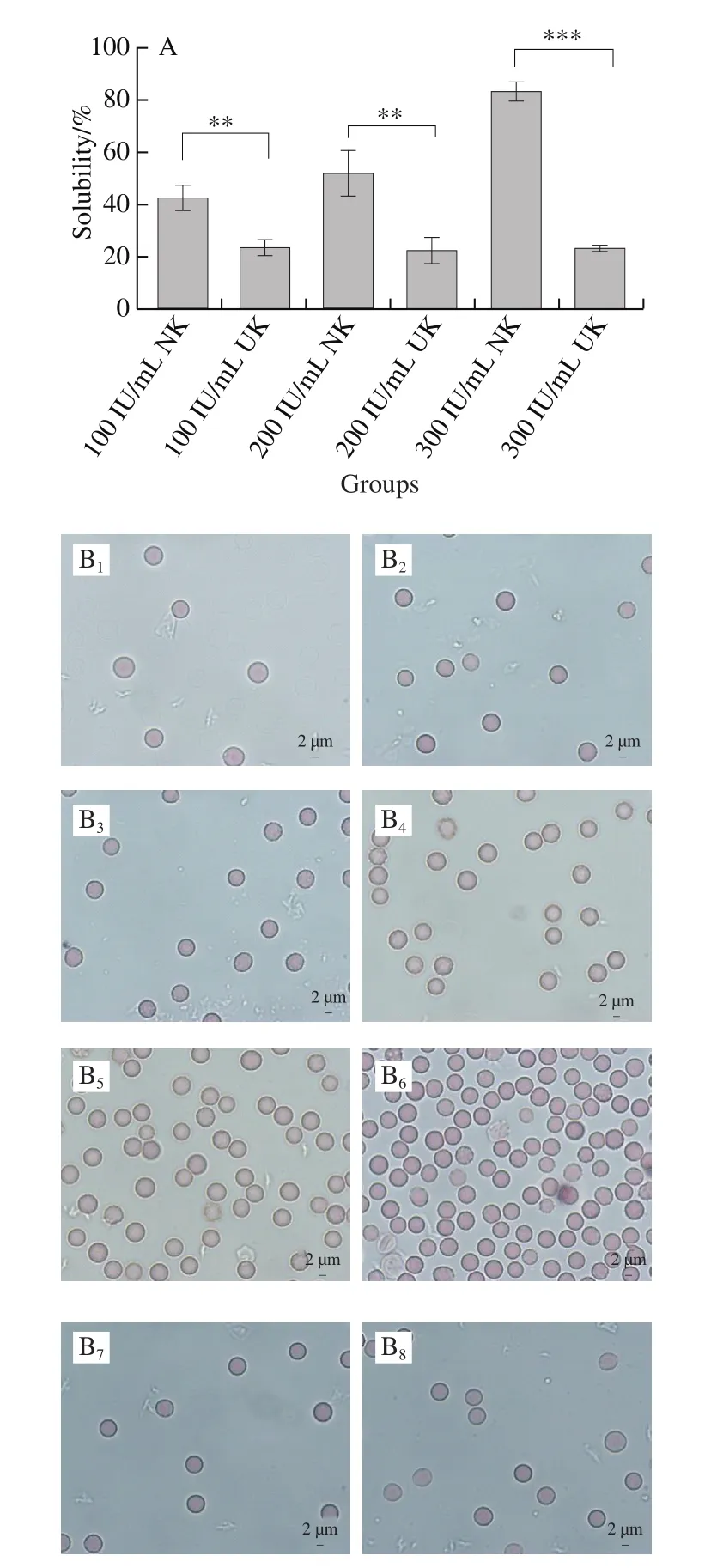
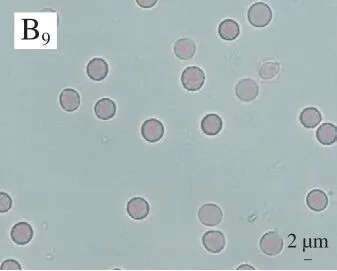
Fig. 2 Thrombolytic activity of different doses of NK on blood clots from rabbits in vitro
2.3 Hemolysis of erythrocytes
Different doses of NK were added to rabbit erythrocytes,and their hemolysis rate was measured. There was no obvious hemolysis or agglutination in initial rabbit erythrocytes. At a NK dose of 280 IU/mL, hemolysis was more than 5%.However, hemolysis was less than 5% at all other NK doses(Fig. 3), which deem them safe for intravenous injection.
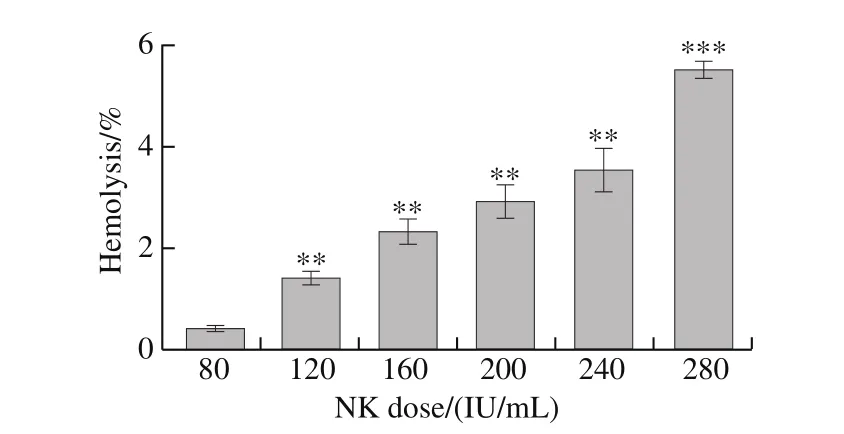
Fig. 3 Hemolysis of rabbit erythrocytes upon the addition of NK
2.4 NK activity in simulated gastric fluid

Fig. 4 NK activity in simulated gastric fluid
NK activity was also evaluated in SGF. After initiating the experiment, samples were taken at different times and spotted on fibrin plates. NK placed in SGF for 3, 5, 10, 15, 30 and 60 min did not lead to formation of dissolved rings (Fig. 4).However, the same concentration SGF-free NK resulted in the formation of dissolved rings. These results indicate that NK activity can be inhibited by SGF. Therefore, NK was administered via the intragastric route using enteric-coated gelatin capsules loaded with freeze-dried NK powder, protect NK from being inactivated by gastric acid and allow it to be released in the intestine.
2.5 Carrageenan-induced rat-tail model
A carrageenan-induced rat-tail thrombosis model was conducted to ascertain the thrombolytic effect of NKin vivo. NK enteric-coated capsules administered via the oral route effectively inhibited thrombus formation in this model(Fig. 5A). Indeed, NK reduced the length of the tail thrombus in a dose-dependent manner (Fig. 5B). The length of the tail thrombus in the NK-administered rats was significantly shorter than that of the NS group. These results suggest that NK can prevent tail thrombosis induced by carrageenan.
2.6 NK increases the levels of FDP and D-dimer in blood
The effect of NK on FDP and D-dimer levels is shown in Fig. 5C and D. Compared with the control group, the blood level of FDP was significantly higher in rats treated with NK at 10 000 (P< 0.01) and 15 000 IU/kg (P< 0.001). Similarly,the amount of D-dimer was significantly higher in rats treated with 5 000, 10 000 and 15 000 IU/kg, compared with the NS-treated group (P< 0.001). The anti-thrombotic activity of NK was significantly stronger than that of UK.
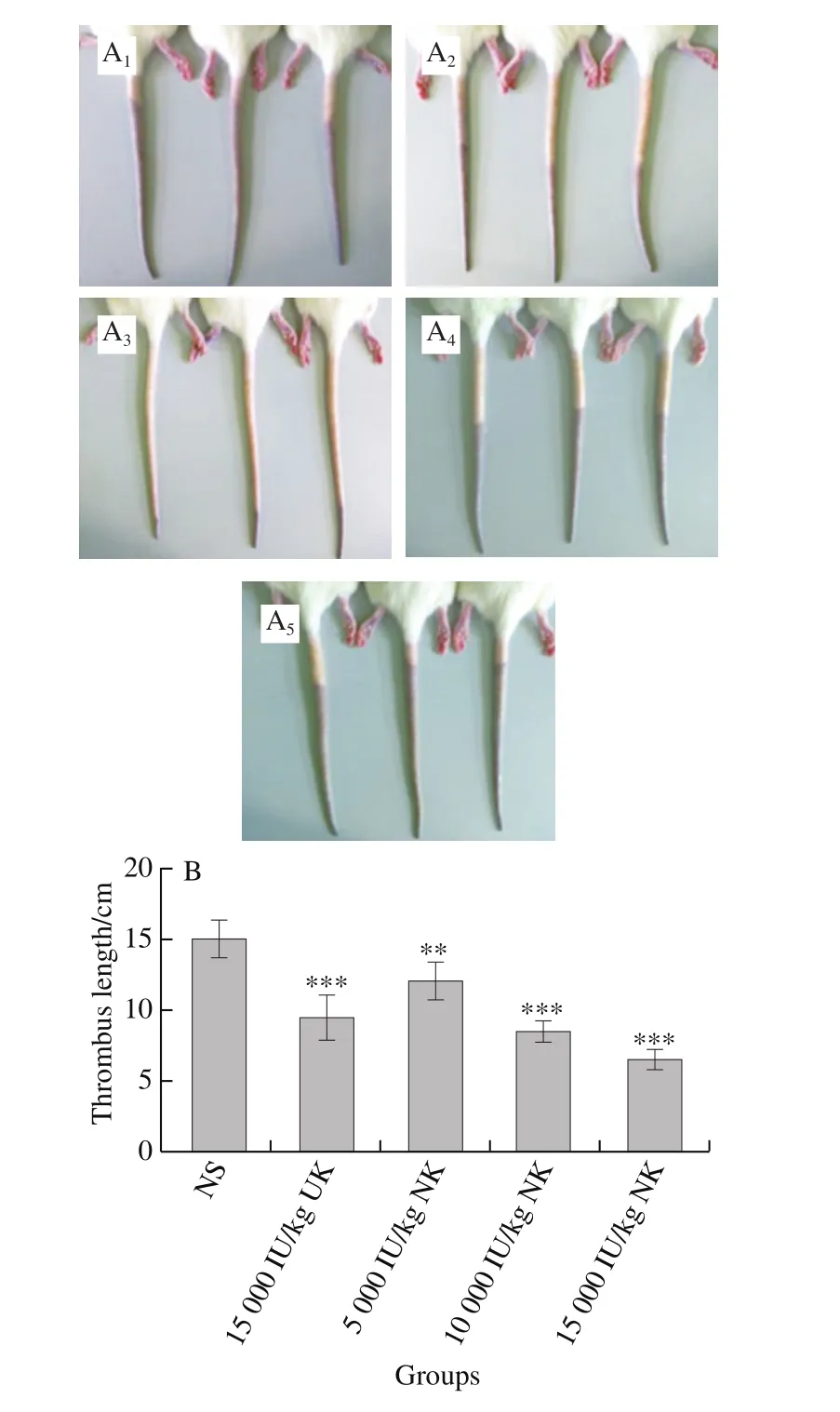
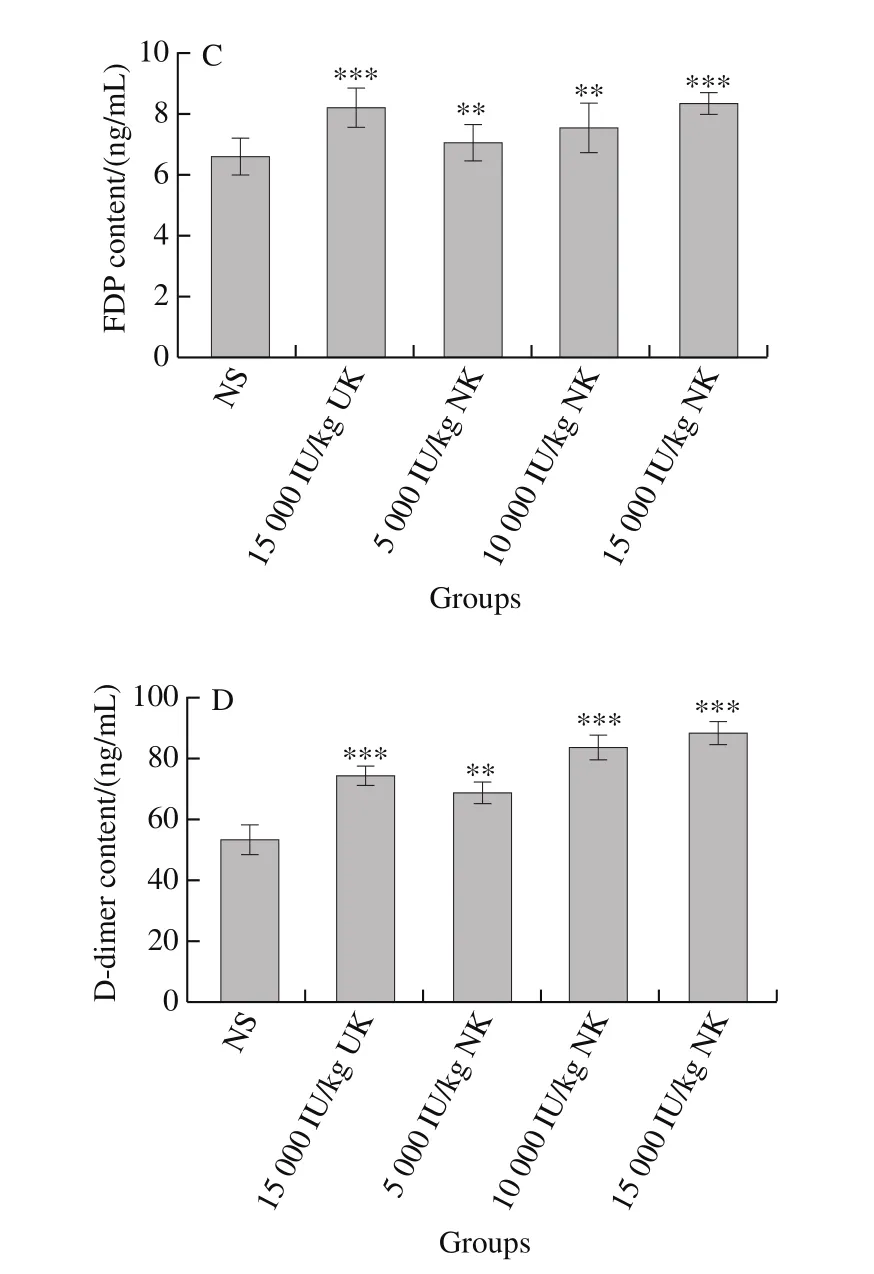
Fig. 5 Thrombolytic effect of NK in a carrageenan-induced rat-tail thrombosis model (n = 6)
2.7 Ferric chloride-induced carotid arterial thrombosis
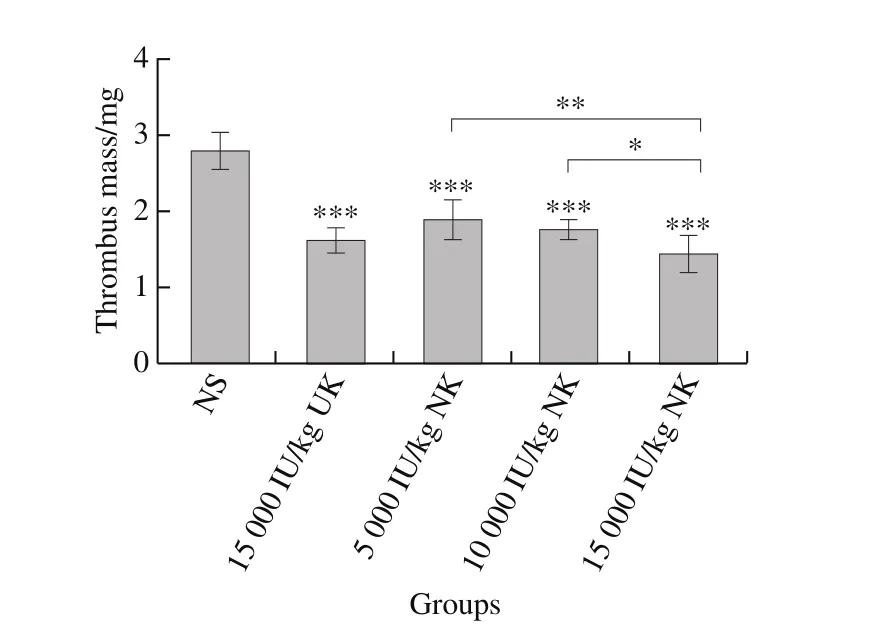
Fig. 6 Measurement of rat arterial thrombus mass (n = 6)
The anti-thrombotic activity of NK was evaluated in a carotid arterial thrombosis model in rats. Vessels were bright-red and blood flowed normally before thrombus formation. The vascular wall darkened after thrombus formation, which was obvious in NS, UK and NK groups(Fig. 6). However, rats pretreated with NK showed a highly significant reduction in thrombus mass, compared with rats pretreated with NS (P< 0.001).
2.8 Tail bleeding time in mice
The effect of NK on bleeding time was determinedin vivoin mice after intraperitoneal administration of NK. The mean bleeding time was 2.5 min in the NS group (Fig. 7), and it was prolonged when mice were administered 5 000, 10 000 and 15 000 IU/kg of NK. Interestingly, at 15 000 IU/kg, NK prolonged the bleeding time to 11.4 min.

Fig. 7 Changes in tail bleeding time after intravenous injection of NK and UK in vivo
2.9 Acute toxicity test results of NK

Fig. 8 Acute toxicity test of NK
Rats were administered with 5 000 mg/kg of NK entericcoated capsules and monitored for 14 days. No abnormalities were observed in the rats during the experiment. The rats were dissected after 14 days of NK administration, and no lesion was observed in the main organs such as the heart, the liver and the lung. In Fig. 8A and B, NK has no significant effect on the mass of the rats, and there is no significant difference in mass between treated and nontreated rats(P> 0.05). These results indicate that NK does not exert risk of acute poisoning.
2.1 0 28-Day toxicity test of NK
No abnormalities were observed in the rats during the experiment. Indeed, no obvious symptoms of drug toxicity and no abnormal sudden death occurred during the experiment time. As seen in Fig. 9A and B, the body mass of the rats increased normally during 28 days of NK administration. There was no significant change in the body mass of rats in each of the drug-administered group,compared with the control NS group (P> 0.05).
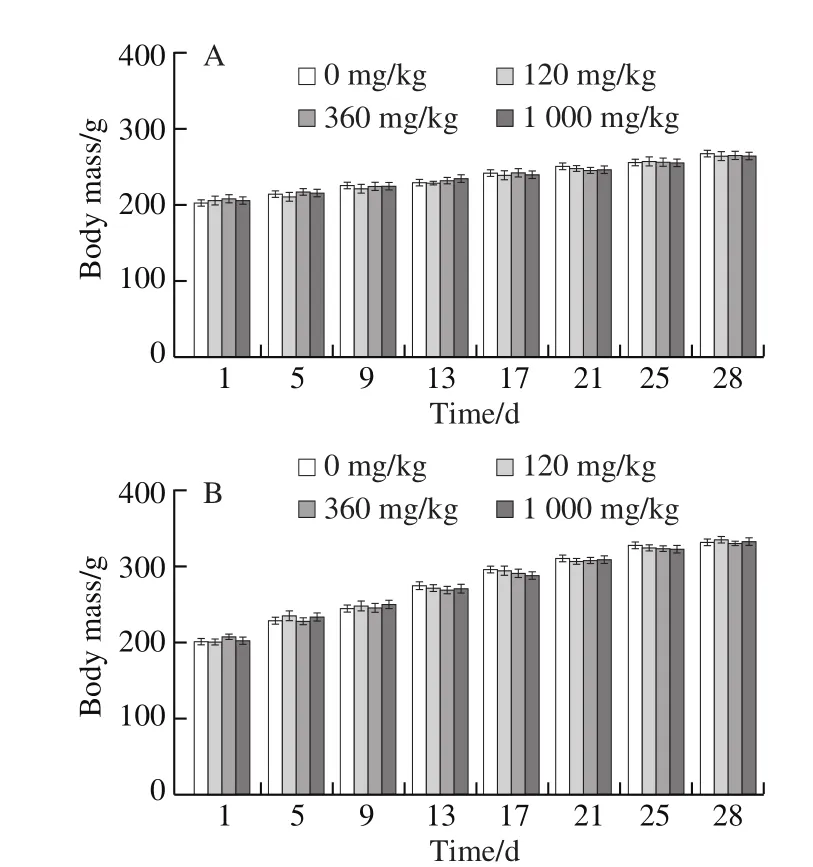
Fig. 9 Long-term effects of NK on rats
As shown in Table 1, mean corpuscular hemoglobin concentrations (MCHC) in male rats after 28 days of NK administration in the middle dose and the high dose groups were significantly lower than that in the control group(P< 0.05). The MCHC index of the high-dose group of female rats was significantly lower than that of the control group (P< 0.05). These indices were all within the normal range of rat MCHC index. There was no significant difference in the other measured hematological parameters between the NK group and the control group (P> 0.05).
As shown in Table 2, after 28 days of administration,blood triglycerides in the NK middle dose group and the high dose female rats were lower than the control group(P< 0.05). On the other hand, there was no significant change between all NK dose groups and the control group in all other biochemical blood measurements (P> 0.05).
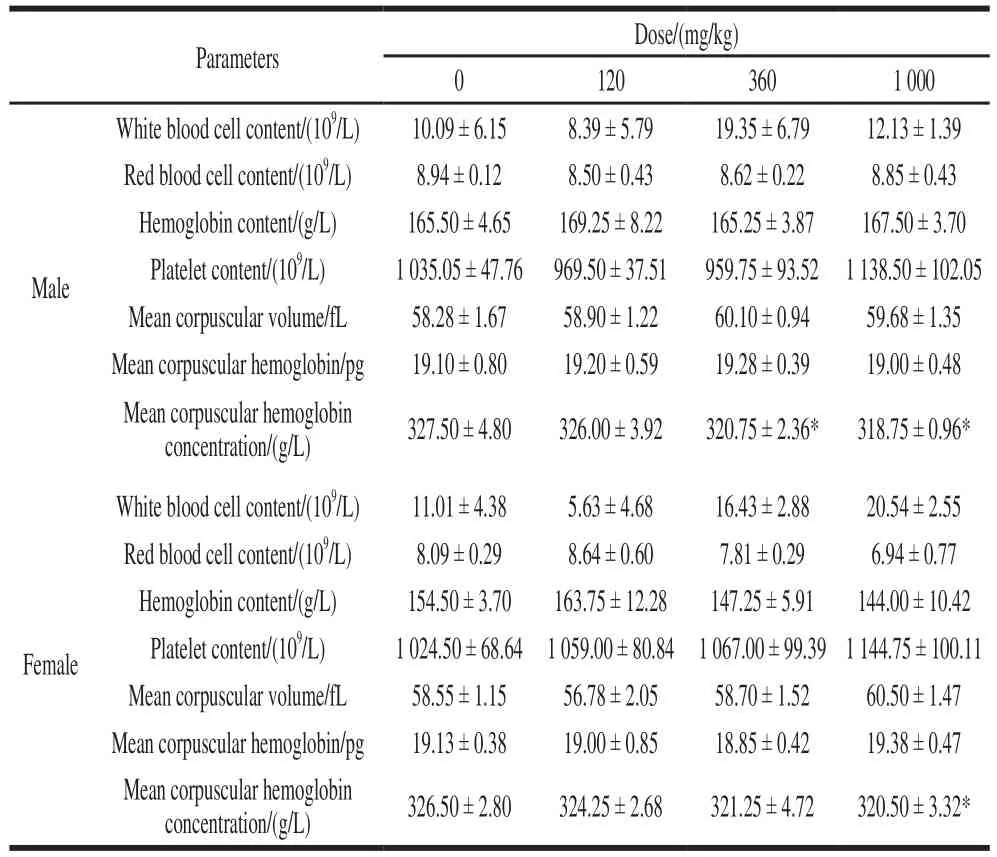
Table 1 Effect of NK administration for 28 days on hematological parameters in rats (n= 6)
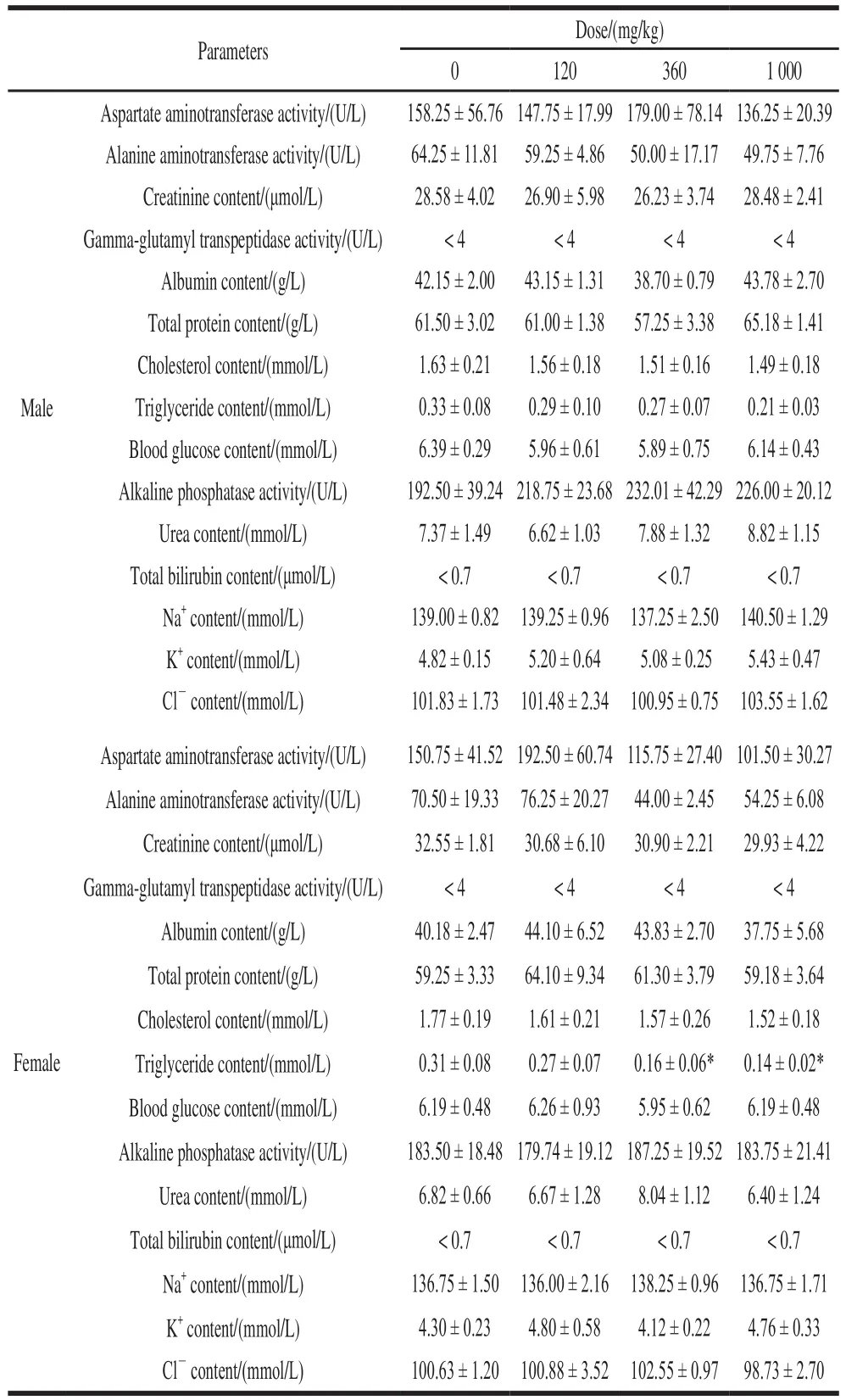
Table 2 Effect of NK administration for 28 days on blood biochemical parameters in rats (n= 6)
As shown in Table 3, the activated partial thromboplastin time (APTT) index in female rats of the high-dose group was significantly higher than that of the control group (P< 0.05).Fibrinogen content extremely significantly decreased in the high dose group (P< 0.01), but values were still within the normal measurement range in rats. The other dose groups did not show any significant change, compared to the control group (P> 0.05).
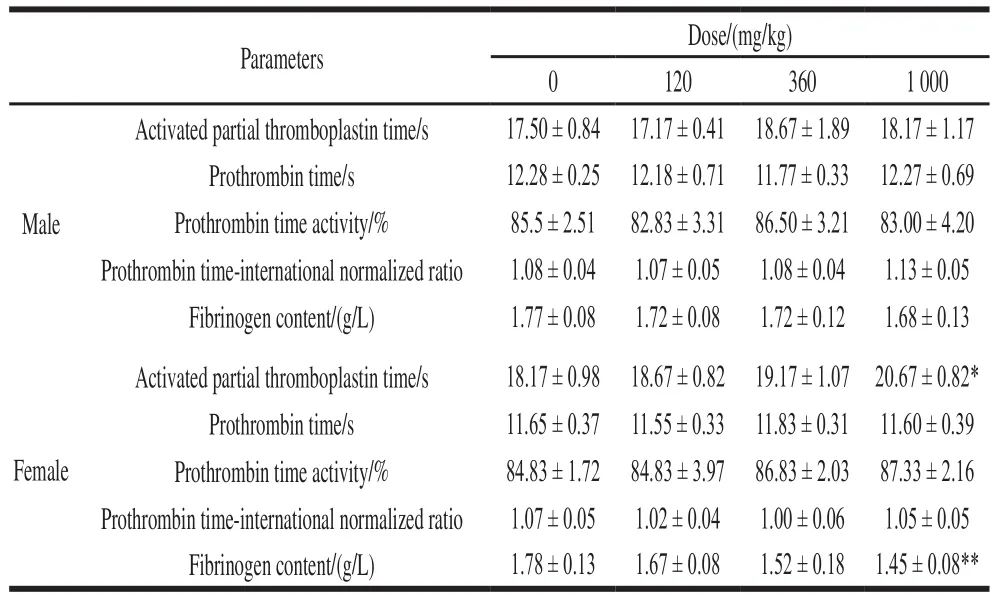
Table 3 Effect of NK administration for 28 days on coagulation parameters in rats (n= 6)
As shown in Table 4, the organ coefficient of the rats in the NK group was not significantly different from that in the control group (P> 0.05), which indicates that NK has no effect on organ damage in rats.
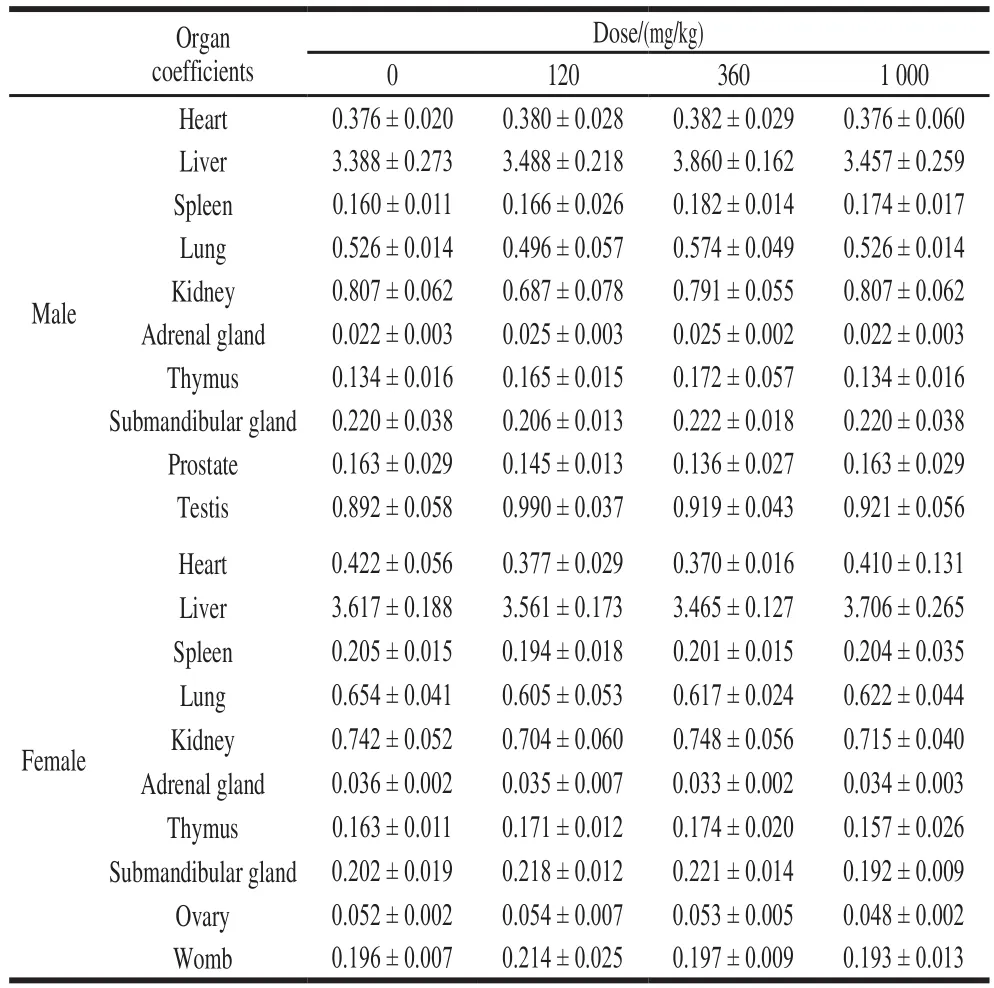
Table 4 Effect of NK administration for 28 days on organ coefficients in rats (n=6)
2.1 1 90-Day toxicity test of NK
During the administration of NK to the rats, behavioral activities, vital signs, glandular secretion, and excretion of the rats in each group were normal, and there was no toxicity or death. As shown in Fig. 10A and B, NK had no significant effect on the body mass of rats during the 90-days administration period. There was no significant difference between the male and female rats in the low, middle and high doses of NK, compared to the control group (P> 0.05).
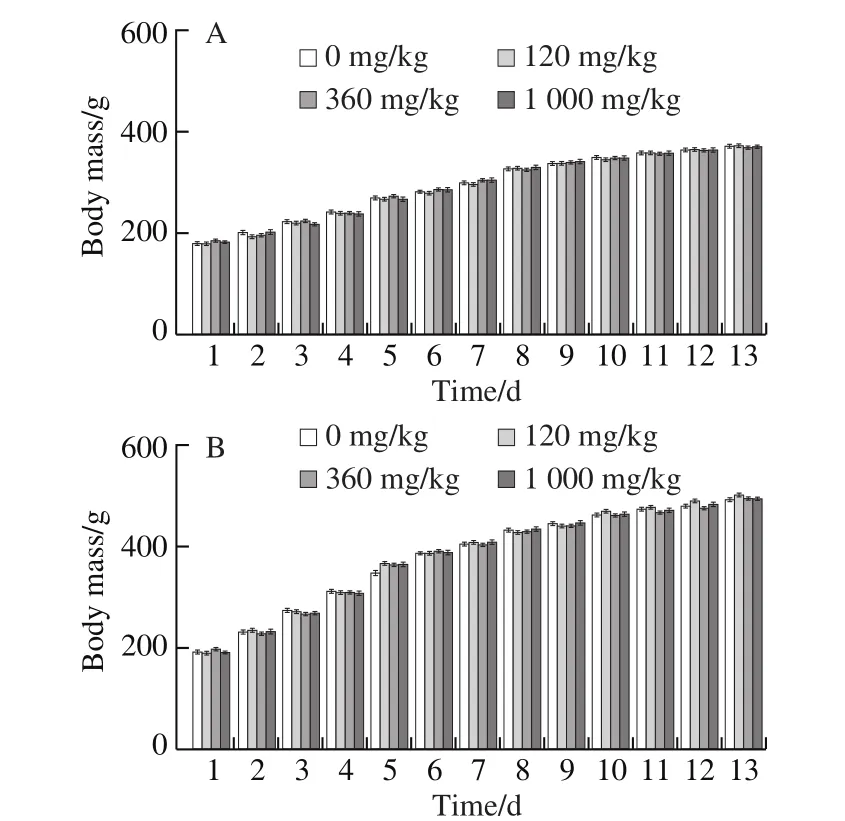
Fig. 10 Long-term chronic effects of NK in vivo
As shown in Table 5, after 90 days of administration,the hematological parameters of the NK group were not statistically significant compared to the control group(P> 0.05), which indicates that NK had noin vivohematological effects.
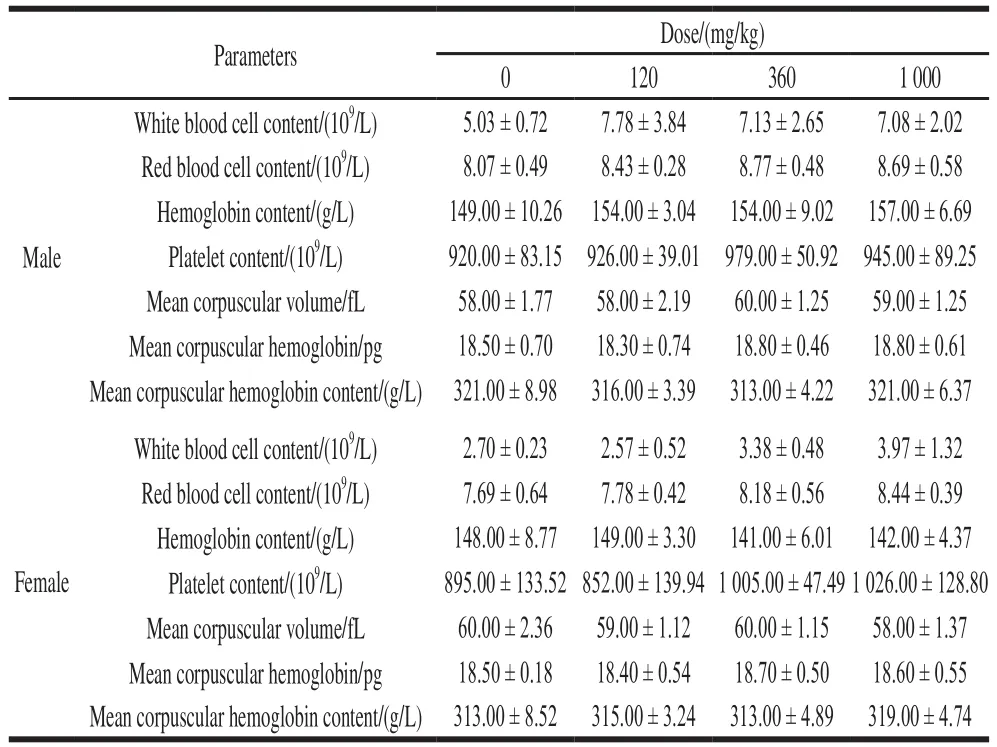
Table 5 Effect of NK administration for 90 days on blood parameters in rats (n= 6)
As shown in Table 6, compared with the control group,triglycerides content of male rats was significantly decreased in the middle and the high dose groups, while cholesterol was only significantly decreased in the high dose group (P< 0.05).There was no significant difference in blood biochemical parameters between the other NK groups and the blank group (P> 0.05).
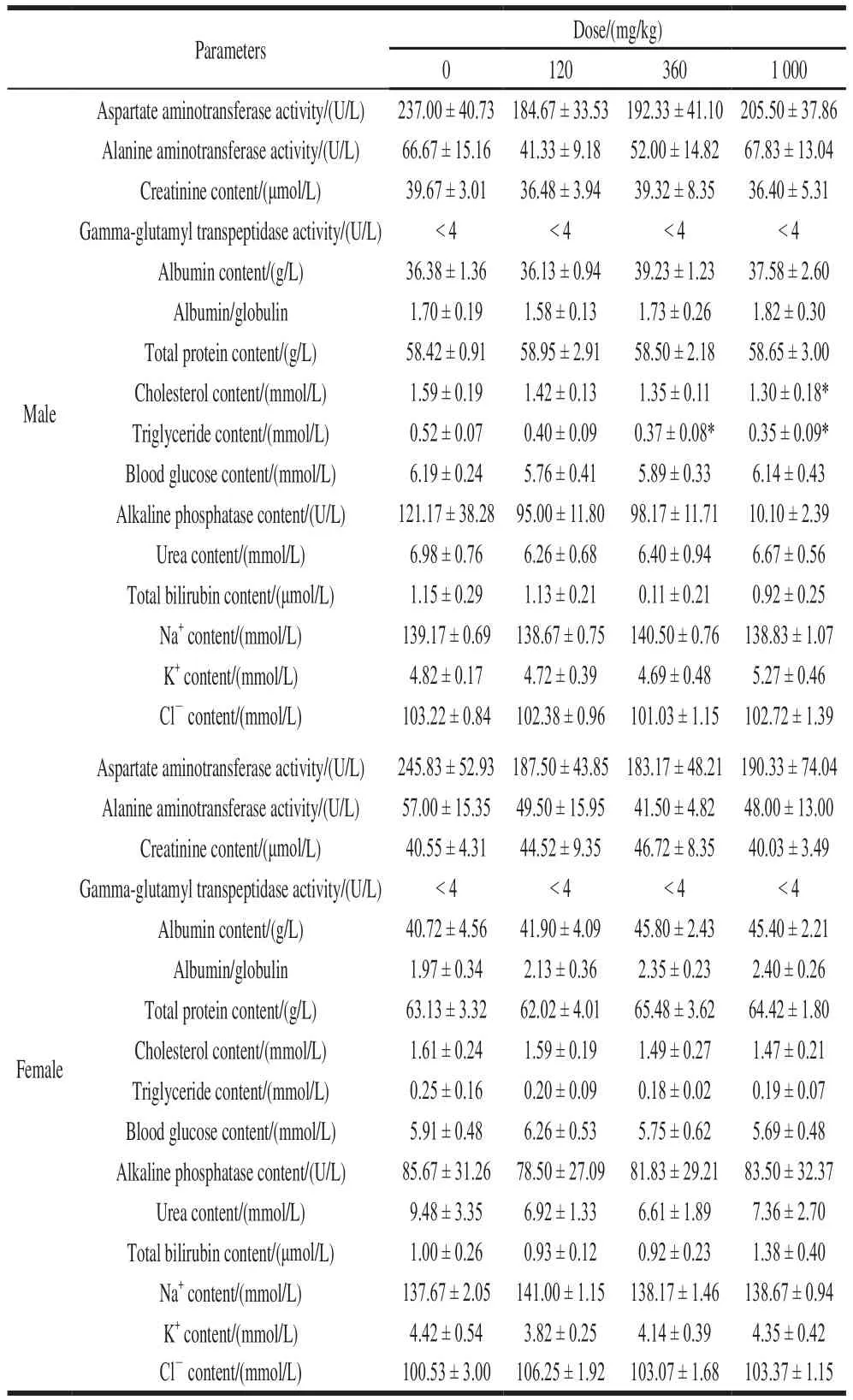
Table 6 Effect of NK administration for 90 days on blood biochemical parameters in rats (n=6)
The APTT of female rats in the high dose NK group was significantly higher than that of the control group(P< 0.05), and its value was within the normal range of rats. The coagulation parameters of the other administration groups were not statistically different from the control group (Table 7).
After 90 days of administration, there was no significant difference in the organ coefficient between the NK-administered group and the control group (P> 0.05).The results showed that NK had no effect on the visceral coefficient of rats (Table 8).
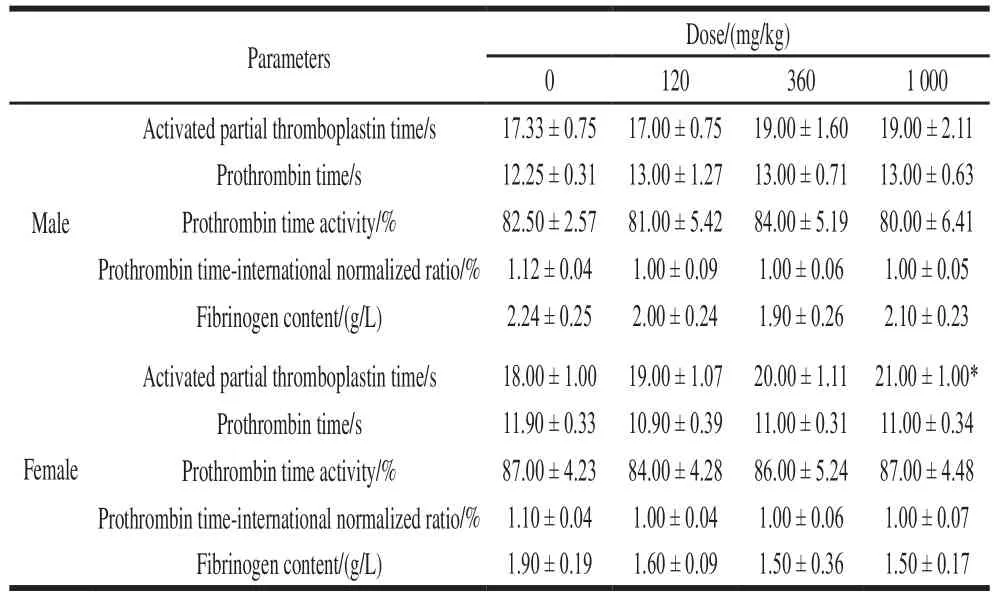
Table 7 Effect of NK administration for 90 days on coagulation parameters in rats (n= 6)

Table 8 Effect of NK administration NK for 90 days on organ coefficients in rats (n= 6)
3 Discussion
The use of fibrinolytic agents, such as plasminogen activators (SK, UK, t-PA), is highly effective for treating thrombosis. However, these agents can only be administered through injection[30]. NK is absorbed effectively across the intestinal tract of rats and it induces fibrinolysis after intraduodenal administration[31]. In the present study, NK administration through enteric-coated capsules, inhibited thrombus formation effectively in rats with tail thrombosis.On the other hand, UK and SK are also used for the treatment of thrombosis, but these enzymes must be given in large quantities and require long periods of administration. One of the reasons is that their half-life is very short and, soon after administration, these enzymes are degraded or eliminated by the kidney and the liver[2]. The molecular mass of NK is lower than that of these fibrinolytic enzymes, and the fibrinolytic activity of NK is retained in the blood for more than 3 h[32]. Our study demonstrated that NK displayed strong thrombolytic activity in rats. The present study shows that NK, produced byBacillus subtilis nattoLNUB236, had high fibrinolytic activity on thrombolysis models bothin vitroandin vivo. NK enhanced clot lysisin vitroand prolonged tail bleeding timein vivoin a dose-dependent manner. NK-loaded enteric-coated capsules, administered via the oral route, inhibited thrombus formation significantly in carrageenan-induced a thrombosis model. Likewise, the levels of FDP and D-dimer were increased significantly in carrageenan-induced a thrombosis model. Moreover, obvious hemolysis or agglutination did not occur in rabbit erythrocytes, and the released erythrocytes were undamaged and had smooth surfaces.
Thrombosis is associated with blood coagulation and endothelial lesions[33]. Pathological studies have revealed that carrageenan can cause inflammation in local blood vessels and injury to endothelial cells by releasing inflammatory factors,which can lead to thrombus formation[34]. A carrageenan-induced rat-tail model was used to reflect peripheral obstructive disease. This thrombosis model did not require traumatic surgery, and drug efficacy could be evaluated by comparing the mean length of the thrombus in rat tails. NK showed a marked prophylactic effect against thrombosis in a dose-dependent manner after 12 days of administration.Fujita et al. reported that NK can hydrolyze thrombi and convert plasminogen to plasminin vivo[3]. A key feature of thrombolysis is fibrinolysis. The effect of NK on fibrinolysis was measured by assessing changes in fibrin/fibrinogen degradation products and D-dimer levels after NK treatment[35]. Levels of D-dimer and FDP were increased significantly at the three tested doses of NK. The present study shows that oral administration of enteric-coated NK-loaded capsules can enhance the disaggregation effect on fibrin/fibrinogen and thereby prevent thrombus formation.
During the 28-day repeated administration of NK, the blood MCHC index of the rats in the drug-administered group was statistically different from that in the control group.This may indicate that NK can improve blood flow state and microcirculation. The blood triglycerides biochemical index of NK-treated rats was lower than that of the control group,which might be caused by a hypolipidemic activity of NK.The APTT coagulation index was prolonged compared to the control group, and the FIB index decreased compared with the control group. This might indicate that NK possess the capacity to inhibit endogenous and exogenous coagulation systems. The hematological parameters and blood biochemical indexes of the other administration groups were not statistically significant compared with the control group.During the repeated administration of NK for 90 days, blood cholesterol and triglycerides of the rats in the administration group were lower than those in the control group. This could be related to the hypolipidemic function of NK. The blood biochemical indicators of other NK administered groups were not statistically significant compared with the control group.The high-dose and control rats were subjected to pathological examination. If tissue lesions appeared in the high-dose group, the other dose groups were examined. The results show that the organs and tissues of the rats were normal, and no toxic reaction or damage to the body was exerted by NK.
Arterial thrombosis is a frequent consequence of atherosclerosis. Arterial thrombosis can occur in regions of moderate-to-high shear stress through adhesion and aggregation of platelets at the luminal surface of damaged vessels, which may lead to their occlusion[8]. In the present study, vascular injury was induced by wrapping a piece of filter paper saturated with ferric chloride around the carotid arteries of rats. This injury triggers the generation of free radicals and leads to the disruption and denudation of the endothelium. These actions activate platelets and the clotting system, leading to thrombus formation in obstructed areas[36].A marked thrombolytic effect was detected in vivo after pretreatment with NK, and the thrombus mass was significantly reduced. NK prolonged the bleeding time significantly in a dose-dependent manner, with greater effects being observed at high doses of NK in comparison with UK. These results suggest that NK possess an anti-coagulant activity.
Taken together, our study suggests that NK produced by Bacillus subtilis natto LNUB236 can perform both thrombolytic and anti-coagulation activities in vitro. NK significantly reduced thrombus mass and length. NK also prolonged the bleeding time in mice in a dose-dependent manner without exerting any toxic reaction or damage to the body. Therefore, NK produced by Bacillus subtilis natto LNUB236 might be an ideal functional food option for the prevention and treatment of thrombosis.

