MnCo Oxides Supported on Carbon Fibers for High-Performance Supercapacitors
Jiu Wang, Nanshi Wu, Tao Liu, Shaowen Cao , Jiaguo Yu
State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology,Wuhan 430070, P.R.China.
Abstract:The development of high-performance supercapacitor electrode materials is imperative to alleviate the ongoing energy crisis.Numerous transition metals (oxides) have been studied as electrode materials for supercapacitors owing to their low cost, environmental-friendliness, and excellent electrochemical performance.Among the developed binary transition metal oxides, manganese cobalt oxides typically show high theoretical capacitance and stable electrochemical performance, and are widely used in the electrode materials of supercapacitors.However, the poor conductivity and active material utilization of manganese cobalt oxide-based electrode materials limit their potential capacitance application.Cotton is mainly composed of organic carbon-containing materials, which can be transformed to carbon fibers after calcination.The resultant carbonaceous material exhibits a large specific surface area and good conductivity.Such advantages could potentially suppress the negative effects caused by the poor conductivity and small specific surface area of manganese cobalt oxides, thereby improving the electrochemical performance.Herein, we firstly deposited manganese cobalt oxides on cotton by a simple hydrothermal method, yielding a composite of manganese cobalt oxides and carbon fibers via subsequent calcination, to improve the electrochemical performance of the electrode material.X-ray diffraction (XRD), field-emission scanning electron microscopy (FESEM), X-ray photoelectron spectroscopy (XPS), Brunauer-Emmett-Teller (BET), thermogravimetric analysis (TGA), and electrochemical characterizations were used to investigate the physical, chemical, and electrochemical properties of the prepared samples.The fabricated manganese cobalt oxides in the composite were uniformly dispersed on the carbon fiber surface, which increased the contact between the interface of the electrode material and electrolyte, and enhanced electrode material utilization.The electrode material was confirmed to have well contacted with the electrolyte during a contact angle test.Hence, a pseudo-capacitance reaction completely occurred on the manganese cobalt oxide material.Moreover, the addition of carbon fibers reduced the resistance of the material, resulting in excellent capacitive performance.The capacitance of the prepared composite was 854 F∙g-1 at a current density of 2 A∙g-1.The capacitance was maintained at 72.3% after 2000 cycles at a current density of 2 A∙g-1.These results indicate that the manganese cobalt oxide and carbon fiber composite is a promising electrode material for high-performance supercapacitors.The findings presented herein provide a strategy for coupling with carbon materials to enhance the performance of supercapacitor electrode materials based on manganese cobalt oxides.Thus, novel insights into the design of high-performance supercapacitors for energy management are provided.
Key Words:Manganese cobalt oxide;Carbon fiber;Supercapacitor;Composite;Contact

1 Introduction
In the modern era, human beings have to develop sustainable and high-efficiency energy conversion/storage devices to address the issues of energy and resource crisis1-8.Among various energy storage devices (Li-ion batteries, fuel cells,supercapacitors, etc.), supercapacitors have attracted increasing attention because of their rapid charging-discharging rates, high power density and long cycle life9-13.Until now, supercapacitors have been applied in a number of fields including electric cars,consumer electronics, memory back-up systems, etc.
According to the difference of working principles, the supercapacitors have been divided as two major types: electric double-layer capacitor (EDLC) and faradaic pseudo-capacitor(PC)14,15.The electrode materials of EDLC consist of carbon materials such as activated carbon, carbon nanotube, carbon aerogel, graphene and graphdiyne16.Researchers have focused on the application of EDLC electrode materials because of their high power density, high cycling life and superior thermal stability17.However, their low capacitance limits their applications.Compared to the electrode materials of EDLC, the electrode materials of PC show higher capacitance18because of the storage of more electronic charges19-22.At the same time,they have rapid charge and discharge property, wide range of working temperature, long cycling life and are also environmental friendly.
Recently, as a promising electrode material of PC, bimetallic manganese cobalt oxides (MnCo) have attracted more and more interest due to their low cost, excellent electrochemical performances and environmentally friendly property23-30.The bimetallic oxides have higher rate capability and more excellent capacitive performance than the monometallic oxides31-36.This is because Co exhibits high oxidation potential and Mn can store and transport more electrons leading to high capacity37.For instance, Liet al.38prepared one-dimensional MnCo2O4 nanowire arrays by a hydrothermal method.The prepared material exhibits good electrochemical performance with a capacitance of 349.8 F∙g-1at 1 A∙g-1.Gomez and Kalu39prepared a thin film binder-free Co-Mn composite oxide electrode with a specific capacitance 832 F∙g-1at a scan rate of 20 mV∙s-1.Despite the bimetallic manganese cobalt oxide electrode materials have shown excellent performance, there still exist several drawbacks, including low utilization of active materials and relatively low conductivity.Cotton is one of the most common and popular natural cellulose fibers with potential bio-renewable, negligible cost and easy availability.Moreover,cotton fibers can be used as a precursor for the production of carbon fibers through pyrolysis, an easy and industrially commonly reported method40.
Herein, we firstly deposited the manganese cobalt composite on cotton by a facile hydrothermal method.Then the composite of manganese cobalt oxides and carbon fibers (MnCo/CF) was successfully fabricated after a calcination process.Due to the presence of carbon fibers, the composite of MnCo/CF shows more excellent conductivity and thus improved electrochemical performance compared to the manganese cobalt oxides.Meanwhile, the carbon fibers could serve as a support, allowing the uniform dispersion of manganese cobalt oxides on the surface.As a result, the electrode material could sufficiently contact with the electrolyte, namely, the utilization of active materials is greatly improved.Due to the improvement in both conductivity and contact, the prepared composite material exhibits excellent electrochemical performance, highlighting the path for its potential in energy management.
2 Experimental
2.1 Materials
Manganese nitrate tetrahydrate (Mn(NO3)2∙4H2O, 99%),cobalt nitrate hexahydrate (Co(NO3)2∙6H2O, 99%), concentrated nitric acid (HNO3, 68%), urea (CO(NH2)2, 99%), absolute ethanol (C2H5OH, 99%) were purchased from Sinopharm Chemical Reagent Co., Ltd.
2.2 Materials characterization
X-ray diffraction (XRD) patterns were recorded on a D/MaxrB X-ray diffractometer (Rigaku, Japan), using CuKαradiation(0.15418 nm) at 40 kV.The morphology was investigated using a JSM 7500F field emission scanning electron microscope(FESEM, JEOL, Japan).The chemical states and compositions of the samples were measured by an X-ray photoelectron spectrometer (XPS, Thermo ESCALA 250, USA) with AlKαradiation.The Brunauer-Emmett-Teller (BET) specific surface area was recorded using a nitrogen adsorption apparatus(Micromeritics ASAP 3020, USA).The resistivity was tested by a four-probe analyzer (ST2263, Suzhou Jingge Electronic Co.,LTD).The contact angle test was measured by a static contact angle/interfacial tension analyzer (Biolin, Attension Theta,Sweden).Thermogravimetric analysis (TGA) was performed to determine the loading ratio of MnCo on the MnCo/CF samples using a Shimadzu DTG-60 instrument (Japan).
2.3 Preparation of MnCo/CF
The sample of MnCo2/CF was prepared using a facile hydrothermal method.Typically, the medical absorbent cotton was soaked into concentrated HNO3overnight to improve the hydrophilicity of the material.The treated cotton was washed using deionized water and absolute ethanol, and dried in a vacuum oven at 60 °C.On the other hand, Mn(NO3)2∙4H2O (0.18 g) and Co(NO3)2∙6H2O (0.15 g) were dissolved in 60 mL deionized water under continuous stirring, followed by the addition of urea (0.5 g).After that, the treated cotton (0.3 g) was added into the above solution, keeping stirring for 10 min.The resultant suspension was transferred into a 100 mL Teflon-lined stainless steel autoclave.After maintained at 100 °C for 12 h,the composite of Mn-Co hydroxide precursor and cotton was obtained.Finally, the precursor was calcinated at 500 °C for 2 h to form MnCo2/CF.
For reference, manganese cobalt oxides (MnCo) were synthesized by a similar method without the addition of cotton.Carbon fibers (CF) were also prepared by direct calcination of cotton at 500 °C for 2 h.MnCo/CF samples with other ratios were prepared by adjusting contents of Mn(NO3)2∙4H2O and Co(NO3)2∙6H2O.When Mn(NO3)2∙4H2O (0.09 g) and Co(NO3)2∙6H2O (0.075 g) were added, the resultant sample was labeled as MnCo1/CF.When Mn(NO3)2∙4H2O (0.27 g) and Co(NO3)2∙6H2O (0.225 g) were added, the resultant sample was labeled as MnCo3/CF.
2.4 Electrochemical measurements
All the electrochemical measurements were tested on a CHI 760E electrochemical workstation (Shanghai Chenhua, China),in a three-electrode system with 2 mol∙L-1KOH aqueous solution as electrolyte.The working electrodes are composed of the as-prepared electrode materials.The reference electrode was a Ag/AgCl electrode.The counter electrode was a Pt foil.Additionally, an asymmetric supercapacitor was assembled by using activated carbon (AC) as negative electrode and MnCo/CF as positive electrode in 2 mol∙L-1KOH aqueous electrolyte.
Galvanostatic charge-discharge (GCD), cyclic voltammetry(CV), electrochemical impedance spectra (EIS) and cycling performance were carried out to evaluate the electrochemical performance.The GCD curves were measured in a voltage window (between 0 and 0.45 V) from 2 to 20 A∙g-1.The CV curves were performed in the voltage window of 0-0.5 V at a wide scan rate ranging from 10 to 100 mV∙s-1.The EIS measurements were tested from 0.01 Hz to 10 kHz.
The composite active material, conductive acetylene black and polyvinylidene fluoride (PVDF) at a weight ratio of 80 : 10 :10 were loaded onto nickel foam (1 cm × 1 cm).Each nickel foam was loaded with about 1 mg of sample.Then the electrodes were dried at 80 °C overnight in an oven.All the measurements were conducted at 25 °C.The specific capacitance was determined from the following equation.

whereC(F∙g-1) represents the specific capacitance,s(V·s-1) is the sweeping rate,m(g) represents the mass of the active material, ΔV(V) is the voltage change,I(A) stands for the discharge current, Δt(s) is the discharge time.
3 Results and discussion
3.1 Structure and morphology of MnCo/CF
Fig.1a shows the FESEM image of CF.The diameter of CF is about 10 μm.It can be clearly seen that the surface of the CF is smooth.Fig.1b reveals that in the as-prepared MnCo/CF sample,manganese cobalt oxide nanorods are evenly distributed across the entire surface of the carbon fiber.Fig.1c shows the stacked sheet-like morphology of pristine MnCo oxides and Fig.1d shows that manganese cobalt oxides still adhere to the surface of the carbon fiber after 2000 cycles at 2 A∙g-1, which proves its good structure stability.
The chemical compositions of as-prepared MnCo/CF were analyzed by X-ray photoelectron spectroscopy (XPS).Fig.2a is the high-resolution XPS spectrum of Mn 2pof MnCo/CF.The peaks of Mn 2p3/2and Mn 2p1/2are located at 642.2 and 653.9 eV, respectively.The binding energy between Mn 2p3/2 and Mn 2p1/2is 11.7 eV, which is consistent with the reported value41.It is proved that Mn element in the sample consists mostly of Mn4+,which is mainly because the sample was easily oxidized during the experimental process.Fig.2b reveals the high-resolution XPS spectrum of Co 2pof MnCo/CF.The two main peaks are at the binding energy of 781.3 and 797.1 eV which correspond to Co 2p3/2and Co 2p1/2, with two small satellite peaks by the sides.The result indicates that Co is mainly in the form of Co2+.Fig.2c is the XPS survey spectrum of MnCo/CF.There are mainly C,O, Mn and Co elements in the sample.In addition, there are small amounts of Cl and N, which originate from the medical absorbent cotton itself.The content of N component is 2.76% in MnCo/CF by XPS result and the existence of nitrogen element can improve both the surface properties and electric conductivity,and thus an additional pseudocapacitance and enhanced electrochemical performance42.The X-ray diffraction (XRD)patterns for MnCo/CF, MnCo and CF are further shown in Fig.2d.It can be seen that the XRD pattern of CF has a broad peak at the position of 22.7° (002), typical for carbon materials.The XRD pattern of MnCo can be indexed as CoMnO3(PDF 65-3696).The peaks at 24.5°, 33.5°, and 36.4° can be assigned to(110), (211), and (1-10) planes.The XRD pattern of the MnCo/CF shows the combination of both peaks of CF and MnCo.
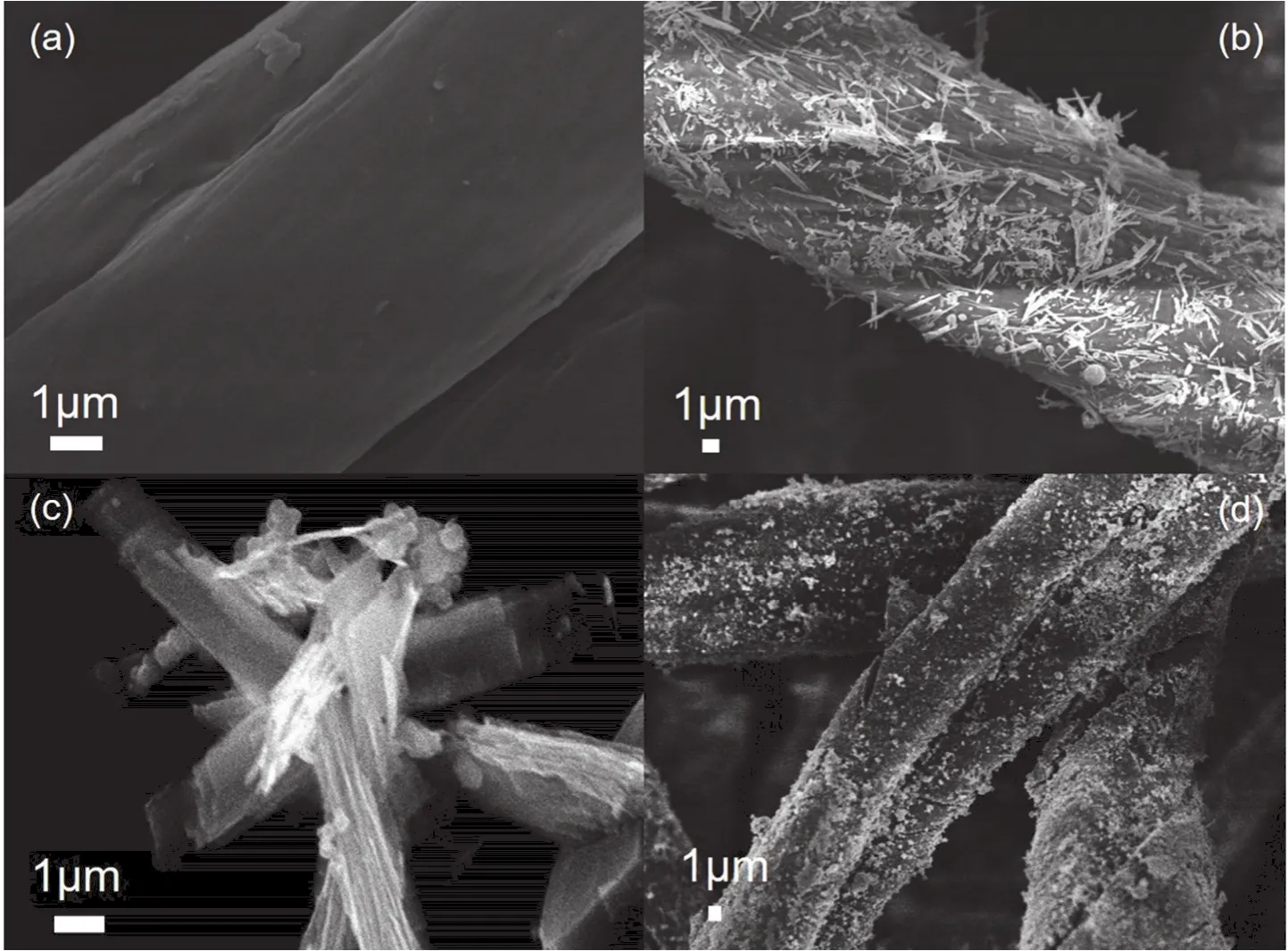
Fig.1 FESEM images of (a) CF, (b) MnCo/CF, (c) MnCo oxides and (d) MnCo/CF after 2000 cycles at 2 A·g-1.

Fig.2 (a) Mn 2p and (b) Co 2p high-resolution XPS spectra of MnCo/CF; (c) XPS survey spectrum of MnCo/CF;(d) XRD patterns of MnCo/CF, MnCo and CF.
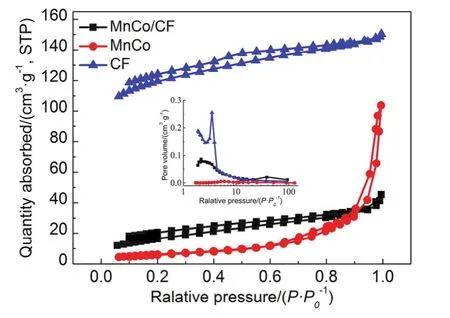
Fig.3 N2 adsorption-desorption isotherms and pore size distributions (inset) of MnCo/CF, MnCo, and CF.
Fig.3 shows the N2adsorption-desorption isotherms and pore size distributions (inset) of MnCo/CF, MnCo and CF.According to the classification of IUPAC (International Union of pure and Applied Chemistry), the isotherm of CF belongs to type I,indicating that there are many micropores in the carbon fibers43.It can be also seen that the pores in the carbon fibers mainly consist of micropores and small mesopores (inset).The specific surface area is determined as 392 m2∙g-1.The isotherm of MnCo sample can be assigned to type IV, with a hysteresis loop of type H3, revealing the existence of large pores caused by the aggregation of nanoparticles.The specific surface area of MnCo sample is only 23 m2∙g-1.Note that the isotherm of MnCo/CF sample can be regarded as the combination of type I and IV,giving rise to a moderate specific surface area of 61 m2∙g-1, as compared to those of separate CF and MnCo samples.The reduced surface area of MnCo/CF compared to CF is possibly because MnCo uniformly dispersed on the surface of CF would block some of the pores.
Thermogravimetric analysis (TGA) was used to determine the amount of deposited MnCo oxides.From Fig.4a-c, the temperature was raised to 650 °C to remove carbon fibers from the samples, thus the deposition of MnCo on MnCo1/CF,MnCo2/CF and MnCo3/CF is 10.7%, 24.6% and 29.9%,respectively.
3.2 Electrochemical analysis
In order to explore the optimal contents of MnCo oxides,different samples were prepared.The calculation of specific capacitance is based on the mass of the active material,i.e.MnCo oxides.Fig.5a shows the CV curves of MnCo1/CF,MnCo2/CF and MnCo3/CF at 20 mV∙s-1.It can be found that MnCo2/CF exhibits the best performance.Hence, this sample was chosen as the typical sample of MnCo/CF for further analysis.Fig.5b shows the CV curves of MnCo/CF, and MnCo at 20 mV∙s-1.The specific capacitance of MnCo/CF and MnCo is calculated to be 481 and 298 F∙g-1from equation (1),respectively.It is noteworthy that the combination of MnCo oxides and carbon fibers greatly improves the electrochemical performance in comparison with pristine MnCo oxides.On one hand, MnCo oxides can be well dispersed on the surface of carbon fibers to improve their utilization.On the other hand, the presence of carbon fibers can enhance the conductivity of the electrode materials.
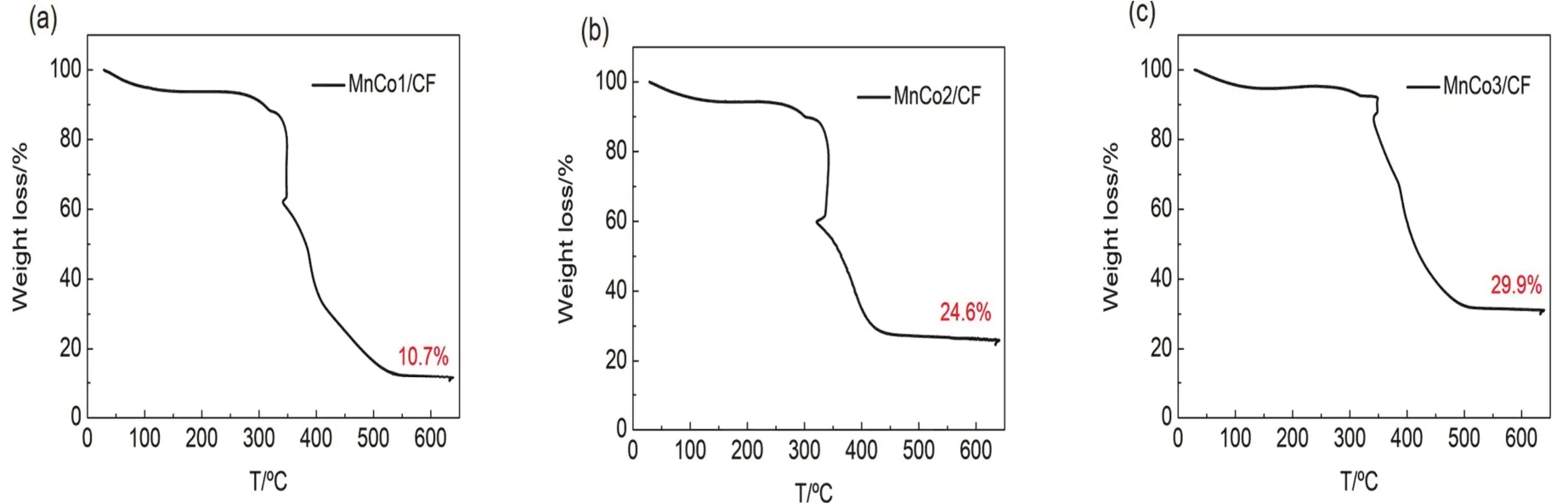
Fig.4 TGA curves of (a) MnCo1/CF; (b) MnCo2/CF and (c) MnCo3/CF with a heating rate of 10 °C·min-1 in air.
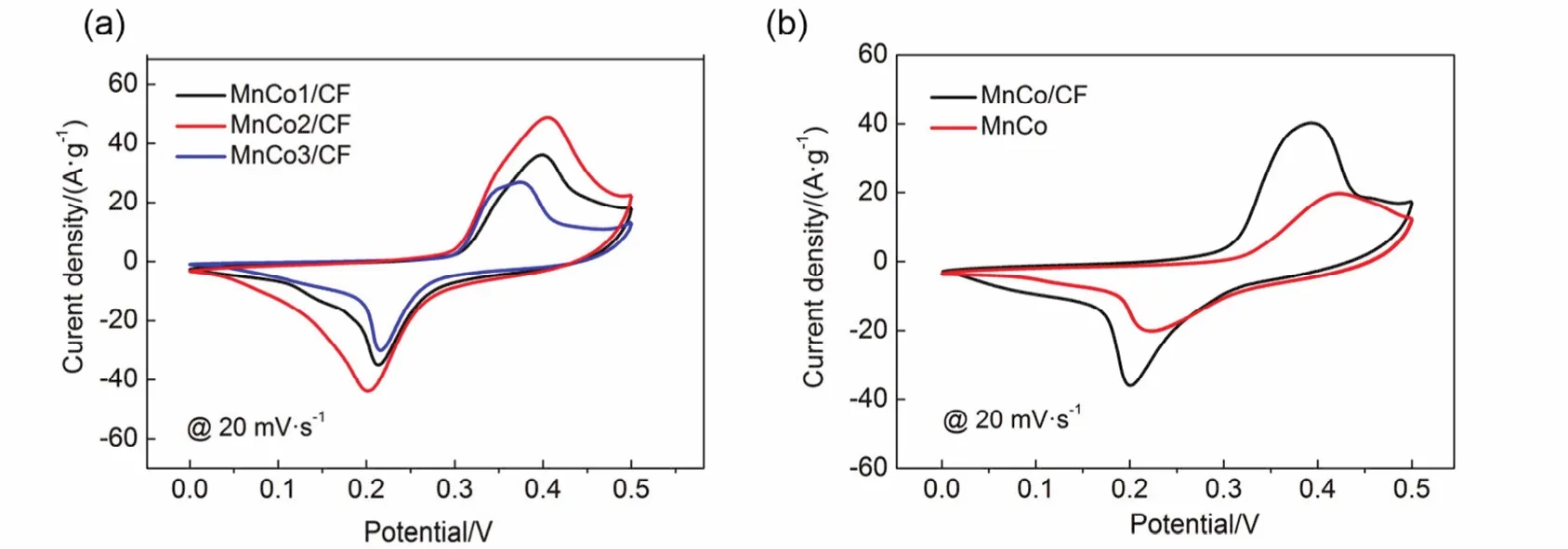
Fig.5 (a) CV curves of MnCo1/CF, MnCo2/CF and MnCo3/CF at 20 mV·s-1; (b) CV curves of MnCo/CF and MnCo at 20 mV·s-1.
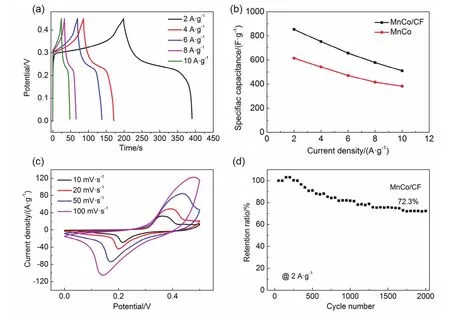
Fig.6 (a) GCD curves of MnCo/CF; (b) specific capacitance changes of MnCo/CF and MnCo at different current densities (2, 4, 6, 8 and 10 A·g-1);(c) CV curves of MnCo/CF at different scan rates (5, 10, 20, 50 and 100 mV·s-1); (d) Long-term cycling stability of MnCo/CF electrodes at the current density of 2 A·g-1 for 2000 cycles.
Fig.6a shows the GCD curves of MnCo/CF at different current densities which were measured by a three-electrode device in a potential range of 0-0.45 V.It can be calculated from equation (2) that, MnCo/CF electrode shows the higher specific capacitances of 854 F∙g-1(2 A∙g-1), 753 F∙g-1(4 A∙g-1), 656 F∙g-1(6 A∙g-1), 578 F∙g-1(8 A∙g-1) and 510 F∙g-1(10 A∙g-1), in comparison with that of MnCo, as shown in Fig.6b.The CV curves of MnCo/CF at different scan rates were also measured in the voltage window of 0-0.5 V (Fig.6c).It can be seen that there are two redox peaks on each curve because the sample is involved in chemical reaction during electrochemical process.The oxidation and reduction peaks are symmetrically distributed,indicating the excellent reversibility of the sample.With increasing the scanning speed, the peak current increases obviously and the redox peaks move slightly.Fig.6d illustrates the cycling stability of MnCo/CF at the current density of 2 A∙g-1for 2000 cycles.The electrode material still shows a specific capacity retention of 72.3% after 2000 cycles.Moreover, the contact angle test was used to illustrate the hydrophilicity of the samples.As seen in Fig.7a, the contact angle of MnCo is 45.8°,which is relatively hydrophilic.Besides, the absence of contact angles of MnCo/CF and CF indicates their hydrophilicity (Fig.7b, c), thus supporting the improvement on the electrode material contacted with the electrolyte.

Fig.7 The contact angle tests of (a) MnCo; (b) MnCo/CF and (c) CF.
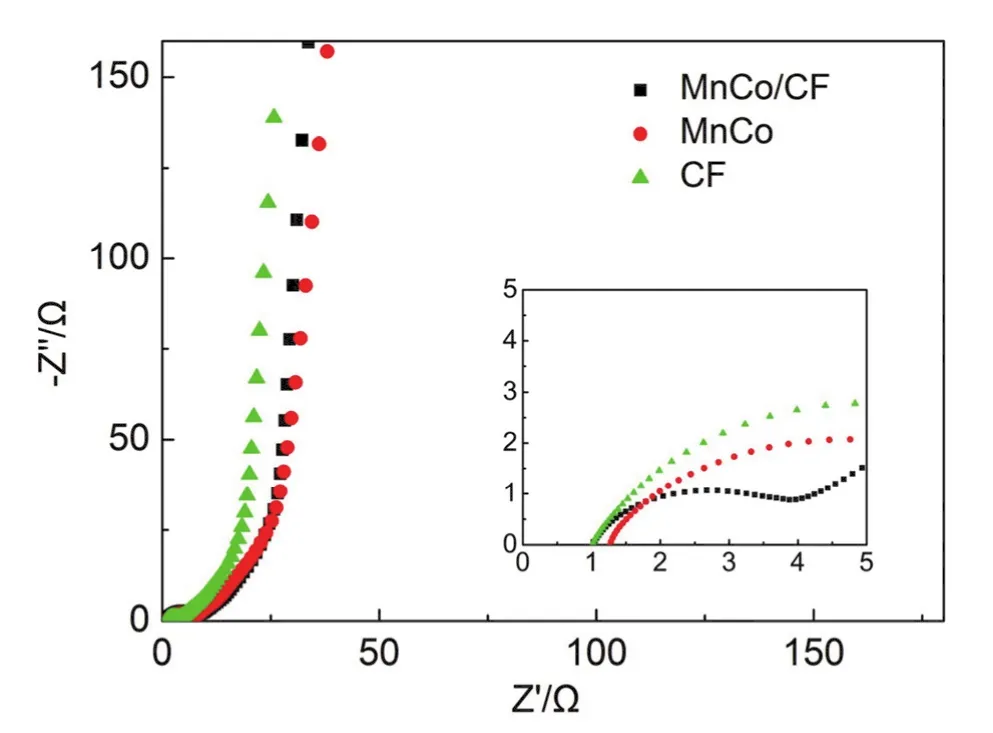
Fig.8 Nyquist plots of MnCo/CF electrodes (the inset reveals the high-frequency region of the Nyquist plots).
The electrochemical impedance spectroscopy (EIS) is one of the important technique in the electrochemical measurements.We set the impedance spectrum frequency from high frequency(10 kHz) to the low frequency (0.01 Hz).As shown in Fig.8, the impedance spectra have a linear part at low frequency, which illustrates the typical capacitor behavior.In the high frequency region, the intercepts of the Nyquist plots on the real axis are total resistance (Rs) including the intrinsic resistance of the electrode material, the resistance of the electrolyte solution and interfacial resistance between the interface of the active material and the current collector.The smaller intercept of the horizontal axis suggests the smaller resistance.From the high frequency region of the figure, it can be seen that the interception of MnCo/CF electrode and CF electrode is smaller than the MnCo electrode, indicating that the conductivity of the MnCo/CF electrode and CF electrode is better than the MnCo electrode.This is consistent with the measured resistivity of MnCo/CF, CF and MnCo of 78.02, 23.52 and 110.2 kΩ∙cm, respectively.The MnCo/CF composite enhance its conductivity compared to MnCo because of the presence of conductive carbon material,thus improving its electrochemical properties.In the middle frequency region, the electrochemical control is the main influencing factor.The charge transfer resistance (Rct) of the electrodes is related to the radius of the circle.The lower charge transfer resistance means faster charge transfer rate.Therefore,the electrochemical performance of MnCo/CF is better than MnCo oxides.In the low frequency region, the slope of the impedance spectra of these three electrode materials is almost the same, revealing that the diffusion rate is almost the same.
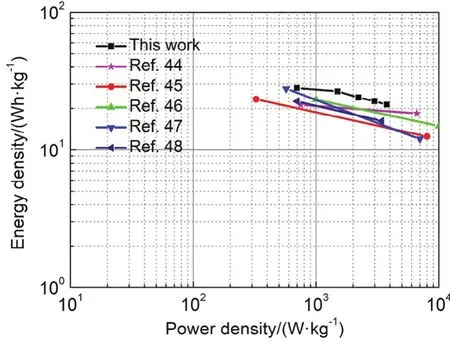
Fig.9 Ragone plots of MnCo/CF//AC in comparsion to the electrodes reported in the literature.
In addition, an asymmetric supercapacitor (ASC) was successfully assembled using MnCo/CF as the positive electrode and activated carbon (AC) as the negative electrode.As shown in Fig.9, the obtained energy density of MnCo/CF//AC is 28.1 Wh∙kg-1at a power density of 699 W∙kg-1.Even at a power density of 3759 W∙kg-1, an energy density of 21.3 Wh∙kg-1was retained.Such energy and power densities are highly competitive with those of previously reported ASC devices such as rGO/MnO2//rGO (21.2 Wh∙kg-1at 750 W∙kg-1)44, NiCo2O4-rGO//AC (23.3 Wh∙kg-1at 325 W∙kg-1)45, GH//MnO2-NF (23.2 Wh∙kg-1at 1000 W∙kg-1)46, Co2MnO4/CHC//AC (27.8 Wh∙kg-1at 571 W∙kg-1)47and rGO/NiMn-LDH//AC (22.5 Wh∙kg-1at 700 W∙kg-1)48.The result indicates MnCo/CF can be a suitable candidate for high-performance electrode materials.
4 Conclusions
The cheap cotton and transition metal compounds are used as raw materials to prepare the composite of manganese cobalt oxides and carbon fibers.Compared with manganese cobalt oxides, the MnCo/CF composite has a larger specific surface area of 61 m2∙g-1.Moreover, the manganese cobalt oxide material is evenly dispersed on the surface of carbon fibers,which enhance the surface contact between the electrode material and electrolyte and improve the utilization of the MnCo oxides.Moreover, the presence of carbon fibers significantly reduces the resistance of MnCo/CF.Therefore, the specific capacitance of MnCo/CF is increased greatly compared to the MnCo oxides.The capacitance of MnCo/CF is 854 F∙g-1at a current density of 2 A∙g-1and remain 72.3% after 2000 cycles.

