Grape Seed Procyanidin Extract Attenuate Sodium Fluoride-induced Oxidative Damage and Apoptosis in Rat Kidneys*
WANG Er Hui, YU Zeng Li, PING Guan Fang, and ZHAI De Sheng
Fluoride is widely distributed in nature, and it can lead to fluorosis if intake is excessive. In addition to its established effects on the skeleton and teeth,fluoride can also exert toxic effects on the kidneys.Fluoride can induce abnormal renal function in humans and other animals[1-3], but the mechanisms through which fluoride exerts such effects remain obscure. Fluoride increases the generation of reactive oxygen species (ROS), causes extensive oxidative stress and excessive lipid peroxidation, and reduces antioxidant enzyme activities in the kidneys[3-5]; furthermore, oxidative stress and apoptosis play fundamental roles in fluoride-induced renal injury[4,5]. Therefore, agents that could exert both antioxidative and antiapoptotic effects on fluoride-induced kidney damage should be identified. Extracts of grape seed proanthocyanidins(GSPE), which are biologically active and have proven antioxidant and antiapoptotic activities[6-9]. These findings suggest that GSPE could treat fluorideinduced oxidative damage and apoptosis in the kidneys. Therefore, we explored the protective role of GSPE against fluoride-induced oxidative stress and apoptosis in rat kidneys, the involvement of apoptosis mechanisms in this process, and the protective role of GSPE against fluoride-induced renal damage.
We purchased > 95% pure GSPE from Tianjin Peak Natural Product Research Development Co.,Ltd. (Tianjin, China), assay kits for malondialdehyde(MDA) (cat. no. S0131) and superoxide dismutase(SOD) (cat. no. S0101) from Beyotime Biotechnology(Shanghai, China), reduced glutathione (GSH) (cat.no. BC1170) and catalase (CAT) (cat. no. BC0200)from Beijing Solarbio Science & Technology Co,(Beijing, China), total protein (cat. no. A045-4-1)from Nanjing Jian Cheng Bioengineering Institute(Nanjing, China), and terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate(dUTP) nick end-labeling (TUNEL) (cat. no. KGA703)from KeyGen Biotechnology (Nanjing, China). Anti-Bcl2 (ab196495), Anti-Bax (ab53154), and anti-GAPDH (ab181602) antibodies were from Abcam(Cambridge, UK).
Male Sprague-Dawley rats (age, 8 weeks; weight,240-260 g) were purchased from Sippr-Bk Lab Animal Co., Ltd. (Shanghai, China). All animals were maintained at 23 ± 2 °C with a 12/12-h light/dark cycle and 60% ± 5% humidity and received humane care according to the criteria outlined in the Guide for the Care and Use of Animals and the Animal Management Rules of the Health Ministry of the People’s Republic of China (document number 55,2001, China). The study protocol was approved by the Animal Care and Use Committees at Xinxiang Medical University. The rats were assigned to the following groups (n= 8 per group). Group 1 (control)was fed with a standard diet for 28 d. Group 2(model) was given 600 ppm of sodium fluoride (NaF)in drinking water for 21 d. Groups 3 and 4 were given NaF (600 ppm in drinking water) for 21 d and treated daily with GSPE (100 mg/kg) or vitamin C(100 mg/kg) by gavage, respectively for one week before NaF intoxication. The animals were then euthanized by anesthetized with sodium pentobarbital (60 mg/kg, ip.) after the 21-d feeding period.
Glutathione (GSH), catalase (CAT), superoxide dismutase (SOD) activities and levels of malonaldehyde (MDA) in kidney tissues, as well as blood urea nitrogen (BUN), serum creatinine (Cr) and urea measured as markers of nephrotoxicity were determined as described by the manufacturers of the assay kits.
The kidneys were excised from sacrificed rats,fixed in 4% paraformaldehyde, embedded in paraffin and sliced into 5-μm serial sections. A pathologist who was blinded to the protocol histologically evaluated morphological changes in hepatocytes on sections stained with hematoxylin and eosin (HE).Kidney cell apoptosis was assessed using TUNEL assay kits. Cells with brown TUNEL-positive granules in nuclei visualized by light microscopy were considered apoptotic. Cell death was assessed in 5 random fields from each tissue section(magnification ×200), then the hepatocyte apoptotic index was calculated. The amount of hepatocytic apoptosis was calculated as the ratio (%) of TUNELpositive cells relative to the total number of cells.
Kidney tissue homogenates generated by suspending tissues in total protein extraction lysis buffer containing phenylmethanesulfonyl fluoride(PMSF), protease inhibitors, and a phosphatase inhibitor, were placed on ice for 30 min, then separated by centrifugation (Thermo Sorvall ST16R)at 12,000 rpm at 4 °C for 10 min. Total protein content in each sample was determined using protein assay kits. Lysates with 30 μg protein were separated by 10% SDS-PAGE, then transferred to PVDF membranes. Non-specific binding on the membranes was blocked with 5% skim milk in Tween 20 Tris-buffered saline (TBST) for 1.5 h, then the membranes were incubated overnight at 4 °C with the primary antibodies anti-Bcl-2 and anti-Bax diluted 1:500, and with polyclonal anti-GAPDH antibodies diluted 1:1,000. The membranes were washed with TBST, incubated in horseradish peroxidase-conjugated secondary antibody (diluted 1:6,000) for 1 h, then washed three times with TBST.Proteins were visualized by enhanced chemiluminescence using the Pierce ECL western blotting detection system (Cat. No: NCI5079ECL;Thermo Fisher Scientific Inc., Waltham, MA, USA).Protein bands were assessed using Quantity One software (Bio-Rad Laboratories Inc., Hercules, CA,USA).
Data were analyzed using Statistical Product and Service Solutions version 11.5 (SPSS Inc., Chicago, IL.,USA) and are expressed as means ± standard deviation (SD). Differences in data homogeneity among groups were analyzed using a one-way ANOVA followed by a least squared differences model or Dunnett multiple comparison tests if the homogeneity significantly varied. Differences were considered statistically significant atP< 0.05
Table 1 shows that the amount of MDA was significantly increased in the kidneys of the model,compared with the control group (P< 0.05), whereas GSPE ameliorated such increases in the kidneys of the group treated with NaF. The MDA levels were significantly decreased in the kidneys of the GSPE,compared with the model group. Antioxidative status was assessed as SOD and CAT activities and GSH levels in the kidneys. All these values were decreased in the model, compared with the control group (Table 1), but significantly increased in the GSPE, compared with the model group (P< 0.05).Vitamin C exerted similar effects. These findings indicated that GSPE protects against fluorideinduced oxidative damage.
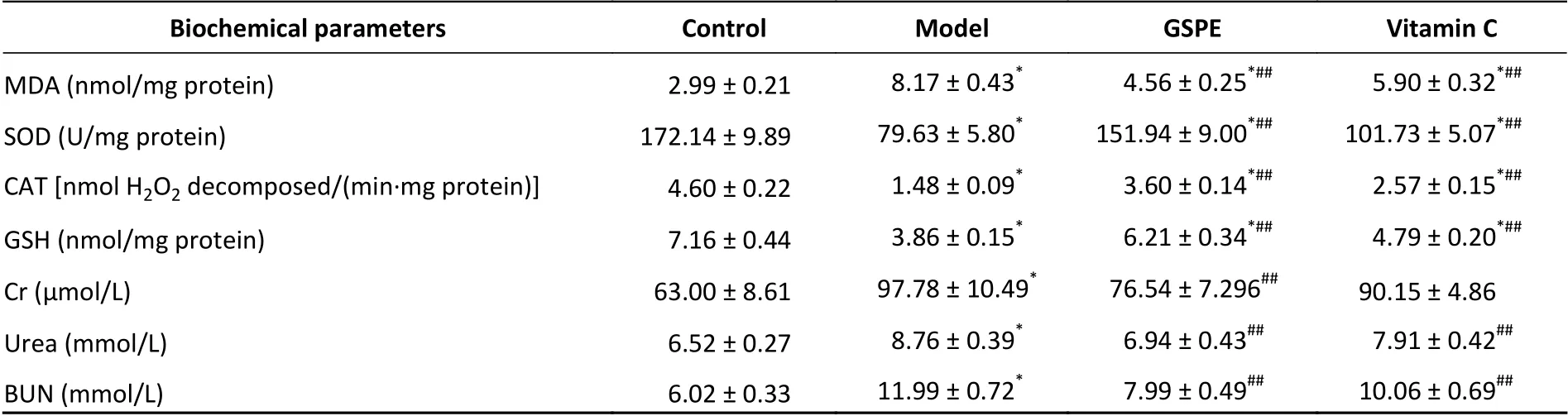
Table 1. Effects of GSPE on NaF-induced renal oxidative and functional damage in rats
Serum levels of renal function markers were measured in all groups to gain further insight into the ability of GSPE to protect the kidneys (Table 1).Notably, the intake of water containing 600 ppm of NaF for 21 d induced significant and deleterious changes in serum markers and biochemical parameters of renal function. Serum levels of BUN,Cr, and urea were significantly restored by 100 mg/kg of GSPE (P> 0.05vs. control). Overall,GSPE and vitamin C mitigated these changes(Table 1).
Kidney sections were histopathologically evaluated by light microscopy. Figure 1a shows intact, uniformly arranged cells, hepatocytes with normal morphology and structure, and no obvious degeneration and necrosis in the control group,whereas the hepatic impellers were ill-defined and inflammatory cells had infiltrated the kidneys of the model group. However, co-administered GSPE and Vitamin C, obviously ameliorated the kidney damage caused by NaF compared with the model group(Figure 1a). We investigated rates of apoptotic cells in the rat kidneys using TUNEL analyses (Figure 1b).Rates of apoptosis were significantly higher in the model, compared with the control group, and GSPE significantly inhibited kidney cell apoptosis induced by NaF (P< 0.05; Figure 1b). Vitamin C again exerted similar effects. Thus, our data suggested that GSPE can protect against histopathologically determined NaF-induced damage and kidney cell apoptosis.
We used Western blots to assay the effects of GSPE on Bax and Bcl-2 expression. Gray scale analyses of Bax and Bcl-2 expression (Figure 2)showed increased Bax and decreased Bcl-2 expression in the model group compared with the control group (P< 0.05 for both). However, the expression of Bax and Bcl-2 was significantly decreased and increased, respectively, in the GSPE,compared with the model group (P< 0.05 for both;Figure 2). Vitamin C also exerted antiapoptotic effects. Collectively, our data suggested that GSPE could inhibit the expression of Bcl-2, which might be involved in the mechanism underlying the antiapoptotic effects of GSPE.
In conclusion, the present findings suggested that GSPE has protective effects against fluorideinduced renal oxidative damage and that it might inhibit fluoride-induced kidney cell apoptosis by interfering with Bax and Bcl-2 expression. The underlying mechanism might be associated with the antioxidant and antiapoptotic capacity of GSPE.However, further investigation is needed to clarify details of the molecular mechanism. Overall, the present findings provide evidence that GSPE can protect against fluoride-induced oxidative stress in the rat kidney and suggest that GSPE could serve as a therapeutic agent to ameliorate fluoride-induced renal injury.
The authors have no conflicts of interest to declare.
#Correspondence should be addressed to WANG Er Hui, Tel/Fax: 86-373-3831802, E-mail: weh1985@126.com
Biographical note of the first author: WANG Er Hui,male, born in 1985, associate professor, majoring in nutrition and food toxicology.
Received: October 10, 2019;
Accepted: April 20, 2020
 Biomedical and Environmental Sciences2020年6期
Biomedical and Environmental Sciences2020年6期
- Biomedical and Environmental Sciences的其它文章
- Gender Differences in the Relationships between Pubertal Stages and the Perpetration of Self-inflicted and lnterpersonal Violence among Middle SchoolStudents in China*
- Capacities and Functionalities Assessment of Veterinary Laboratories in South-west Nigeria Using the FAO Laboratory Mapping Tool
- Prevalence of Hyperuricemia and Associated Factors in the Yi Farmers and Migrants of Southwestern China: A Cross-sectional Study
- Detection of Pseudorabies Virus Antibodies in Human Encephalitis Cases*
- Risk or Beneficial Factors Associated with Unplanned Revascularization Risk Following Percutaneous Coronary lntervention: A Large Single-Center Data*
- Epidemiological Characteristics of Notifiable lnfectious Diseases among Foreign Cases in China,2004-2017*
