The Status Quo and Countermeasures of “4+7”Quantity Purchase in China
Zhou Yusheng,Wei Xingchen,Piao Huiling,Wang Nan,Wang Xing,Dong Li*
(School of Business Administration,Shenyang Pharmaceutical University,Shenyang 110016,China)
Abstract Objective To understand the effectiveness and problems of centralized drug procurement in China by analyzing the implementation of the “4+7” pilot work for centralized drug procurement in China.Methods The current situation and existing problems of drug procurement in China were analyzed through the literature research method.Results and Conclusion At present,good social benefits have been achieved for China’s drug procurement through pilot projects in several cities,but there are some serious problems.The proposed countermeasures will help to improve the procurement of pharmaceuticals in China.
Keywords:quantity procurement; 4+7; drug bidding
1 “4+7” quantity procurement implementation process
On November 14,2018,the Central Committee of the Comprehensive Deepening Reform Committee reviewed and approved the “National Organizational Drug Centralized Procurement Pilot Program”,which clarified the overall plan of national organizations,alliance procurement,and platform operations.On November 15,2018,11 cities such as Beijing,Tianjin,Shanghai,Chongqing,Shenyang,Dalian,Xiamen,Guangzhou,Shenzhen,Chengdu,and Xi’an were taken as the first procurement pilots by National Medical Insurance Bureau (hereinafter referred to as “4+7” cities).A total of 31 generic drugs that had passed consistency evaluation of quality and efficacy were selected and 60%-70% of annual drug use in pilot cities was used to estimate purchase volume[1].Then the pilot work on centralized drug purchase was carried out.On January 17,2019,the General Office of the State Council issued the “Notice on the National Organizational Plan for Centralized Purchasing and Use of Drugs”,which marked the start of the centralized drug procurement at the national level.
The trial of centralized drug purchase in the early stage has already achieved the initial effect,such as reducing the transaction cost of enterprises,drug prices,and guiding hospitals to regulate drug use.
2 Implementation status and performance of“4+7” quantity procurement
2.1 The implementation of centralized quantity procurement
At the present stage,the successful implementation of centralized drug procurement in public hospitals are the three batches of procurement carried out by the Shanghai Municipal Medical Insurance Bureau and the “4+7” quantity procurement pilot conducted by the National Medical Security Bureau in early 2019.The medical insurance department has been in charge of drug procurement since 2010 in Shanghai and it is the first city where the medical insurance department is responsible for drug procurement in China[2].China’s joint procurement pilot project was carried out in 11 cities in early 2019,and the Shanghai Pharmaceutical Affairs Institute was in charge of the daily management and implementation of this volume procurement.
2.2 Range of varieties for centralized quantity procurement
On December 7,2018,Shanghai Sunshine Bidding Purchasing Network publicized the results of the proposed drug centralized procurement,a total of 25 products won the bid[3].This was another bid after the implementation of three batches of purchases in Shanghai.The selected varieties were mainly competitive drugs with large clinical doses,which had been widely used in clinical applications and produced by more than three pharmaceutical companies.Specific varieties are shown in Table 1.

Table 1 Table of selected varieties of drug centralized procurement [4]
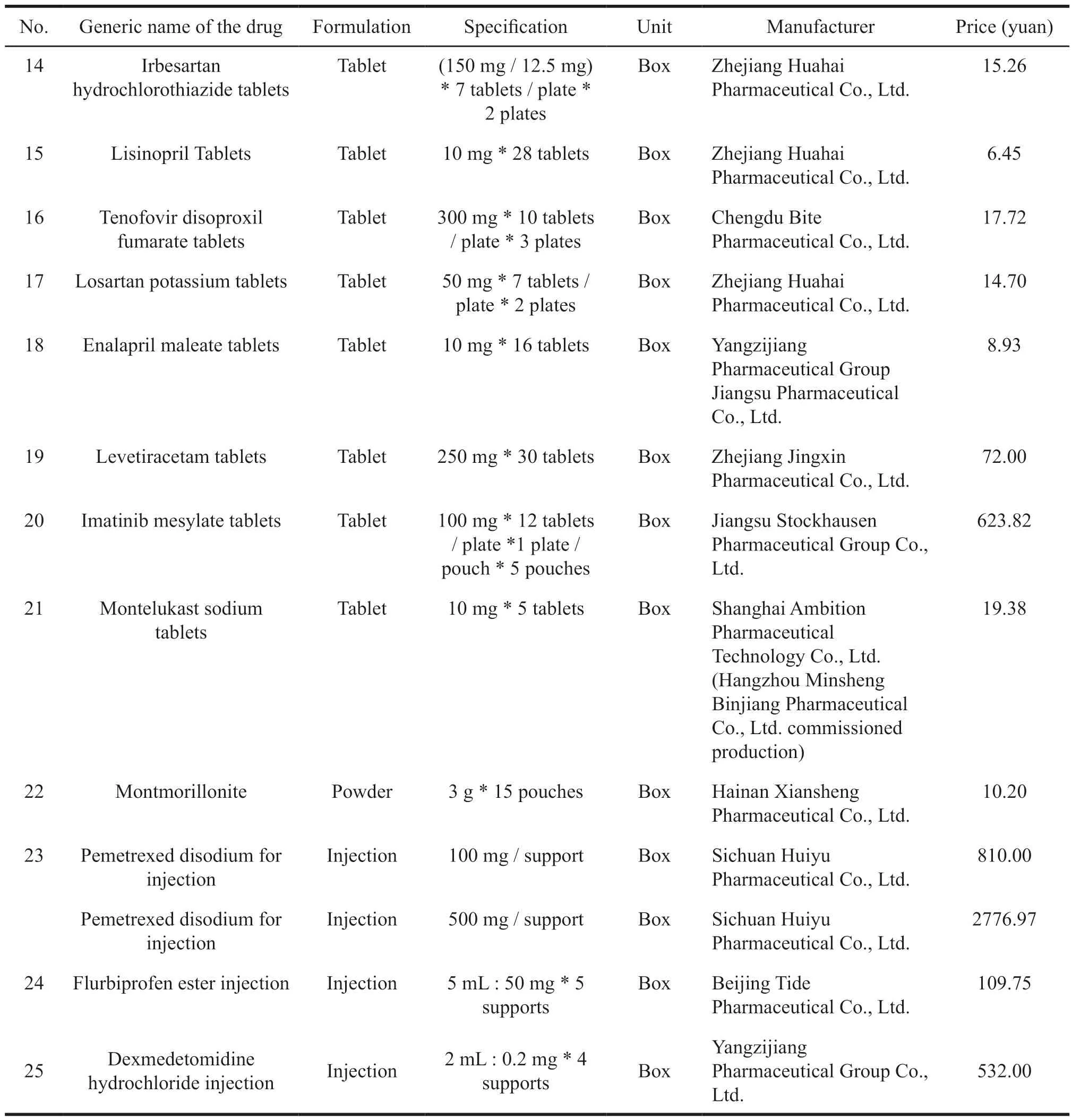
Continued Table 1
The varieties selected by the National Health and Welfare Bureau at the beginning of 2019 for the pilot procurement were mainly generic drugs that had passed the consistency evaluation of the quality and efficacy,including drugs that were deemed to pass the consistency assessment.Therefore,they were mainly the original drugs,the reference preparation,and the generic drugs approved by the new registration classification of chemical drugs through the consistency evaluation of generic drugs.A total of 31 varieties were selected as pilot species for the national centralized procurement.However,at the later stage,6 products such as Alfacalcitol tablets,Azithromycin tablets,Tramadol hydrochloride tablets,Captopril tablets,Amoxicillin capsules,and Azithromycin for injection failed the bid[5].Therefore,in the later centralized procurement,the remaining 25 varieties were still used as bidding drugs.
2.3 Bidding rules and requirements for procurement quantity
The bidding rules for “4+7” quantity procurement adopted the principle of bidding first,and then bargaining,and finally the lowest price winning the bid.
Different from the previous drug bidding procurement policy,the quantity procurement can encourage pharmaceutical companies to fully compete in the bidding process,which will drive drug price reduction through the state-led quantity-for-price exchange.The previous method of drug bidding was only reflected in the highest selling price set by the provincial bidding platform and pharmaceutical companies.Because the quantity of drugs purchased cannot be guaranteed,pharmaceutical companies are not willing to cut prices.Table 2 shows the changes in procurement patterns.
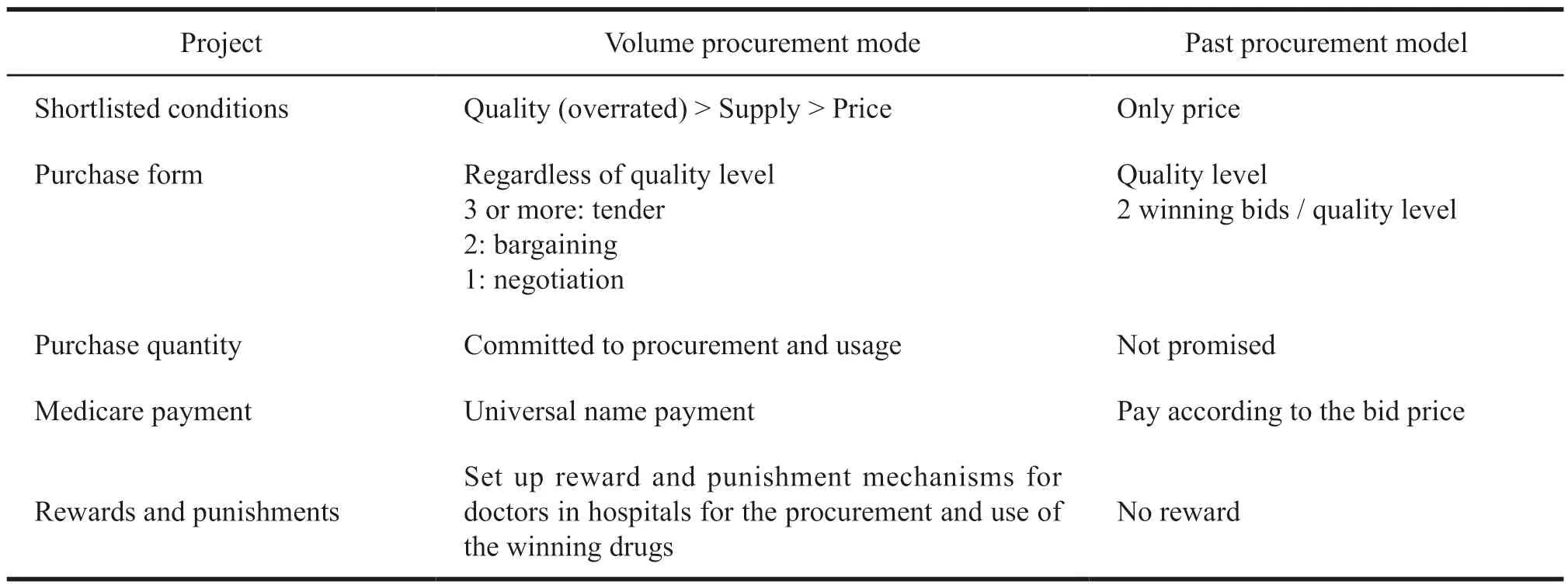
Table 2 Difference between quantity procurement and previous procurement model
The most important thing for “4+7” quantity procurement is the determination and execution of the purchase quantity.The purchase quantity is determined mainly by a certain proportion of the drug consumption in the previous year in the pilot area,and the company is committed to a certain market share to ensure the profit of the enterprise.The price is linked to the specific purchase volume of each drug which should be reported by the public hospitals in the pilot areas according to the drug use records.The agreed purchase volume accounts for 60%-70% of the annual use of drugs in the pilot areas,which is about 30% of the national market share.For the quantity regulation of volume procurement,the published specific list data include the basic quantitative data of the agreed purchase amount in the first year,50% purchase quantity summary value,the 60% purchase quantity summary value and 70% purchase quantity summary value[6].
2.4 Regulatory measures for winning varieties
The supervision measures for the winning varieties include the assessment of hospitals and medical personnel.A series of assessments are applied to ensure the use of winning drugs in public hospitals.The State Health Insurance Administration put forwards the following indicators for assessment in the Opinions on the Centralized Procurement of Medicines and the Use of Measures for Pilot Medical Insurance.Then,the procurement and completion of the selected drug should have monthly monitoring.Besides,it is not allowed to impede the admission of the winning products by taking the proportion of medicines and the total cost control.Second bargaining is strictly forbidden.In addition,there are incentives for the use of medical insurance,such as the use of medical insurance fund balance retention and medical insurance performance assessment.Public hospitals are required to use the winning drugs,the prescription review system is formulated and strengthened,and interviews with illegal medical personnel are carried out to have the later supervision of procurement.
2.5 Performance of centralized procurement
Through the pilot of quantity procurement,25 of the 31 varieties were selected.The average decline was 52%,with a maximum of 96%.Among them,22 generics had passed the consistency evaluation.See Table 1 above.
As of April 14,2019,the total purchase amount of 25 winning bidders in the “4+7” city has reached 438 million pieces,and the total cost was 533 million.A total of 27.31% of the agreed purchase volume was completed[7].The bid-winning price of the quantity procurement was generally lower than the price before the implementation.After bargaining,drug price was greatly reduced.Figure 1 shows the original bid price and the purchase price of the 25 purchased varieties.
The follow-up effect is getting stronger and better.Under the drive of “4+7” cities,Fujian has already followed the step.In addition,Qinghai,Tangshan,Inner Mongolia,Taiyuan,Wuxi,etc.are also preparing to join the league for the drug price reduction.In addition,the following benign development trends will become obvious.(1) The price of unselected products will decrease year-onyear,especially the price of the original drugs.(2)Drug prices in the retail industry will cut as well.(3)It can further promote the consistency evaluation of drug standardization.(4) Medical insurance payment standard and centralized purchase price tend to be the same.(5) It will promote the transformation of pharmaceutical enterprises to adapt to the development of modern market.(6) The role of logistics is increasing,and pharmaceutical business will rely more on logistics.(7) The payment settlement method is falling behind,and settlement innovation will become popular.(8) Management of supply chain becomes important,and pharmaceutical companies will become the main body of the supply chain.
3 Problems in the pilot
Through the trial implementation of centralized drug procurement in “4+7” cities,the following problems were found from the previous performance.
3.1 The vague forecast of centralized procurement volume
The initial forecast of the procurement method is uncontrollable because it is hard to control doctor’s medication process.The most important thing for centralized drug procurement is to determine the quantities first so that the price can be negotiated with a specific amount.However,the number of previous purchases was only from a province or a city,and there was no clear data.Moreover,it is uncontrollable to negotiate with the proportion of drug use.This proportion cannot control the actual doctor’s medication and hospital procurement,which leads to a certain gap between the amount of declaration and the actual use.
3.2 Administrative intervention affects doctors’prescription rights
Quantity procurement has the characteristics of administrative enforcement.For example,a series of monitoring measures are taken to ensure the procurement quantity,such as regular monitoring of the use of the quantity purchased.Besides,the quantity of purchased products should be applied to the performance evaluation standard of medical insurance.Measures like strengthening prescription reviews and interviewing medical staff will interfere with doctor’s prescription rights and it is absurd to a certain degree.
3.3 Quantity procurement can lead to monopoly
The principle of only winning bid with the lowest price can lead to product monopoly.The only company that wins the bid has to bear a certain market supply independently.It means the winning bidder has a great influence on the supply of certain drugs,and it is easy to control the market.At the same time,the vicious price competition will not be conducive to the formation of a benign pharmaceutical competition environment.Due to the particularity of drugs,if the supply company has problems in the production process or raw materials,it will bring great problems to the drug supply,and even lead to a shortage of drugs.Therefore,the patient’s medication will not be guaranteed.
3.4 The quality and market share of the winning varieties cannot form a long-term mechanism
First of all,the varieties purchased with quantity are original drugs and generic drugs that have been evaluated.Their price competitions are based on the same level,but the international evaluation standards are more stringent.The quality of domestically reviewed generic drugs should be accepted by doctors and patients,and they must have further clinical efficacy testing.
Secondly,the long-term guarantee of market share of quantity procurement may also cause some problems.Because the winning enterprise can obtain a certain market share,and the defeated companies must compete in the remaining market share.If the unsuccessful enterprises give up producing the drugs but make new drugs with better efficacy,the original market share may change.Therefore,it is difficult for the winning enterprise to maintain the market share in the long run.
4 Suggestions on improving the centralized procurement of drugs in China
4.1 Responsibilities of the parties involved in volume of procurement
At present,responsibilities of the parties involved in centralized drug procurement is clear,the National Health Insurance Bureau is in charge of drug procurement,and the State Health Care Commission is responsible for the use of the winning drugs.The National Medical Products Administration supervises and inspects the qualification of the winning enterprises and the consistency evaluation of drug quality.However,it is necessary to carry out the responsibility of the parties involved in the procurement process to ensure the smooth implementation of the centralized drug procurement.Meanwhile,coordination and cooperation should be strengthened among various parties to realize their respective supervisory functions that can prevent monopoly in procurement.
4.2 Improving the accurate prediction of purchase volume
Pre-published centralized bidding for pharmaceuticals is the basis for volume procurement,which requires accuracy and reasonability of its predictions.Therefore,it is necessary to strictly focus on the scientific nature of its initial procurement forecast to ensure the later effect.Comprehensive prediction and estimation should be made with the information of the drug variety and supplier system,standardized clinical medication habits,and analysis of past clinical drug data.It is vital to accumulate and analyze the data of drug procurement for many years.Therefore,we should improve the data accumulation function of medical institutions and procurement platforms to optimize the prediction model with reasonability and accuracy.
4.3 Improving the clinical efficacy feedback mechanism of drugs
Clinical efficacy is the true feedback on the quality of the drug,so the right to choose drugs should be adjusted through real clinical practice.Establishing and improving the clinical efficacy feedback mechanism of drugs can better collect the real drug data of medical institutions,evaluate the efficacy and judge the quality and value of drugs.The adjustment of these varieties of drug purchased in the late stage of centralized bidding can also play an important role,which will make the quantity procurement more reasonable and effective.Therefore,the level and efficacy of the medical institutions can be improved.
4.4 Improving the rulemaking of the winning bidding
Since China has not established a relatively complete drug price formation mechanism yet,the principle of the lowest bid is the only one that can be used in the procurement of drugs.However,this principle will have some negative effects.If we rely too much on price factors,we cannot guarantee the formation of a long-term mechanism for drug supply because we pay no attention to the quality of drugs,clinical efficacy and drug supply.Therefore,in addition to the drug prices,it is necessary to include several other indicators,such as quality,clinical efficacy,and drug supply capacity to make the gradual improvement of the bidding rules,which can form a long and secure mechanism of drug supply.
In short,through the “4+7” pilot of China’s centralized procurement of drugs we can get the social benefits that have been formed.The obvious effect is to achieve a general reduction in drug prices,guarantee the supply of drugs,and indirectly to promote consistency evaluation of generic drugs in China.At the same time,the price of the original drug has also dropped sharply.Among the 31 pilot generic drugs,there are 25 centralized purchases with a success rate of 81%.Among them,there are 3 original research drugs,accounting for 12%,22 generic drugs,and accounting for 88%.The price of the selected products will be the same in these cities.Through continuous improvement and adjustment,the quantity procurement of drugs will develop fast.
- 亚洲社会药学杂志的其它文章
- Research on Social Medical Insurance of College Students in Shenyang
- The Present Situation,Problems and Countermeasures of Continuing Education for Licensed Pharmacists
- Reference of New Zealand’s Drug Shortage Management Measures
- The Construction of Drug Traceability System Based on Block Chain Technology
- Research on Technology Opportunity ldentification of Small andMedium-sized Vaccine Enterprises Based on Patent Analysis
- An Analysis of Marketing Trend of IVD in China

