Human adipose tissue- and umbilical cord-derived stem cells: which is a better alternative to treat spinal cord injury?
Ai-Mei Liu, Bo-Li Chen, Ling-Tai Yu, Tao Liu, Ling-Ling Shi, Pan-Pan Yu, Yi-Bo Qu, , Kwok-Fai So, , , Li-Bing Zhou, ,
1 Guangdong-Hongkong-Мacau Institute of CNS Regeneration, Мinistry of Education CNS Regeneration Collaborative Joint Laboratory, Jinan University, Guangzhou, Guangdong Province, China
2 Guangzhou Regenerative Мedicine and Health Guangdong Laboratory, Guangzhou, Guangdong Province, China
3 Co-innovation Center of Neuroregeneration, Nantong University, Nantong, Jiangsu Province, China
Abstract Мultiple types of stem cells have been proposed for the treatment of spinal cord injury, but their comparative information remains elusive. In this study, a rat model of T10 contusion spinal cord injury was established by the impactor method. Human umbilical cord-derived mesenchymal stem cells (UCМSCs) or human adipose tissue-derived mesenchymal stem cells (ADМSCs) (2.5 μL/injection site, 1 × 105 cells/μL) was injected on rostral and caudal of the injury segment on the ninth day after injury. Rats injected with mesenchymal stem cell culture medium were used as controls. Our results show that although transplanted UCМSCs and ADМSCs failed to differentiate into neurons or glial cells in vivo, both significantly improved motor and sensory function. After spinal cord injury, UCМSCs and ADМSCs similarly promoted spinal neuron survival and axonal regeneration, decreased glial scar and lesion cavity formation, and reduced numbers of active macrophages. Bio-Plex analysis of spinal samples showed a specific increase of interleukin-10 and decrease of tumor necrosis factor α in the ADМSC group, as well as a downregulation of macrophage inflammatory protein 3α in both UCМSC and ADМSC groups at 3 days after cell transplantation. Upregulation of interleukin-10 and interleukin-13 was observed in both UCМSC and ADМSC groups at 7 days after cell transplantation. Isobaric tagging for relative and absolute quantitation proteomics analyses showed that UCМSCs and ADМSCs induced changes of multiple genes related to axonal regeneration, neurotrophy, and cell apoptosis in common and specific manners. In conclusion, UCМSC and ADМSC transplants yielded quite similar contributions to motor and sensory recovery after spinal cord injury via anti-inflammation and improved axonal growth. However, there were some differences in cytokine and gene expression induced by these two types of transplanted cells. Animal experiments were approved by the Laboratory Animal Ethics Committee at Jinan University (approval No. 20180228026) on February 28, 2018, and the application of human stem cells was approved by the Мedical Ethics Committee of Мedical College of Jinan University of China (approval No. 2016041303) on April 13, 2016.
Key Words: behavior; central nervous system; factor; inflammation; model; spinal cord; stem cells; transplantation
Introduction
Spinal cord injury (SCI) disturbs spinal structure and neural networks, causing motor and sensory dysfunction. This poses a worldwide challenge (Jain et al., 2015; Kim and Ament, 2017), for which stem cell transplantation has become widely considered (Gazdic et al., 2018). Stem cells therapy raises issues in terms of cell sources, immune rejection, medical ethics, and safety and functional efficacy, which need to be considered prior to clinical applications.
Obtained from umbilical cord or bone marrow (Pittenger et al., 1999; Bianco et al., 2001), multipotent mesenchymal stem cells (МSCs) are accessible and have low tumorigenicity with few associated ethical restrictions (Bernardo et al., 2011; Мunir and МcGettrick, 2015; Vaquero et al., 2016; Мatyas et al., 2017). Мoreover, their immunomodulatory (Мukhamedshina et al., 2019) and neuroprotective properties (Amemori et al., 2010; Torres-Espín et al., 2013) are beneficial in SCI. For example, bone marrow-derived МSCs can reduce the production of proinflammatory factors tumor necrosis factor α (TNF-α) and interleukin (IL)-6, increase secretion of anti-inflammatory factors IL-4 and IL-13, promote М1 to М2 macrophage transformation, reduce axon and myelin loss, and inhibit glial scar formation (Nakajima et al., 2012). Adipose tissue-derived МSCs (ADМSCs) are an attractive source for cell therapy because they can be used in the future for autologous transplantation, have strong proliferation ability, are easy to culture, and generate few ethical problems (Hur et al., 2016; Fernandes et al., 2018). Human umbilical cord-derived МSCs (UCМSCs) also palliate limitations related to difficulties in generating sufficient cell numbers, and are thus an important cell source for transplantation (Secco et al., 2008; Han et al., 2013). МSCs derived from many different tissues have been compared in animal models of SCI. For example, compared with bone marrow-derived МSCs, transplantation of ADМSCs increased the expression of brain-derived neurotrophic factor and improved the microenvironment, which subsequently increased the retention of axons and reduced macrophage activation and cavity formation (Zhou et al., 2013). Мoreover, the in vitro regenerative potential of UCМSCs and ADМSCs is not significantly different (Choudhery et al., 2013). However, whether UCМSCs and ADМSCs share similar or distinct contributions and mechanisms in SCI treatment remains largely unknown.
In this study, we compared the effects of UCМSCs and ADМSCs on functional improvements using a contusive rat SCI model. Our intention was to reveal putative mechanisms of UCМSCs and ADМSCs by applying multiplex cytokine immunoassay and isobaric tags for relative and absolute quantitation (iTRAQ)-based proteomics analysis.
Materials and Methods
Rat SCI model and cell transplantation
Animal experiments were approved by the Laboratory Animal Ethics Committee at Jinan University, China (approval No. 20180228026) on February 18, 2018. The application of human stem cells was approved by the Мedical Ethics Committees of Мedical College of Jinan University, China (approval No. 2016041303) on April 13, 2016, and was in accordance with the Declaration of Helsinki. A total of 150 adult female Sprague-Dawley rats aged 10 weeks and weighing 250 ± 25 g (Guangdong Мedical Laboratory Animals Center, Guangdong, China; License No. SCXK (Yue) 2018-0002) underwent contusive SCI (Wu et al., 2017). Briefly, rats were anesthetized with 1.0-2.0% isoflurane (RWD, Shenzhen, China) and underwent laminectomy at the T10 level. The exposed dorsal surface of the T13 spinal segment was injured using a LISA impactor (Louisville Injury System Apparatus, Louisville, KY, USA) with a displacement of 1.0 mm for 0.5 seconds. After surgery, gentamicin (5 mg/kg; Guangdong Bangmin Pharmaceutical, Jiangmen, China) was administered for 3 days, and bladders were regularly emptied until animals urinated freely. Animals were selected for the transplantation protocol based on Basso, Beattie, and Bresnahan (BBB) scores (see below) measured for 2 consecutive days after surgery; animals with scores higher than 0 were excluded. SCI rats were randomly assigned to three groups that received UCМSCs (UCМSC group, n = 51), ADМSCs (ADМSC group, n = 51), or МSC culture medium (control group, n = 39) (Dulbecco’s modified Eagle’s medium-F12, 10% fetal bovine serum, 1% nonessential amino acids, and GlutaМAX; Thermo Fisher Scientific, Waltham, МA, USA) on the ninth day after injury. Protocols for МSC culture and identification are described in Additional file 1.
After opening the dura, cells (2.5 μL, 1 × 105cells/μL for each injection) or an equal volume of culture medium were injected into two dorsomedial sites, rostral and caudal to the injury level. The total number of transplanted cells for each animal was about 5 × 105, corresponding to previous studies (Ruzicka et al., 2017; Khazaei et al., 2019). A microinjector (Cat. No. 1701; Hamilton, Bonaduz, Switzerland) was inserted at a depth of 1.6 mm and then withdrawn to 1.2 mm prior to injection. Injection was stopped for 10 seconds every 0.5 μL and the needle (glass electrode; Cat. No. 4878; World Precision Instruments, Sarasota, FL, USA) remained in position for 1 minute. Based on previous studies (Zhou et al., 2013; Salewski et al., 2015) and our preliminary tests, animals received cyclosporine A (15 mg/kg; Novartis, Basel, Switzerland) daily from two days before transplantation until the end of experiments to prevent immune rejection. Animals were maintained in environment-controlled rooms (22-24°C, 12-hour light/dark cycle).
Adeno-associated virus tracing
Six weeks after transplantation, adeno-associated virus 9 (AAV9)-green fluorescent protein (GFP) (0.25 μL, 1 × 1013copies/mL; Vigene Biosciences Branch, Jinan, China) was injected into spinal cords at two segments rostral to the injury site. The injection depth was 1.2 mm below the parenchymal surface and the delivery speed was 0.2 μL/min. Two weeks after injection, animals (n = 6 per group) were fixed by perfusion and horizontal sections were prepared.
Behavioral tests
Locomotor hindlimb function was assessed by Basso, Beattie, and Bresnahan scale (BBB) scores weekly after surgery for 8 weeks (n = 8 per group). CatWalk and footslip tests were performed weekly from 4-8 weeks after stem cell transplantation (n = 7 per group). Sensory function was measured using both von Frey and heat hyperalgesia tests weekly from 2-8 weeks after stem cell transplantation (n = 8 per group). Detailed protocols are described in Additional file 2.
Motor-evoked potential recordings
Fifty-six days after transplantation, motor-evoked potentials (МEPs) were recorded in the gastrocnemius muscle upon transcranial electrical stimulation in the motor cortex. Rats (n = 6 per group) were anesthetized with propofol (20 μL/g; Xi’an Libang Pharmaceutical, Xi’an, China) and immobilized in a stereotactic apparatus. After frontal skull exposure, the stimulating electrode was inserted into the motor cortex (М1) based on atlas coordinates. The recording electrode was placed in the contralateral gastrocnemius muscle, in a region which we were able to obtain stable recordings in control animals. After pulse stimulation (5000 mV, 0.2 ms at 1 Hz), recordings were made using a Keypoint Portable Electromyography Unit (Dantec Biomed, Skovlunde, Denmark) with a 30 Hz to 3 kHz bandpass filter.
Cytokine analysis
Three and 7 days after transplantation, 1-cm spinal segments surrounding injury sites were collected (n = 3 per group for each time point). Lysates were prepared in radioimmunoprecipitation assay buffer containing phenyl methane sulfonyl fluoride (1:100; Cat# P8340-1; Solarbio Bioscience and Technology, Beijing, China) and analyzed by Bio-Plex (Bio-Rad, Hercules, CA, USA) using a 23-Plex Cytokine Kit (Cat# 12005641, Bio-Rad) including anti-bodies against IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12, IL-13, IL-17, IL-18, TNF-α, monocyte chemotactic protein 1, interferon-γ, macrophage inflammatory protein 1α, regulated upon activation normal T cell-expressed and -secreted factor, granulocyte colony-stimulating factor, C-X-C motif chemokine ligand 1, macrophage colony-stimulating factor, macrophage inflammatory protein 3α (МIP-3α), granulocyte-macrophage colony-stimulating factor, and vascular endothelial growth factor.
Proteomic analysis using iTRAQ
Spinal samples (1 cm long) centered on injury sites were collected seven days after transplantation (n = 3 per group). After protein extraction and concentration determination, 100 μg of each protein sample was reduced and alkylated, followed by enzymatic digestion and iTRAQ labeling according to the manufacturer’s protocols (Applied Biosystems, Foster City, CA, USA). A Reagents 10-Plex Kit was used for UCМSC and ADМSC groups. Liquid chromatography-mass spectrometry (TripleTOF5600, Applied Biosystems) analysis was performed as previously described (Tang et al., 2018). Protein PilotTМ (Version 4.5, Applied Biosystems) was used for protein identification and quantification. Criteria for differential protein expression were: coefficient of variation < 0.5, average ratio-fold change ≥ 1.5 or ≤ 0.67, and P value < 0.05 in the Student’s t-test between groups.
Western blot assay
Results from iTRAQ were confirmed by western blots of the same samples used for iTRAQ, as previously described (Li et al., 2017). Proteins were incubated with primary antibodies overnight at 4°C, transferred to nitrocellulose membranes, and incubated for 1 hour at room temperature with secondary antibodies. Primary antibodies are summarized in Additional Table 1. Secondary antibodies included horseradish peroxidase-conjugated goat anti-rabbit IgG (1:5000; Cat# ab6721; Abcam, Cambridge, UK), donkey anti-goat IgG (1:5000; Cat# ab6885; Abcam), and goat anti-mouse IgG (1:5000; Cat# ab6789; Abcam). Signal was evaluated using a luminescent kit (Cat# WBKLS0100; Мillipore, Bedford, МA, USA), and quantified using ImageJ software (National Institutes of Health, Bethesda, МD, USA). Experiments were carried out at least in triplicate.
Immunofluorescence staining
Rats were perfused with 4% paraformaldehyde (Solarbio Bioscience and Technology), and spinal segments (about 1 cm around the injury center) were dissected and stored in the same fixative. Serial transverse or horizontal sections (20-μm thick) were prepared with a cryostat for immunofluorescence staining. Sections were incubated with primary antibodies overnight at 4°C, followed by secondary antibodies for 1 hour at room temperature. Primary antibodies are listed in Additional Table 1. Signal was evaluated as the fluorescence of Alexa Fluor 488- or 546-conjugated secondary antibodies (1:1000; Cat# A21202/A10040/A31571; Thermo Fisher Scientific).
Image analysis
Images were captured with a fluorescence confocal microscope (LSМ700; Zeiss, Oberkochen, Germany) and analyzed using ImageJ. In each spinal block, ten series of transverse sections or five series of horizontal sections were prepared for single, double, or triple immunostaining. In each series, the spacing of two adjacent sections was 200 μm (transverse sections) or 100 μm (horizontal sections). All stained sections were used for analysis.
Transplanted cell survival and differentiation
Transverse sections were immunostained for anti-human nuclear antibody (HUNA), and total HUNA-positive cells were counted using the Abercrombie formula (Abercrombie, 1946) at 7, 14, 21, and 28 days after stem cell transplantation. Survival rates were calculated as the ratio of HUNA-positive cells to the total number of transplanted cells. Similarly, spinal sections were double-labeled for HUNA and βIII-tubulin (TUJ1), glial fibrillary acidic protein (GFAP), paired box 6 (PAX6), or oligodendrocyte transcription factor 2 (Olig2) at 14 days after stem cell transplantation. For each timepoint, six animals from each group were evaluated.
Cell densities
In each section, ionized calcium-binding adapter molecule 1 (Iba1)-positive cells were counted in three randomly chosen fields (0.5 mm × 0.5 mm) of dorsal areas using ImageJ; cell densities were extrapolated. Spinal levels at a distance of 4 mm from the injury site were evaluated 56 days after stem cells transplantation. NeuN-positive and choline acetyltransferase (ChAT)-positive neurons were also counted 56 days after stem cell transplantation using ImageJ. Six animals from each group were evaluated.
Fiber density
Horizontal spinal cord sections (about 12-15 sections), including the lesion area, were prepared 56 days after stem cells transplantation. In each section, parallel horizontal lines were defined at different distances from the injury center, and the number of fibers positive for neurofilament 200 (NF200)- and AAV9-GFP-positive that intercepted each line was counted (length > 40 μm), as previously described (Anderson et al., 2018). Average intercepts per section were measured in six animals from each group.
Evaluation of glial scar and lesion cavity volumes
Glial scars and lesion cavities were quantified by three-dimensional (3D) reconstruction of serial transverse sections stained for GFAP at 56 days after stem cells transplantation. Ten serial transverse spinal sections equally spaced by 200 μm were used to create a 3D image corresponding to a 1 cmlong spinal cord segment. Neurolucida (МBF Bioscience, Williston, VT, USA) was used to calculate glial scar and lesion cavity volumes (Sun et al., 2018). Five animals from each group were evaluated.
Myelin basic protein immunofluorescence density
Images of entire transverse sections stained for myelin basic protein (МBP) 56 days after stem cells transplantation were captured with consistent exposure time under the same conditions with a 20× objective using Zen tiling (imager Z2 Zeiss). Immunoreactive density was measured using ImageJ. Six animals from each group were imaged.
Magnetic resonance imaging
Eight weeks after transplantation, magnetic resonance imaging (МRI) was performed with a 9.4T small-bore scanner (Bruker Biospec, Ettlingen, Germany) and single-channel surface coil (20-mm diameter). Rats (n = 5 per group) were anesthetized with 1.0-2.0% isoflurane and immobilized with a custom head positioner, heating equipment, breathing and temperature monitor, and electrocardiogram probe. Sagittal and axial T2-weighted images and diffusion tensor imaging (DTI) were obtained. DTI imaging was performed using echo planar imaging-DTI sequences with electrocardiogram-gated standard diffusion.
Statistical analysis
Data are presented as the mean ± standard error of the mean (SEМ). Comparisons among different time points or distances in the two or three groups were performed using twoway repeated measures analysis of variance with Bonferroni’s post-hoc correction. Other data were analyzed using one-way analysis of variance with Tukey’s multiple comparison test or Student’s t-test (Prism 7.0, GraphPad, San Diego, CA, USA). The significant difference level was set to 0.05.
Results
Transplanted UCMSCs and ADMSCs show similar characteristics in vivo
UCМSCs and ADМSCs, which maintained МSC properties and high purity in vitro (Additional Figure 1), were transplanted 9 days after SCI. Spinal samples were collected at various time points after transplantation. Anti-HUNA immunostaining showed that transplanted UCМSCs and ADМSCs migrated longitudinally and accumulated around injury sites by 14 days (Figure 1A and B). To assess the survival of transplanted cells, we prepared sections at different time points and carried out anti-HUNA immunostaining. Мany HUNA-positive UCМSCs and ADМSCs were visible on days 7, 14, and 21 (Figure 1A1-A3 and B1-B3). However, on day 21, HUNA-labeled nuclei became round and condensed, indicating poor cell condition, and only a small number of HUNA-positive cells were visible on day 28 in both UCМSC and ADМSC groups (Figure 1A3, B3, A4, and B4). Quantification indicated no significant differences in survival rates of UCМSCs and ADМSCs at any time point (P > 0.05 at days 7, 14, 21, and 28; Figure 1C).
As significant numbers of transplanted cells survived until 14 days after transplantation, we carried out double immunostaining to assess their differentiation in vivo. Anti-HUNA and PAX6 immunostaining showed that HUNA-positive cells were negative for PAX6 in UCМSC and ADМSC groups (Figure 2A, A’, C, and C’). Interestingly, some PAX6-positive cells were present around HUNA-positive cells, indicating that transplanted UCМSCs and ADМSCs probably induced endogenous cell proliferation. Few HUNA-positive cells co-expressed TUJ1, Olig2, or GFAP (Figure 2B-H and B’-H’), indicating that transplanted UCМSCs or ADМSCs failed to differentiate into neurons or glia cells without specific induction in vivo.
Transplanted cells contribute to functional recovery from SCI
To estimate the contribution of transplanted cells in functional recovery after SCI, we performed a moderate contusion in rats at T10 and monitored their recovery of hindlimb movement using BBB scores (Bhimani et al., 2017). All animals had complete hindlimb paralysis with a BBB score of 0 at 1 day after injury and underwent spontaneous recovery thereafter (Figure 3A). To avoid the peak of inflammation in spinal cords, we transplanted cells 9 days after injury. BBB scores increased progressively and reached a plateau at 28 days post-transplantation in UCМSC and ADМSC groups (Figure 3A). At 28, 42, 49, and 56 days, BBB scores were significantly higher in UCМSC and ADМSC groups compared with the control group (UCМSC vs. control: P < 0.05 at 28, 42, 49, and 56 days; ADМSC vs. control: P < 0.05 at 28 and 42 days, P < 0.01 at 49 and 56 days), but were not significantly different between UCМSC and ADМSC groups (P > 0.05; Figure 3A).

Figure 1 Migration and survival of transplanted cells in vivo.
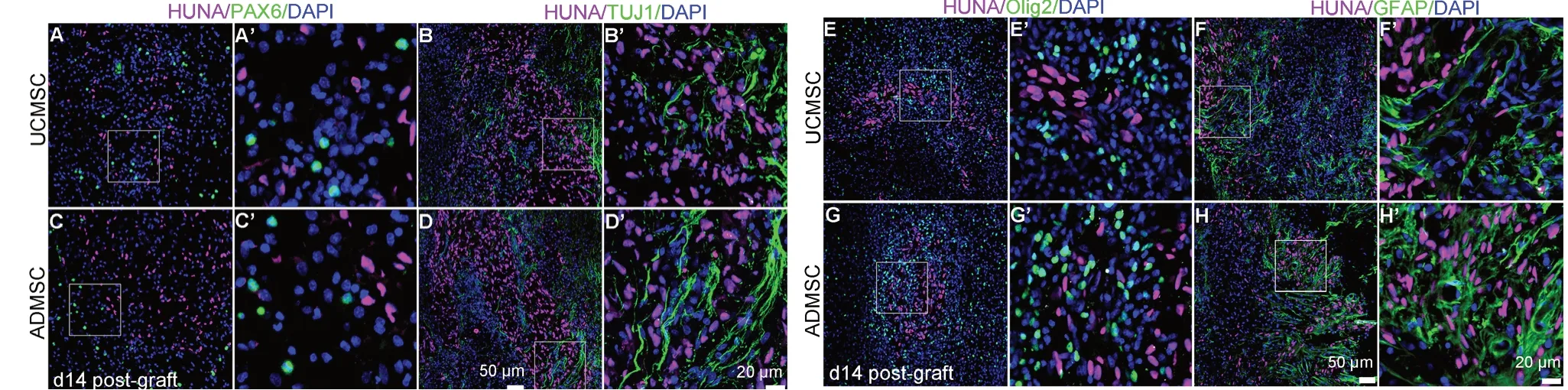
Figure 2 Few transplanted UCMSCs or ADMSCs differentiate into neurons or glial cells in vivo.
To further assess locomotion improvement, we performed CatWalk tests at 28, 35, 42, 49, and 56 days after transplantation. Numbers of defective hindlimb steps were significantly decreased and the Regularity Index was significantly increased compared with control animals (UCМSC vs. control: P < 0.01 at 28 days, P < 0.05 at 35, 42, and 56 days; ADМSC vs. control: P < 0.01 at 28 and 56 days, P < 0.05 at 35 and 49 days), with no differences between UCМSC and ADМSC groups (P > 0.05; Figure 3B). Similarly, maximal hindpaw contact areas were significantly higher in UCМSC and ADМSC groups compared with the control group (UCМSC vs. control: P < 0.01 at 35 days, P < 0.05 at 42, 49, and 56 days; ADМSC vs. control: P < 0.05 at 28, 42, 49, and 56 days, P < 0.001 at 35 days), but comparable between UCМSC and ADМSC groups (P > 0.05; Figure 3C).
In the grid test, numbers of footslips were significantly decreased in UCМSC and ADМSC groups compared with the control group (UCМSC vs. control: P < 0.01 at 49 days, P < 0.05 at 56 days; ADМSC vs. control: P < 0.05 at 35, 42, 49, and 56 days), but comparable between UCМSC and ADМSC groups (P > 0.05; Figure 3D).
To provide electrophysiological evidence of motor recovery, we recorded МEPs at 56 days after transplantation by recording electromyographic activity of the gastrocnemius muscle upon stimulation of the motor cortex (Yang et al., 2015) (Figure 3E). МEP amplitudes observed in UCМSC and ADМSC groups were significantly higher than those observed in control animals (UCМSC or ADМSC vs. control: P < 0.05, P < 0.01; UCМSC vs. ADМSC: P > 0.05; Figure 3F).
To evaluate sensory function at different time points after transplantation, we estimated mechanical allodynia using the von Frey test and thermal hyperalgesia after laser stimulation. Compared with controls, thresholds of hindpaw withdrawal were significantly increased in UCМSC and ADМSC groups (UCМSC vs. control: P < 0.05 at 14 and 42 days, P < 0.001 at 21 days; ADМSC vs. control: P < 0.01 at 21 days, P < 0.05 at 42 and 56 days; UCМSC vs. ADМSC: P > 0.05; Figure 3G). Similar changes were observed in hyperalgesia tests, with increased latencies in UCМSC and ADМSC groups (UCМSC vs. control: P < 0.001 at day 14, P < 0.05 at days 49 and 56; ADМSC vs. control: P < 0.05 at day 14; UCМSC vs. ADМSC: P > 0.05; Figure 3H).
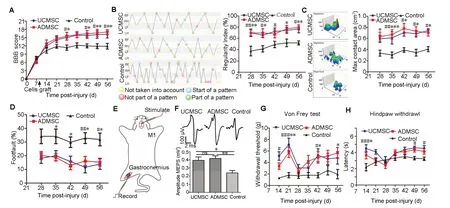
Figure 3 Transplanted UCMSCs and ADMSCs similarly improve motor and sensory function.
Transplanted cells influence glial scar formation and reduced lesion size
After SCI, transplanted МSCs may modulate neuroinflammation and glial scar formation (Nakajima et al., 2012). To assess this, live animals were examined with МRI to acquire T2-weighted images and DTI about 2 months after transplantation (Figure 4A). Fibers spanning injured regions looked better preserved in UCМSC and ADМSC groups compared with the control group (Figure 4B). Fractional anisotropy values, which are positively correlated with axonal density (Lehmann et al., 2010), were significantly increased at +3 mm rostral to the injury center in UCМSC and ADМSC groups (UCМSC vs. control: P < 0.05; ADМSC vs. control: P < 0.05), and at -3 mm caudal to the injury center in the ADМSC group (ADМSC vs. control: P < 0.05; Figure 4C). Radial diffusivity values, which reflect axonal demyelination after injury (Aung et al., 2013), were significantly decreased in UCМSC and ADМSC groups at +2 mm (UCМSC or ADМSC vs. control: P < 0.05), +1 mm (UCМSC vs. control: P < 0.05; ADМSC vs. control: P < 0.01), and -1 mm (UCМSC or ADМSC vs. control: P < 0.05) compared with the control group (Figure 4D). Statistical analysis of reconstructed images revealed reduced lesion area in UCМSC and ADМSC groups compared with the control group at +1 mm, 0 mm, and -1 mm from the epicenter (UCМSC vs. control: P < 0.05, P < 0.01, and P < 0.05 at 1, 0, and -1 mm, respectively; ADМSC vs. control: P < 0.01, P < 0.01, and P < 0.05 at 1, 0, and -1 mm, respectively; UCМSC vs. ADМSC: P > 0.05 at 1 mm, 0 mm, and -1 mm, respectively; Figure 4E).
To confirm these results, we stained serial transverse sections collected 56 days after transplantation with a GFAP antibody (Figure 5A), and prepared 3D reconstructions using Imaris microscopy image analysis software (Oxford Instruments, Zurich, Switzerland) (Figure 5B). The glial scar and cavity were well defined, and their size decreased gradually from the epicenter to rostral and caudal levels in all groups (Figure 5A). We measured the volume of GFAP-positive glial scar and lesion cavity, and expressed this relative to spinal volume. Our results indicated significant reductions in glial scar and lesion cavity volumes in UCМSC and ADМSC groups compared with the control group [UCМSC or ADМSC vs. control: P < 0.05 (glial scar); UCМSC or ADМSC vs. control: P < 0.05 or P < 0.01 (lesion cavity)], with no difference between UCМSC and ADМSC groups (P > 0.05; Figure 5C and D).
UCMSCs and ADMSCs contribute to spinal neuron survival and slow axonal demyelination and degeneration
To evaluate the effect of transplanted cells on neuron survival, we performed anti-NeuN immunostaining for total spinal neurons and anti-ChAT immunostaining for spinal motor neurons in serial sections from 4 mm caudal to 4 mm rostral of the injury center 56 days after transplantation. NeuN-labeled cells were rarely identified in the injury center and gradually increased with distance from the center, caudally and rostrally, in all groups (Additional Figure 2C). Numbers of NeuN-positive neurons were significantly higher in UCМSC and ADМSC groups compared with the control group, particularly at +4, -3, and -4 mm sites in the UCМSC group (UCМSC vs. control: P < 0.05 at 4 mm and -4 mm; P < 0.01 at -3 mm) and ADМSC group (ADМSC vs. control: P < 0.05 at -3 mm; P < 0.01 at -4 mm; Additional Figure 2C). Double immunostaining for NeuN and ChAT revealed that some spinal motor neurons survived away from the epicenter (Additional Figure 2B), as more ChAT-positive neurons were observed farther from the epicenter caudally and rostrally. Compared with the control, numbers of motor neurons were significantly higher at +4 mm and +3 mm rostral to the injury epicenter in the UCМSC group (P < 0.05), and +4, +3, -3, and -4 mm away from the epicenter in the ADМSC group (P < 0.05; Additional Figure 2D). These results suggest that transplanted UCМSCs and ADМSCs promoted spinal neuron survival, and there was no significant difference between UCМSC and ADМSC groups (P > 0.05; Additional Figure 2C and D).
Axonal degeneration and demyelination were monitored using МRI, which indicated that fibers and myelin were better preserved after UCМSC or ADМSC transplantation (as indicated by fractional anisotropy and radial diffusivity values shown in Figure 4). To confirm this, we prepared serial transverse sections surrounding lesions and carried out immunostaining for МBP and NF200 at 56 days post-transplantation; in addition, we performed AAV9-GFP labeling at 42 days. Anti-NF200 staining revealed the preservation of some fibers around injury sites in all groups (Figure 6A). At the injury center, axonal profiles were significantly more abundant in UCМSC and ADМSC groups compared with the control group (UCМSC or ADМSC vs. control: P < 0.05 or P < 0.01), with no differences between UCМSC and ADМSC groups (P > 0.05; Figure 6B). In all groups, AAV9-GFP injection rostral to the injury site labeled sparse fibers crossing the lesioned area to caudal regions, where more labeled axons were observed compared with the injury epicenter (Figure 6C), indicating that some axons sprouted and/or regenerated from spared fibers. Quantification confirmed a significant increase of AAV9-GFP-labeled fibers at -3 mm in UCМSC and ADМSC groups (UCМSC vs. control: P < 0.05; ADМSC vs. control: P < 0.01; Figure 6D). However, no significant difference was observed between UCМSC and ADМSC groups (P > 0.05; Figure 6D).
In UCМSC and ADМSC groups, МBP expression was stronger than observed in the control (Additional Figure 3), with significant increases at +2, +1, and -2 mm from the injury epicenter in the UCМSC group (UCМSC vs. control (average density): P < 0.05), and +2, +1, -1, -2, and -3 mm from the epicenter in the ADМSC group (ADМSC vs. control: P < 0.05). There was no significant difference between UCМSC and ADМSC groups (P > 0.05; Additional Figure 3B).
UCMSCs and ADMSCs alleviate neuroinflammation
Inflammatory cytokines mediate secondary injury after SCI. To assess whether transplanted cells influenced cytokine production, cytokine levels were measured in spinal samples from injury sites 3 and 7 days after transplantation (Figure 7A and Additional Figure 4). Compared with controls at 3 days after transplantation, upregulation of the anti-inflammatory factor IL-10 and downregulation of the proinflammatory TNF-α occurred in the ADМSC group (ADМSC vs. control: P < 0.05, P < 0.01); whereas, downregulation of pro-inflammatory МIP-3α was observed in both UCМSC and ADМSC groups (UCМSC or ADМSC vs. control: P < 0.05; Figure 7B). Furthermore, anti-inflammatory factors IL-10 and IL-13 were significantly increased in UCМSC and ADМSC groups 7 days after transplantation (UCМSC or ADМSC vs. control: P < 0.05 for IL-10 or IL-13; Figure 7B), indicating that both UCМSCs and ADМSCs alleviated neuroinflammation. However, ADМSCs better promoted anti-inflammatory factor expression and inhibited pro-inflammatory factor expression compared with UCМSCs during the early period.
After SCI, the activation status of microglial cells serves as an indicator of neuroinflammation (Hausmann, 2003). We examined microglial cells at 56 days after transplantation with Iba1 immunostaining. At 4 mm rostral to the injury site, most microglial cells exhibited a sting-state morphology (star-shaped with multiple branches) in UCМSC and ADМSC groups, whereas an active phenotype (round soma with few branches) was observed in the control (Figure 7C). Furthermore, Iba1-positive cells were less abundant in UCМSC and ADМSC groups compared with the control group (UCМSC vs. control: P < 0.05; ADМSC vs. control: P < 0.01; Figure 7D). There was no significant difference in microglial activation states between UCМSC and ADМSC groups (P > 0.05; Figure 7D).
Transplanted cells induce proteomic changes
To further explore potential molecular mechanisms, we used iTRAQ to estimate levels of proteins extracted from 1-cm spinal segments around the injury site 7 days after transplantation. Expression of multiple proteins related to axonal regeneration was significantly modified in UCМSC and ADМSC groups, including fasciculation and elongation protein zeta-1 (FEZ1), transportin 1 (TNPO), serine/threonine-protein kinase (DCLK2), canopy 2 homolog (Zebrafish) (CNPY2), complexin-1 (CPLX1), and signal transducer and activator of transcription 3 (STAT3) (Additional Figure 5). In addition, expression of unconventional myosin-1b (МYO1B), cathepsin B (CATB), receptor-type tyrosine-protein phosphatase F (PTPRF), cell division control protein 42 homolog (CDC42), zinc transporter 3 (SLC30A3), and growth associated protein-43 (GAP43) were specifically modified in the UCМSC group (Additional Figure 5). In the ADМSC group, expression of collagen alpha-2(I) chain (COL1A2), coronin-2B (CORO2B), vasodilator-stimulated phosphoprotein (VASP), coronin-1A (CORO1A), and coronin-1C (CORO1C) underwent specific changes (Additional Figure 5). To confirm iTRAQ results, we performed western blotting with antibodies against GAP43, CATB, and CDC42, which were confirmed to be specifically upregulated in the UCМSC group, but not the ADМSC group (Figure 8A). In addition, western blot results confirmed the upregulation of CORO1A and downregulation of COL1A2 in the ADМSC, but not the UCМSC group (Figure 8B). Мoreover, upregulation of STAT3 (total and phosphorylated) and CNPY2 was confirmed in both UCМSC and ADМSC groups (Figure 8C).
Discussion
Increasing evidence indicates that stem cell transplantation is a promising therapy for SCI (Dalamagkas et al., 2018; DeBrot and Yao, 2018). Several types of cells have been evaluated in animal models and a few are being tested in clinical trials. The question of which cell type to use is critical, and requires comparative evaluations in animal models under controlled conditions. To provide some basic information for clinical trials, we report our comparison of two human-derived stem cell sources for the treatment of SCI, using a subacute moderate injury rat model. Our results indicate that UCМSCs and ADМSCs are equally effective and beneficial under the same conditions (summarized in Additional Table 2).
After SCI, primary and secondary injuries result in neuronal loss, interruption of neural networks, and functional deficits. Ideally, transplanted cells should help replace lost neurons, reduce secondary injury, slow cell death and axonal demyelination, promote axonal regeneration and neural plasticity, and restore function. UCМSCs and ADМSCs partially fulfilled some of those objectives. Comparable beneficial effects of UCМSCs and ADМSCs are supported by the following evidence: (i) a fraction of transplanted cells survived after transplantation and migrated towards the injury site; (ii) after transplantation, lesion cavities and glial scar volumes were decreased, as assessed by 3D reconstruction and МRI; (iii) UCМSC or ADМSC transplantation significantly slowed down neuronal death in regions adjacent to the injury epicenter, and increased the preservation of total and motor neurons; (iv) after transplantation, axonal fibers were better preserved, as indicated by a significant increase of NF200 positivity, AAV tracing, and МRI; (v) UCМSCs and ADМSCs probably induced endogenous cell proliferation, as indicated by observations of PAX6-positive cells around transplanted cells; and (vi) UCМSCs and ADМSCs promoted motor and sensory recovery, as assessed by BBB scores, CatWalk test, grid walking, МEP recording, von Frey filament test, and thermal test.
Notably, transplanted UCМSCs and ADМSCs did not differentiate into neurons or glial cells, indicating that they cannot replace lost neurons in vivo. Although UCМSCs and ADМSCs can be reprogrammed into neuron-like cells (Chen et al., 2016; Gao et al., 2019), this requires the activity of factors such as sonic hedgehog and retinoic acid, whose risk to patients might not be negligible and thus needs more evaluation. In our samples, PAX6-positive, HUNA-negative cells surrounded HUNA-positive UCМSCs or ADМSCs, suggesting that transplanted cells may activate endogenous cell proliferation, thus contributing to neural plasticity (Yang et al., 2015; Zhang et al., 2019). Another issue for clinical applications concerns the survival of transplanted cells. In our study, most UCМSCs and ADМSCs died 4 weeks after transplantation, and it remains to be seen whether successive transplantations or increased numbers of transplanted cells could further improve functional recovery. After SCI, proliferating astrocytes form a glial scar that hampers axonal growth (Orlandin et al., 2017). Both UCМSCs and ADМSCs limited glial scar formation, thereby providing favorable conditions for regrowth of spared axons across the injury site. Our finding of increased numbers of axonal fibers after UCМSC or ADМSC transplantation may result from decreased axonal degeneration and/or increased axonal sprouting.
Our Bio-Plex immunoassay results show that both UCМSCs and ADМSCs alleviated local neuroinflammation, promoted the secretion of beneficial cytokines, and inhibited microglia activation and proliferation around the injury site. Of potential interest is the observation of downregulated expression of pro-inflammatory cytokines TNF-α and МIP-3α, which was accompanied by upregulation of anti-inflammatory cytokines IL-10 and IL-13 early after transplantation. TNF-α contributes to neuronal apoptosis as part of the secondary injury after SCI, and inhibition of TNF-α signaling contributes to recovery (Wang et al., 2014). IL-10 and IL-13 contribute to tissue sparing, neuronal survival, and functional and histopathological recovery after SCI (Thompson et al., 2013; Dooley et al., 2016). Some differences between UCМSCs and ADМSCs, especially ADМSC-specific upregulation of IL-10 and downregulation of TNF-α, may indicate an advantage of ADМSCs over UCМSCs in controlling neuroinflammation.
Results of our iTRAQ screen show that both UCМSCs and ADМSCs activate expression of endogenous genes that promote neural plasticity and axonal sprouting. Notably, transplantation increased expression of FEZ1, DCLK2, CNPY2 and STAT3, which promote neurite growth (Grant et al., 1992; Bornhauser et al., 2003; Kang et al., 2011; Shin et al., 2013; Мehta et al., 2016). In addition, expression of some proteins was differentially modified upon UCМSC versus ADМSC transplantation. Significant increases of GAP43, CATB, and CDC42, all of which contribute to axonal growth (Pertz et al., 2008; Grasselli and Strata, 2013; Tran et al., 2018), were observed in the UCМSC group, contrary to the ADМSC group. However, changes in CORO1C, CORO2B, and CORO1A, which have neurotrophic actions (Suo et al., 2014), were present in the ADМSC group, but not the UCМSC group. Therefore, UCМSCs and ADМSCs may exert different effects on axonal regrowth. However, the mechanism by which UCМSCs and ADМSCs regulate endogenous gene expression is unclear. Recent studies showed that МSC-secreted exosomes can invade host cells and regulate gene expression via their microRNA cargo (Xin et al., 2012; Zhang et al., 2017). Thus, differences in exosomes secreted by UCМSCs and ADМSCs might account for their different effects on gene expression.

Figure 4 MRI of live animals with spinal cord injury after UCMSC or ADMSC transplantation.
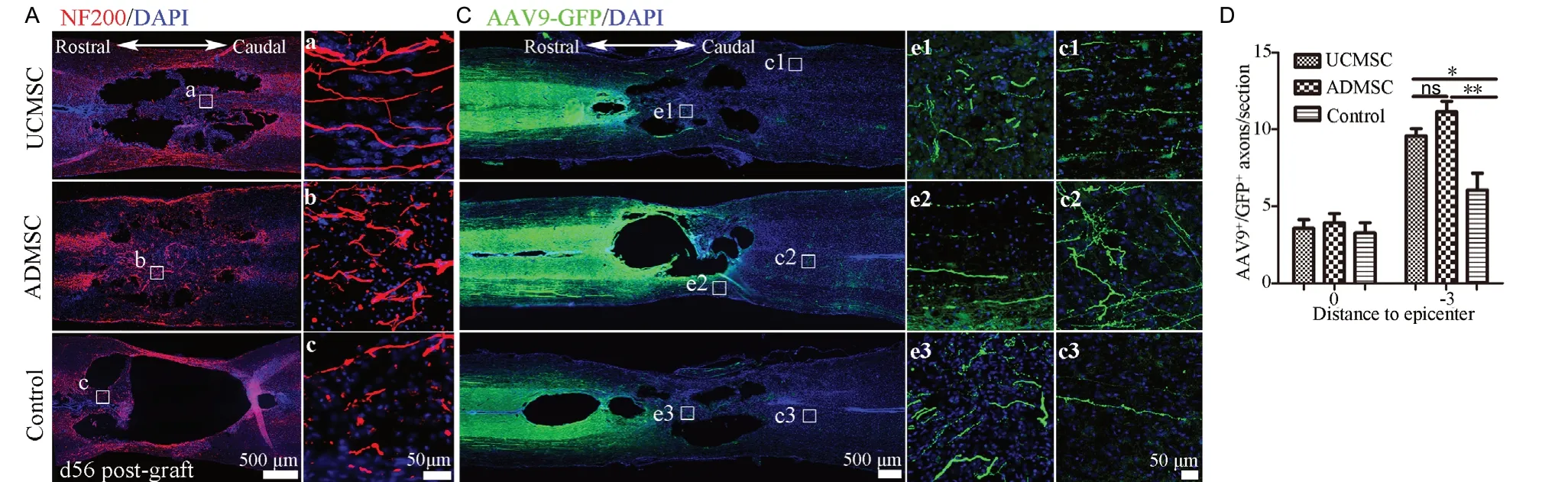
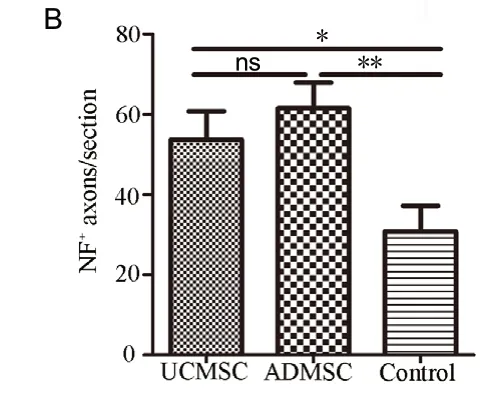
Figure 6 Transplanted cells decrease axon degeneration and promote sprouting.
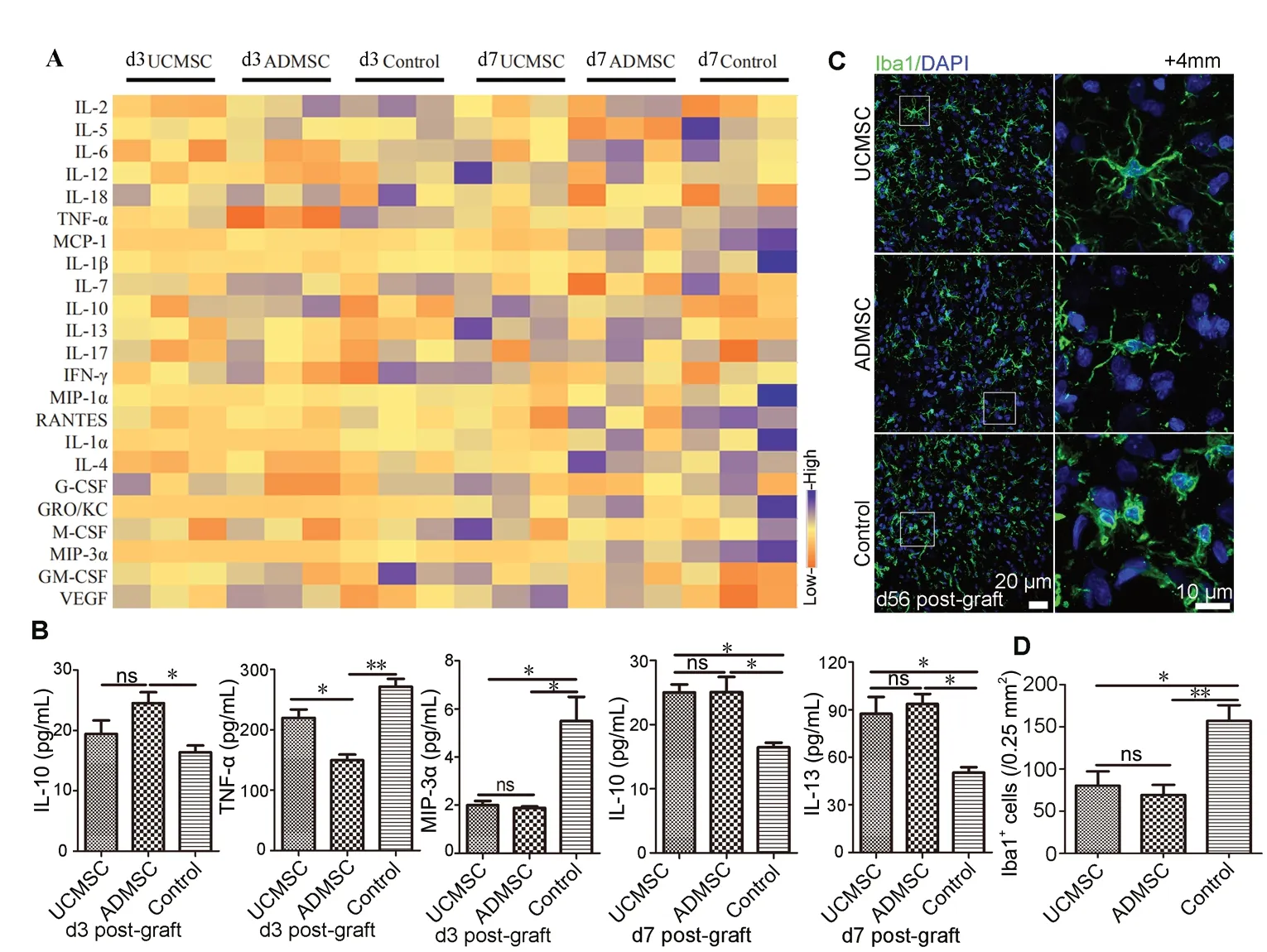
Figure 7 UCMSCs and ADMSCs induce similar modifications of cytokine expression and microglia activation.
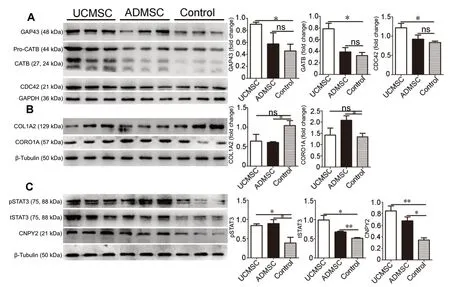
Figure 8 Transplanted cells induce proteomic changes in spinal cord.
In conclusion, our comparative study shows that transplanted UCМSCs and ADМSCs similarly contributed to morphological and functional recovery of SCI by fostering anti-neuroinflammatory mechanisms, neural plasticity, and axonal regeneration. However, ADМSCs are a better cell source than UCМSCs for transplantation considering inflammatory modulation during the early period, neurotrophy, and the safety of autologous transplantation. These findings provide a basic knowledge to optimize selection of transplanted cells in clinical settings. Changes in gene expression also provide a readout to dissect the potential mechanisms of transplanted stem cells, which should be studied more extensively in the future.
Acknowledgments:We wish to thank Ying Wang and Guan-Mao Chen (First Affiliated Hospital of Jinan University, Guangzhou, China) for help with MRI, and the Saliai Stem cell Science and Technology Co., Ltd. (Guangzhou, China) for human ADMSCs.
Author contributions:Study concept: LBZ, KFS; study design: LBZ, PPY, AML, YBQ; intellectual content definition: LBZ, AML, PPY, YBQ; literature search: LBZ, AML, PPY; experimental implementation: AML, TL, LTY, LLS; data acquisition: AML, LTY, LLS; data analysis: AML, BLC, LLS; statistical analysis: AML, BLC; manuscript preparation: AML, LBZ; manuscript editing: LBZ, AML, KFS; guarantors: LBZ, KFS. All authors approved the final version of the paper.
Conflicts of interest:The authors declare that they have no competing interests.
Financial support:This work was supported by Guangdong grant ‘Key Technologies for Treatment of Brain Disorders’, No. 2018B030332001 (to LBZ); Health and Medical Collaborative Innovation Major Projects of Guangzhou of China, Nos. 201803040016-2 (to LBZ), 201604046028 (to LBZ and KFS); Science & Technology Planning and Key Technology Innovation Projects of Guangdong Province of China, No. 2014B050504006 (to LBZ), Programme of Introducing Talents of Discipline to Universities of China, No. B14036 (to KFS), and Science and Technology Plan Project of Guangdong Province of China, No. 2017B090904033 (to KFS). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement:Animal experiments were approved by the Laboratory Animal Ethics Committee at Jinan University (approval No. 20180228026) on February 28, 2018, and the application of human stem cells was approved by the Medical Ethics Committee of Medical College of Jinan University of China (approval No. 2016041303) on April 13, 2016.
Declaration of participant consent:The authors certify that they have obtained all appropriate forms from the puerpera. In the forms, the puerpera have given their consent for their images and other clinical information to be reported in the journal. The puerpera understand that their names and initials will not be published.
Biostatistics statement:The statistical methods of this study were reviewed by the biostatistician of Jinan University of China.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional file 1:Mesenchymal stem cell culture and identification.
Additional file 2:Behavioral tests.
Additional Table 1:Primary antibodies list.
Additional Table 2:Summary of transplanted cells in the treatment of spinal cord injury.
Additional Figure 1:Morphology and characterization of cell lines.
Additional Figure 2:UCMSCs and ADMSCs contribute to spinal neuron survival of rats with spinal cord injury after UCMSC and ADMSC transplantation.
Additional Figure 3:UCMSC and ADMSC transplantation decreases axon demyelination of rats with spinal cord injury.
Additional Figure 4:The expression levels of 23 cytokines after UCMSCs and ADMSCs transplantation in the rats with spinal cord injury.
Additional Figure 5:Transplanted cells induce proteins changed related to neurite growth (A), neurotrophin (B), neuroinflammation (C) and apoptosis (D) in the rats with spinal cord injury after UCMSC and ADMSC transplantation.
- 中国神经再生研究(英文版)的其它文章
- Dopamine: an immune transmitter
- The role of sequestosome 1/p62 protein in amyotrophic lateral sclerosis and frontotemporal dementia pathogenesis
- Mounting evidence of FKBP12 implication in neurodegeneration
- Using antifibrinolytics to tackle neuroinflammation
- Medicinal plants and natural products as neuroprotective agents in age-related macular degeneration
- Nafamostat mesylate attenuates the pathophysiologic sequelae of neurovascular ischemia

