Annexin A2 promotion of hepatocellular carcinoma tumorigenesis via the immune microenvironment
Li-Wei Qiu, Yi-Fei Liu, Xiao-Qing Cao, Yan Wang, Xiao-Hong Cui, Xian Ye, Shuo-Wen Huang, Hong-Jun Xie,Hai-Jian Zhang
Abstract Hepatocellular carcinoma (HCC) is the most common primary liver cancer with a dismal prognosis, especially when diagnosed at advanced stages. Annexin A2(ANXA2), is found to promote cancer progression and therapeutic resistance.However, the underlining mechanisms of ANXA2 in immune escape of HCC remain poorly understood up to now. Herein, we summarized the molecular function of ANXA2 in HCC and its relationship with prognosis. Furthermore, we tentatively elucidated the underlying mechanism of ANXA2 immune escape of HCC by upregulating the proportion of regulatory T cells and the expression of several inhibitory molecules, and by downregulating the proportion of natural killer cells and dendritic cells and the expression of several inhibitory molecules or effector molecules. We expect a lot of in-depth studies to further reveal the underlying mechanism of ANXA2 in immune escape of HCC in the future.
Key words: Annexin A2; Hepatocellular carcinoma; Immune microenvironment; Overall survival; Chemotherapy resistance; Checkpoint
INTRODUCTION
As an aggressive malignancy, liver cancer is the fifth leading cause of death from cancer globally[1]. Hepatocellular carcinoma (HCC), the most common type of primary liver cancer, has a poor prognosis, especially when diagnosed at the advanced stages[2]. One of the leading risk factors for HCC is infection with the hepatitis B virus,particularly in East Asia[3]. Although surgical treatment for HCC may be effective in the early stages, the 5-year overall survival (OS) rate is only 50%-70%[4,5]. According to a recent study based on proteomic and phosphoproteomic profiling, early-stage HCC can be further stratified into three subtypes with different clinical outcomes[6]. The third subtype, which is characterized by disrupted cholesterol homeostasis, is associated with the lowest postoperative OS rate and the greatest risk of a poor prognosis[7,8]. Despite global advancement of social development and implementation of the annual physical examination program to increase the diagnosis of patients with early-stage HCC, the proportion of patients with advanced HCC at first diagnosis remains high[9]. Although first-line drugs such as sorafenib and lovatinib were used in the treatment of advanced HCC, the landscape of advanced HCC management was not optimistic until the advent of immunotherapy and the knowledge gained about the molecular pathogenesis of the disease[2,10,11].
HCC is considered to be an immunogenic tumor resulting from diseases that lead to chronic inflammation of the liver[12]. Therefore, immunotherapeutic strategies may represent a key treatment direction for improving the clinical outcomes of patients with HCC[13,14]. In recent years, immune checkpoint inhibitors have emerged as potential drugs with promising therapeutic effects against advanced HCC[15-17];examples include nivolumab[18-20]or pembrolizumab[21,22]for programmed cell death protein 1 (PD-1) blockade, atezolizumab (MPDL3280A) for programmed cell deathligand 1 blockade[23,24], and ipilimumab for cytotoxic T-lymphocyte-associated protein 4 blockade[25]. Of course, the combination of different immunotherapies or of immunotherapies with conventional therapeutic approaches may provide synergistic effects and facilitate the development of personalized medicine[16,26]. However, the molecular mechanisms underlying HCC immune escape remain poorly understood[27-29].
Annexin A2 (ANXA2, also termed annexin II, p36, calpactin 1 heavy chain, or lipocortin II) was originally extracted from human placenta as an inhibitor of phospholipase A2[30]. The humanANXA2gene, which is located on chromosome 15q21, is 40 kb in length and has 13 exons. It can be cleaved by chymotrypsin into a 3 kDa amino-terminal domain and a 33 kDa carboxyl-terminal domain. The ANXA2 protein can exist as a monomer, heterodimer, or heterotetramerin vivo. The heterodimeric form consists of one subunit of ANXA2 in complex with a molecule of 3-phosphoglycerate kinase, whereas the heterotetrameric form consists of two ANXA2 subunits combined with an S100A10dimer[31].
The function of ANXA2 is closely related to the form in which it exists. It has been shown that the ANXA2 monomer is localized mainly in the cytoplasm but may transition to the intracellular membrane in response to signals such as changes in the Ca2+concentration, pH, or membrane phospholipid composition. However, the specific biological roles it plays in the subsequent processes are still unclear[32]. The ANXA2 dimer is involved in the formation of intracellular vesicles through combination with multiple endosomes and mediation of membrane fusion[33].Additionally, the dimer is required for the biogenesis of multivesicular bodies as well as being a constituent of exosomes that are frequently cited in proteomic studies[34].The ANXA2 heterotetramers are the most well studied of the three forms, and it is now well established that they serve as an assembly site for plasminogen and tissue plasminogen activator on the endothelial cell surface, thereby promoting the generation of plasmin and allowing the clearance of fibrin formed on the blood vessel surface in response to more subtle forms of vascular injury[35,36].
In recent years, increasingly more studies have focused on the relationship between ANXA2 and immune-related diseases, such as lupus nephritis, rheumatoid arthritis,and cancer[37-39]. ANXA2 was found to promote various processes related to cancer progression, such as cancer proliferation, migration, epithelial-mesenchymal transition (EMT), invasion, and stem cell formation, as well as their resistance to radiotherapy, chemotherapy, and immunotherapy[40]. There is growing evidence that ANXA2 plays an important role in tumor immune escape[41].
LITERATURE SEARCH
A scientific literature search was conducted using the PubMed, Web of Science, and Google Scholar databases. The keywords used included “cancer,” “hepatocellular carcinoma,” “ANXA2,” “immune escape,” “immunotherapy,” “overall survival,” and combinations of the aforementioned terms.
MOLECULAR FUNCTIONS OF ANXA2 IN CANCER
Upregulation of ANXA2
A high level of ANXA2 is characteristic of malignant salivary gland tumors[42]and pulmonary invasive mucinous adenocarcinoma[43]and is associated with DNA repair as well as metabolic alteration in pancreatic ductal adenocarcinoma[44]. ANXA2 is highly expressed in gastric cancer tissues and is related to the tumor size, histological differentiation, tumor-node-metastasis stage, and lymph node metastasis[45]. The ANXA2-S100A10 heterotetramers are upregulated by the promyelocytic leukemia-retinoic acid receptor alpha fusion protein and promotes plasminogendependent fibrinolysis and matrix invasion in acute promyelocytic leukemia[46].ANXA2 overexpression contributes to the aggressive phenotype of triple-negative breast cancer in the African American population[47]. The ANXA2 protein content harbored by extracellular vesicles represents a promising prognostic biomarker in endometrial cancer[48]. The ginsenoside compound K inhibits nuclear factor-kappa B by targeting ANXA2[49,50].
In a rat model of cirrhosis, after 30 wk of thioacetamide induction, the level of ANXA2 in the liver increased three times over the level before modeling with the dynamic increasing trend being positively correlated with immune factors, such as interleukin and transforming growth factor-beta, indicating the close relationship between ANXA2 and precancerous lesions of HCC[51]. Our previous study results suggested that the circulating levels of ANXA2 in patients with HCC were significantly higher than those in patients with other liver diseases[52]. ANXA2 was frequently found to be upregulated in HCC tissues compared with its levels in benign liver disease tissues and was significantly correlated with the degree of histological differentiation, intrahepatic metastasis, portal vein thrombosis, and tumor-node-metastasis stage[53]. From our present search of The Cancer Genome Atlas database, we have further confirmed that ANXA2 is upregulated in HCC tissues compared with its level in normal tissues (Figure 1A,P< 0.001). In addition,ANXA2is a critical differentially expressed gene in nonalcoholic fatty liver disease, where it is associated with the disease severity and modifiable lifestyle factors[54].
Signaling pathways
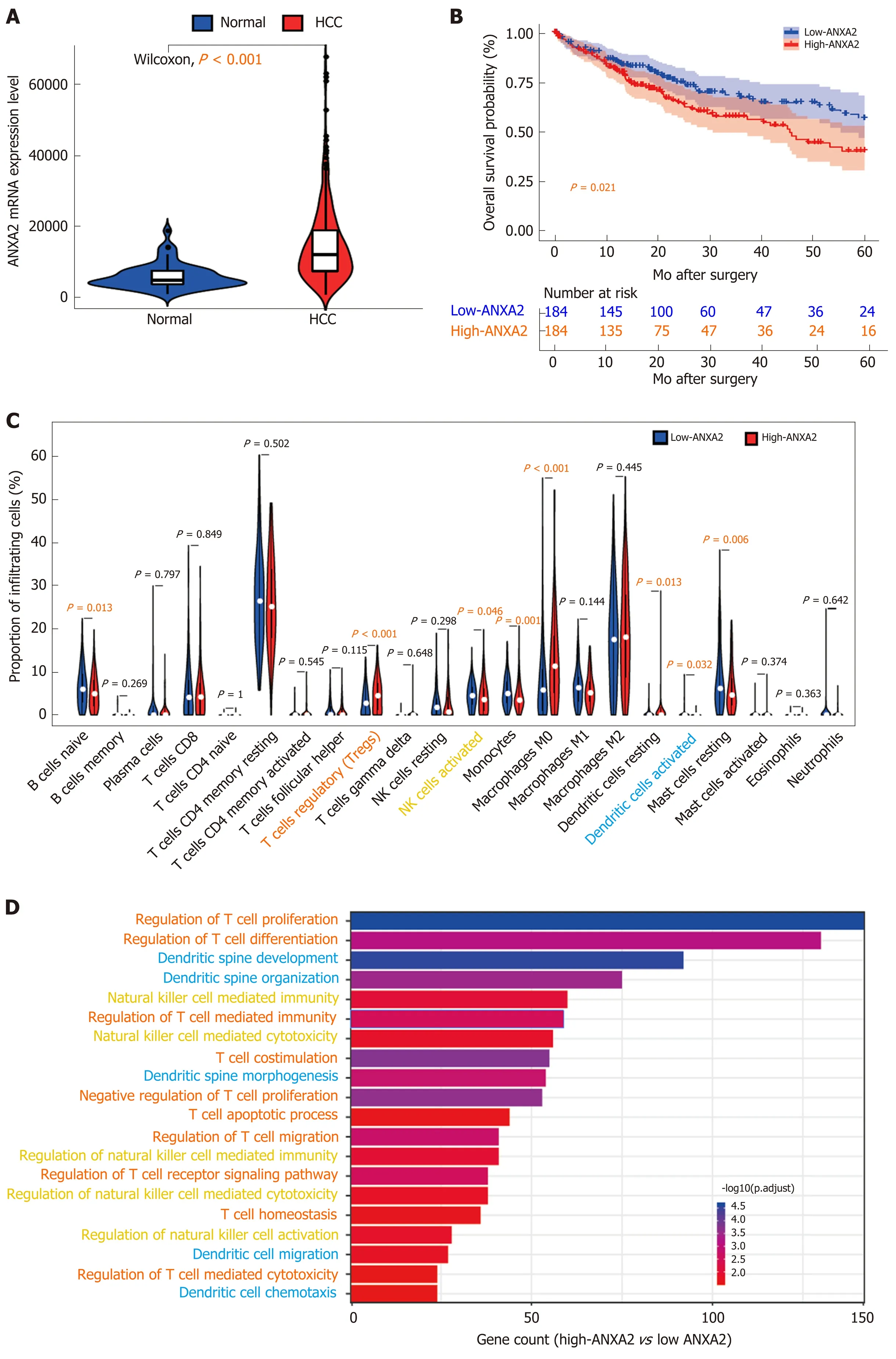
Figure 1 lnvolvement of Annexin A2 in the change of immune microenvironment. A: The expression of ANXA2 mRNA according to The Cancer Genome Atlas database; B: Increased ANXA2 results in poorer 5-year overall survival; C: The proportion of 22 infiltrating immune cells; D: Gene ontology analysis of differentially expressed genes. ANXA2: Annexin A2; HCC: Hepatocellular carcinoma.
The interaction of human epididymis protein 4 with ANXA2 promotes the migration of various malignant cells[55]. ANXA2 enhances the progression of colorectal cancer and HCCviastructural rearrangement of the cytoskeleton[56]. The protein also promotes glioma cell proliferation through the signal transducer and activator of transcription 3-cyclin D1 pathway[57]. ANXA2 has been shown to be a specific target of bleomycin, where its binding with the drug impeded the induction of pulmonary fibrosis mediated by the transcription factor EB-induced autophagic flux[58]. The miR155HG-miR-185-ANXA2 loop contributes to glioblastoma progression[59].The long noncoding (lnc) RNA lung cancer-associated transcript 1 promotes tumorigenesis by inhibiting ANXA2 phosphorylation in HCC[60]. The miR-23b-3p-ANXA2 axis inhibits the development and progression of pancreatic ductal adenocarcinoma[61]. ANXA2 was found to promote cancer progression and therapeutic resistance in nasopharyngeal carcinoma[40]. The lncRNA cytoskeleton regulator RNA induces the upregulation of ANXA2 by binding competitively to miR-613, leading to nasopharyngeal carcinoma metastasis[62]. Another lncRNA, colon cancer-associated transcript 1, interacts with ANXA2 to promote beta-catenin translocation to the nucleus where it then activates T-cell factor 4, leading to breast cancer progression[63,64]. The lncRNA small nucleolar RNA host gene 14 potentiates pancreatic cancer progressionviaANXA2 expression upregulation by acting as a competing endogenous RNA for miR-613[65]. Our previous results have suggested that ANXA2 silencing inhibits the invasion, migration, and tumorigenic potential of hepatoma cells[66].
Epithelial-mesenchymal transition
ANXA2 overexpression is associated with colorectal cancer invasiveness and TGFßinduced EMT through the Src-ANXA2-signal transducer and activator of transcription 3 axis[67]. Mesenchymal stem cells promote hepatocarcinogenesisviathe interaction of ANXA2 with a novel lncRNA termed mesenchymal stem cell-upregulated factor[68].ANXA2 inhibition suppresses ovarian cancer progression through the control of betacatenin and hence EMT[69]. ANXA2 silencing inhibits the proliferation, invasion, and migration of gastric cancer cells[70]as well as non-small cell lung cancer proliferation and EMT through a p53-dependent pathway[71].
Posttranslational modification
The phosphorylation of ANXA2 at its tyrosine residue promotes the invasion and metastasis of drug-resistant breast cancer cells[72]. Highly expressed phosphorylated ANXA2 (Tyr23) also promotes esophageal cancer progression by activating the MYChypoxia-inducible factor 1 alpha-vascular endothelial growth factor axis[73]. The tumor suppressor sirtuin 6, which is ubiquitylated and degraded by E3 ubiquitin ligase,contributes to liver tumorigenesis in an ANXA2-dependent manner[74]. Tripartite motif-containing, a novel marker of poor prognosis in ovarian cancer, promotes the malignant progression of the disease by inducing ANXA2 expression[75]. Likewise,tripartite motif-containing 65 supports the aggressiveness of bladder urothelial carcinoma cells by promoting ANXA2 ubiquitination and degradation[76].
ASSOCIATION OF ANXA2 WITH POOR PROGNOSIS
Reduced overall survival
ANXA2 is an independent prognostic biomarker for the malignant progression of laryngeal cancer[77]. The protein may also be a potential prognostic biomarker of liver cancer[78]. The high expression level of ANXA2 in stromal tissue is associated with a reduced OS rate in patients with epithelial ovarian cancer[79], and when highly expressed in cancer cell membranes is associated with poor prognosis in pancreatic cancer[80]. ANXA2 overexpression is predictive of decreased survival in patients with pancreatic cancer[81]and triple-negative breast cancer[82]. According to a quantitative phosphoproteomic analysis, the phosphorylation of ANXA2 Tyr23 is associated with poor prognosis in HCC[83]. Our previous research results confirmed that an increased level of ANXA2 was closely associated with a shortened OS rate in patients with HCC and was therefore identified as an independent prognostic factor of this disease[53].Herein, working with data from the Cancer Genome Atlas database, we further confirmed that patients with HCC with high ANXA2 expression levels had a shorter OS (Figure 1B,P< 0.001).
Drug resistance
A combinedANXA2-N-Myc downstream regulated 1-signal transducer and activator of transcription 1gene signature predicts the response of patients with cervical cancer to chemoradiotherapy[84]. The interaction of P37 with ANXA2 is required for the mycoplasma-associated multidrug resistance of hepatocarcinoma cells[85]. ANXA2 contributes to cisplatin resistance in cells of non-small cell lung cancer by activating the c-Jun N-terminal kinase-p53 pathway[86]and enhances multidrug resistance in pediatric neuroblastoma by regulating the nuclear factor-kappa B signaling pathway[87]. MiR-101 alleviates the chemoresistance of gastric cancer cells by targeting ANXA2[88]. Chemotherapy combined with bevacizumab can effectively destroy advanced lung adenocarcinoma cells harboringepidermal growth factor receptor-ANXA2mutations[89].
ROLE OF ANXA2 IN THE CHAOTIC IMMUNE MICROENVIRONMENT
A study of the antitumor effect of a vaccine prepared from H22 hepatocarcinoma cells induced by cartilage polysaccharides found ANXA2 to be closely related to oncogenesis and cancer development, invasion, and metastasis. A major increase in ANXA2 mRNA was found in the cartilage polysaccharide-induced H22 cells. The data suggested that ANXA2, a specific antigen, may play a key role in the antitumor immune response of HCC and in activating the immune system[90]. ANXA2 was found to be a tumor-associated antigen in patients with lung cancer who had been exposed to asbestos[91]. It has also been implicated in the attachment and entry, genome replication and expression, assembly, and egress of viruses[92].
ANXA2 is essential for the trafficking and capsid disassembly of oncogenic human papillomavirus and protects the virions from lysosomal degradation[93]. The cellsurface translocation of ANXA2 contributes to bleomycin-induced pulmonary fibrosis through its mediation of the inflammatory response in mice[94]. Stromal cell-derived factor-1 alpha triggers theengulfment and cell motility 1gene-dependent membrane translocation of ANXA2 for the regulation of HCC chemotaxis and metastasis[95].
Cancer-associated fibroblasts promote EMT and epidermal growth factor receptor-tyrosine kinase inhibitor resistance in non-small cell lung cancersviahepatocyte growth factor-insulin-like growth factor-1-ANXA2 signaling[96]. A study on the pathogenesis of immune-mediated liver fibrosis found that after modeling of the disease in rats by injection with pig serum, the ANXA2 concentration increased continually in the rat liver during the process of fibrosis. Similarly, the serum levels of ANXA2 in patients with liver fibrosis were upregulated by 1.4-fold compared with the levels in healthy individuals. When Huh7 cells were exposed to the hepatitis B virusin vitro, ANXA2 translocated from the nucleus and cytoplasm to the cytoplasmic membrane, which suggested that it was involved in the immune-mediated liver injury caused by the virus[97]. Dendritic cells (DCs) respond to nasopharyngeal carcinoma cells through an ANXA2-recognizing C-type lectin, named DC-specific intercellular adhesion molecule 3-grabbing nonintegrin[98].
In this present review, our analysis of Cell-type Identification By Estimating Relative Subsets Of RNA Transcripts data[99]revealed that elevated ANXA2 levels resulted in a higher proportion of Treg cells (P< 0.001) and lower proportions of activated natural killer (NK) cells (P= 0.046) and DCs (P= 0.032) than those found in the low-ANXA2 group and in some nonfunctional immune cells (Figure 1C).Furthermore, our Gene Ontology analysis of differentially expressed genes (false discovery rate < 0.05; fold change = 2) suggested that signatures of functional regulation in Treg, NK, and DC cells were enriched (Figure 1D) in patients with HCC.
In addition, ANXA2 plays a key role in nontumorous immunological diseases.Soluble ANXA2 activates human macrophagesviamitogen-activated protein kinases and may be capable of acting as an inflammatory mediator[100]. ANXA2 expression was downregulated in myeloid cells that had been induced to differentiate through stimulation with all-trans retinoic acid[101]. An immune response mediated by ANXA2 autoantibodies resulted in high circulating levels of interleukin-6 in serum samples from patients with lung cancer[102].
ROLE OF ANXA2 IN IMMUNE ESCAPE
A recent study has shown that T-cell activation, proliferation, and cluster formation are dependent on the proteases tissue plasminogen activator and plasmin[103]. The tissue plasminogen activator treatment of T cells increased the cleavage of ANXA2,which regulates the actin cytoskeleton. Live cell imaging of the activated T cells further indicated a negative role of the ANXA2-regulated actin cytoskeleton in T-cell clustering. This may be one of the mechanisms by which the upregulation of ANXA2 in tumors leads to decreased T-cell activation and an imbalance of the tumor microenvironment[103].The soluble ANXA2 released by tumor cells has novel immunosuppressive properties in patients with renal cell carcinoma[104]. Elevated serum levels of ANXA2 may be important for the suppression of the immune response[105]. AListeria-based ANXA2-targeting immunotherapy in combination with anti-PD-1 antibodies demonstrated high efficacy against pancreatic tumors[39]. ANXA2 in the cancerous cell membrane was identified as the direct antigenic ligand of the Vγ8Vδ3 T-cell receptor of γδ T cells, which make up the first line of defense of stressed cells[41].
At present, there are limited published studies on the role of ANXA2 in immune escape. In this review, we analyzed the correlations of partially labeled genes of Treg cells (Figure 2A), activated NK cells (Figure 2B), and DCs (Figure 2C). We further confirmed that an elevated ANXA2 level results in the upregulation of several checkpoint molecules, such as PD-1, hepatitis A virus cellular receptor 2, gelectin-9,cytotoxic T-lymphocyte-associated protein 4, CD86, and CD80 (Figure 2D). Moreover,we also found that elevated ANXA2 levels result in the downregulation of several inhibitory molecules (e.g., TGFβ and interleukin-10), and effector molecules (e.g.,perforin 1, granzyme B, interferon-gamma, and tumor necrosis factor-alpha; Figure 2E). These results suggest that elevated ANXA2 levels contribute to HCC immune escape.
CONCLUSION
ANXA2 is usually overexpressed in cancerous tissue and results in shorter OS and chemotherapy resistance in patients with HCC[106]. Furthermore, an elevated ANXA2 level results in the upregulation of both the proportion of Treg cells and the expression of several checkpoint molecules as well as the downregulation of both the proportions of activated NK cells and DCs and of several inhibitory molecules.Although there are few research studies to date on the role of ANXA2 in tumor immune escape, we expect a future increase in the number of in-depth studies being carried out to reveal the mechanism through which ANXA2 mediates the immune escape of HCC.
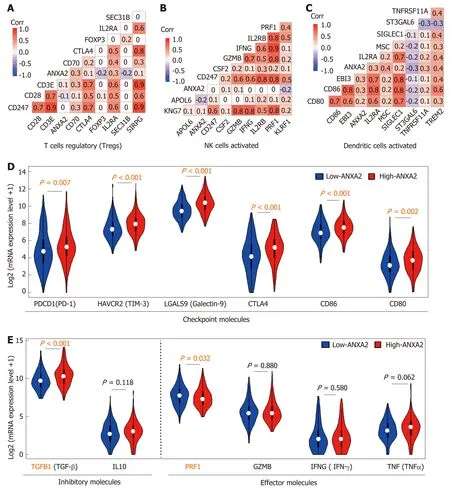
Figure 2 lncreased Annexin A2 promotes immune escape in hepatocellular carcinoma patients. A: Correlation of partial labeled genes of regulatory T cells; B:Correlation of partial labeled genes of activated natural killer cells; C: Correlation of partial labeled genes of activated dendritic cells; D: The expression of partial checkpoint molecules; E: The expression of partial inhibitory molecules and effector molecules. ANXA2: Annexin A2; NK: Natural killer.
 World Journal of Gastroenterology2020年18期
World Journal of Gastroenterology2020年18期
- World Journal of Gastroenterology的其它文章
- Folic acid attenuates high-fat diet-induced steatohepatitis via deacetylase SlRT1-dependent restoration of PPARα
- Genetic association analysis of CLEC5A and CLEC7A gene singlenucleotide polymorphisms and Crohn's disease
- Hepatitis B virus recurrence after liver transplantation: An old tale or a clear and present danger?
- Natural products that target macrophages in treating non-alcoholic steatohepatitis
- Ectopic hepatocellular carcinoma mimicking a retroperitoneal tumor:A case report
- Computed tomography vs liver stiffness measurement and magnetic resonance imaging in evaluating esophageal varices in cirrhotic patients: A systematic review and meta-analysis
