Prognostic value and nomograms of proximal margin distance in gastric cancer with radical distal gastrectomy
Jun Luo,Yuming Jiang,Xinhua Chen,Yuehong Chen,Jhang Lopsang Gurung,Tingyu Mou,Liying Zhao,Guoqing Lyu,Tuanjie Li,Guoxin Li,Jiang Yu
1Department of General Surgery,Nanfang Hospital,Southern Medical University,Guangzhou 510515,China;2Department of Gastrointestinal Surgery,Peking University Shenzhen Hospital,Shenzhen 518036,China
Abstract Objective:The proximal margin(PM)distance for distal gastrectomy(DG)of gastric cancer(GC)remains controversial.This study investigated the prognostic value of PM distance for survival outcomes,and aimed to combine clinicopathologic variables associated with survival outcomes after DG with different PM distance for GC into a prediction nomogram.Methods:Patients who underwent radical DG from June 2004 to June 2014 at Department of General Surgery,Nanfang Hospital,Southern Medical University were included.The first endpoints of the prognostic value of PM distance(assessed in 0.5 cm increments)for disease-free survival(DFS)and overall survival(OS)were assessed.Multivariate analysis by Cox proportional hazards regression was performed using the training set,and the nomogram was constructed,patients were chronologically assigned to the training set for dates from June 1,2004 to January 30,2012(n=493)and to the validation set from February 1,2012 to June 30,2014(n=211).Results:Among 704 patients with pTNM stage I,pTNM stage II,T1−2,T3−4,N0,differentiated type,tumor size≤5.0 cm,a PM of(2.1−5.0)cm vs.PM≤2.0 cm showed a statistically significant difference in DFS and OS,while a PM>5.0 cm was not associated with any further improvement in DFS and OS vs.a PM of 2.1−5.0 cm.In patients with pTNM stage III,N1,N2−3,undifferentiated type,tumor size>5.0 cm,the PM distance was not significantly correlated with DFS and OS between patients with a PM of(2.1−5.0)cm and a PM≤2 cm,or between patients with a PM>5.0 cm and a PM of(2.1−5.0)cm,so there were no significant differences across the three PM groups.In the training set,the C-indexes of DFS and OS,were 0.721 and 0.735,respectively,and in the validation set,the C-indexes of DFS and OS,were 0.752 and 0.751,respectively.Conclusions:It is necessary to obtain not less than 2.0 cm of PM distance in early-stage disease,while PM distance was not associated with long-term survival in later and more aggressive stages of disease because more advanced GC is a systemic disease.Different types of patients should be considered for removal of an individualized PM distance intra-operatively.We developed a universally applicable prediction model for accurately determining the 1-year,3-year and 5-year DFS and OS of GC patients according to their preoperative clinicopathologic characteristics and PM distance.
Keywords:Gastric cancer;margin distance;nomograms;distal gastrectomy
Introduction
Gastric cancer(GC)has become a significant cause of cancer-related deaths worldwide(1-3).Radical resection is still the only proven and potentially curative treatment for GC without distant metastasis(4,5).A range of proximal margin(PM)distance has been advocated for distal gastric adenocarcinoma in previous studies(6-10);however,the specific margin width remains debatable.To ensure radical resection of the tumor,some surgeons prefer to get a wider margin,but reducing the margin width during resection has been associated with better quality of life,easier digestive tract reconstruction and a lower incidence of complications.Therefore,it is imperative to identify optimal margins of excision to reduce postoperative recurrence and complications and to increase postoperative quality of life in patients with GC.
The aim of the current study was to assess the relationship between PM distance and clinicopathological variables and to evaluate the prognostic value of PM distance for disease-free survival(DFS)and overall survival(OS)in patients after radical distal gastrectomy(DG)in order to provide a reasonable reference for the surgeon to select a suitable PM distance intra-operatively.In addition,this study combined preoperative clinicopathologic variables associated with DFS and OS with PM distance into a prediction nomogram for GC based on the data from Department of General Surgery,Nanfang Hospital,Southern Medical University.
Materials and methods
Patient population
This retrospective study included 704 consecutive patients with gastric adenocarcinoma underwent treatment between June 1,2004 to June 30,2014 at Department of General Surgery,Nanfang Hospital,Southern Medical University,Guangzhou,China.The baseline characteristics for each variable are listed inTable 1.Among the patients,those who underwent DG with R0 resection were selected for analysis.To eliminate the interference of residual neoplasm in this study,patients with microscopic residual tumor(R1)and macroscopic residual tumor(R2)were excluded.We thoroughly and systematically analyzed the patients’clinical basic data and clinicopathological parameters.The preoperative TNM stage and the final pathologic TNM stage were classified according to the AJCC Cancer Staging Manual,7th Edition(11).During the study period,patients were followed up from the date of surgery until July 1,2017 or their death.The follow-up period for survivors ranged from 5 to 133 months,with a mean period of 48 months.Each patient’s follow-up data were collected from hospital records.
Margin analysis
The distribution of PM distance in 0.5 cm increments is shown inFigure 1A.Margin distance was measured by surgeon within half an hour after the stomach cancer specimen was removed.The first objective was to evaluate the prognostic value of PM distance for DFS and OS after DG with R0 resection.DFS in this current study was defined as the time to recurrence at any site(such as anastomosis,gastric remnant,perigastric regional lymph nodes and distant metastasis to the pancreas,liver,peritoneum,or other sites).We also conducted multiple subgroup analysis based on PM distance in DFS and OS among different clinicopathological variables.We analyzed the survival of patients for the three intervals beyond which no apparent further increase in DFS and OS was observed(PM≤1.0 cmvs.1.1−1.5 cmvs.1.6−2.0 cm;DFS,P=0.168;OS,P=0.062;Figure 1B),while the PM in 0.5 cm increments,these four groups showed a statistically significant difference in DFS and OS(PM≤1.0 cmvs.1.1−1.5 cmvs.1.6−2.0 cmvs.2.1−2.5 cm;DFS,P=0.022;OS,P=0.003;Figure 1B).Thus,the entire cohort was finally divided into three PM distance groups(≤2.0 cm,2.1−5.0 cm,and>5.0 cm).
Construction of nomogram
For nomogram construction and validation,we chronologically assigned patients from June 1,2004 to January 31,2012 to the training set(n=493)and patients from February 1,2012 to June 30,2014 to the validation set(n=211).The preoperative clinicopathologic characteristics and the PM distance in the training and validation set were evaluated.For the current study,9 variables were included in the initial analysis:age,sex,carcinoembryonic antigen(CEA),carbohydrate antigen(CA)199,CA724,cT stage,cN stage,biopsy histology typeand PM.Variables were selected by the forward stepwise selection method using the Cox regression model.On the basis of the predictive model with the prognostic factors,a nomogram was constructed for predicting 1-year,3-year and 5-year DFS and OS.
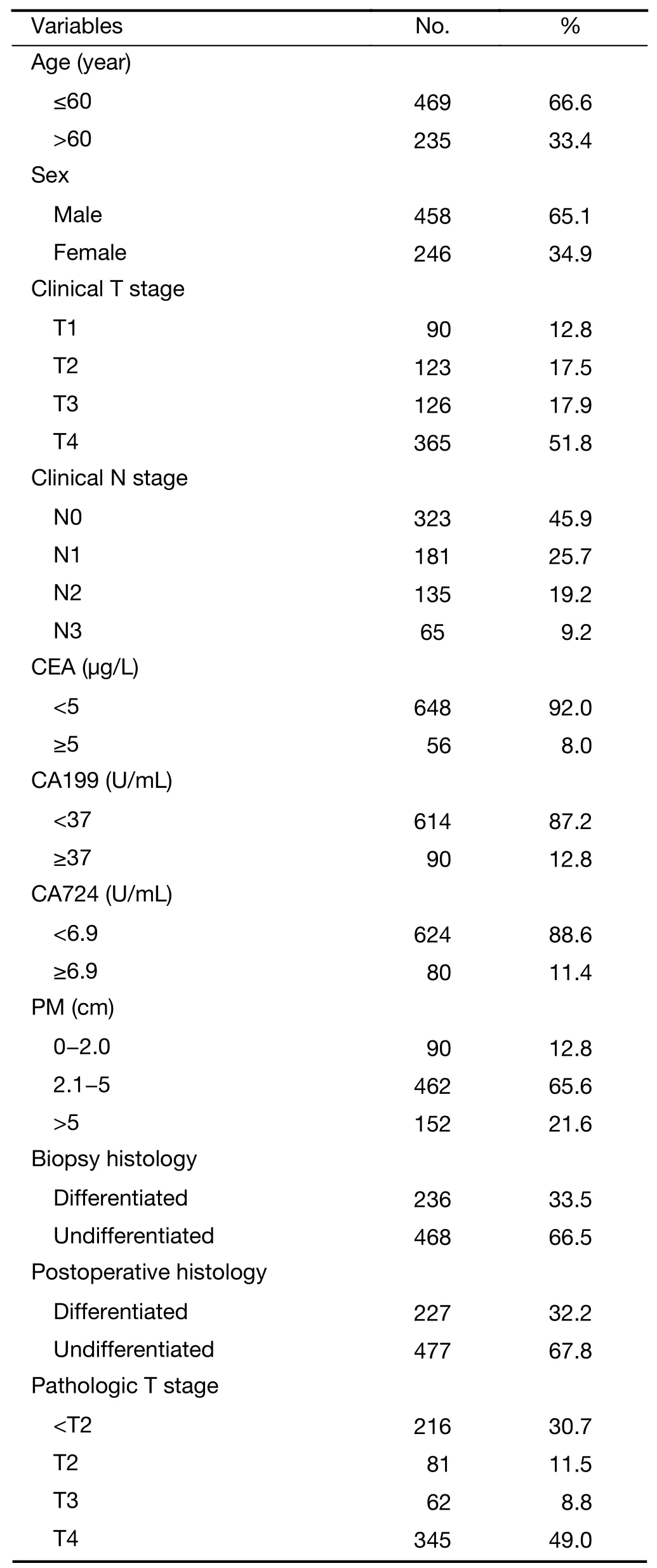
Table 1 Clinicopathological features of gastric cancer patients(N=704)
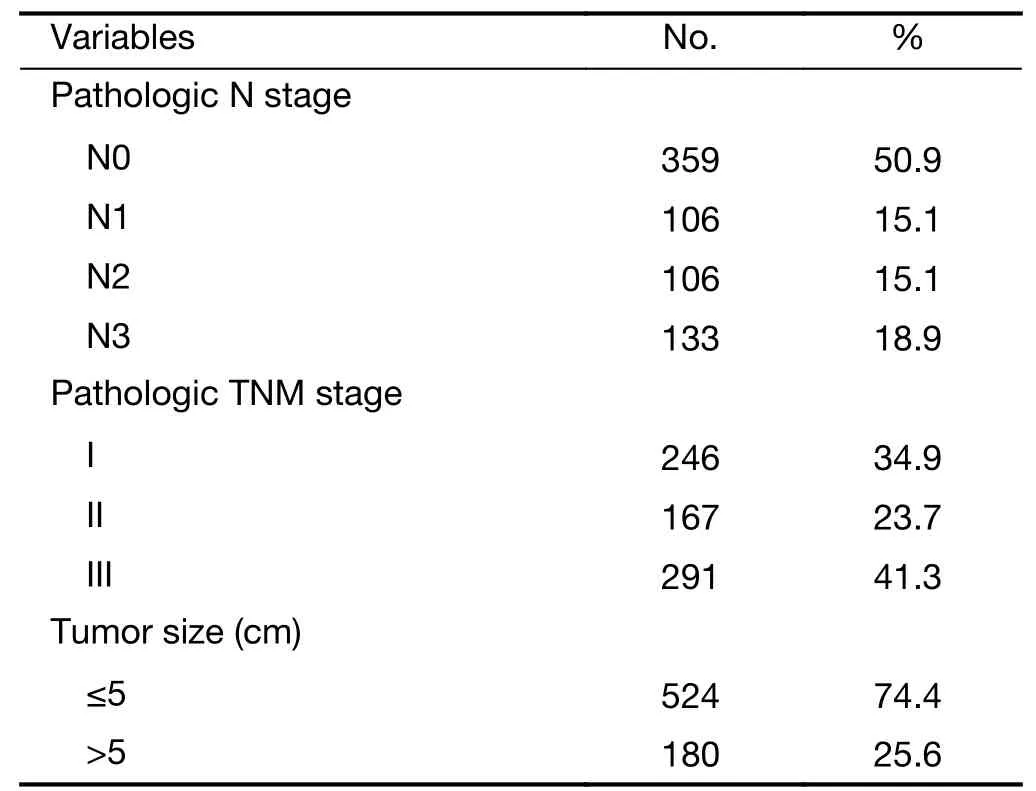
Table 1 (continued)
Validation of nomogram
Nomogram validation consisted of a discrimination and calibration curve.Discrimination was assessed using the concordance index(C-index).To do so,a relatively unbiased estimate was obtained with the bootstrap method.The data were randomly drawn with replacement from the original data set,and the parameters were recalculated.The C-index was then computed using the new parameters in the bootstrap random samples.After grouping of the nomogram predicted survival by decibel,calibration was carried out by comparing the means of predicted survival with actual survival evaluated with the Kaplan-Meier method.
Statistical analysis
DFS and OS were evaluated using the Kaplan-Meier method.A multivariate predictive model was constructed using a Cox proportional hazards model based on univariate and multivariate Cox regression analyses of risk factors associated with DFS and OS,and P<0.05 was considered statistically significant.All calculations were performed with SPSS version 20.0(IBM Inc.,New York,USA)and R software version 3.4.0(http://www.rproject.org)with the design and survival packages.Survival analysis was performed with the“survival”package.Multivariate Cox regression,nomograms and calibration plots were generated with the“rms”package.Comparisons between C-indexes were performed with the“Hmisc”package.Decision curve analysis was performed with the function of“dca.R”.Reported statistical significance levels were all two-sided.The statistical significance level was set at 0.05.
Results
Entire cohort analysis
The PM distances were divided into three groups for analysis(PM≤2.0 cm,n=90;PM:2.1−5.0 cm;n=462,and PM>5.0 cm,n=152).For 704 patients,Kaplan-Meier analysis showed an extended survival time,while patients with a PM of 2.1−5.0 cm and those with a PM≤2.0 cm demonstrated significant differences in DFS and OS(P<0.001 for both)(Figure 2A,E).However,the difference was not significant when the two groups were compared to those with a PM of 2.1−5.0 cmvs.a PM>5.0 cm(P=0.181 and P=0.492,respectively)(Figure 2A,E).
Subgroup stratification analysis
Among patients with pTNM stage I and TNM stage II disease,a PM of 2.1−5.0 cmvs.PM≤2.0 cm showed a statistically significant difference in DFS(P<0.001 and P=0.022,respectively)(Figure 2B,C)and OS(P<0.001 and P=0.027,respectively)(Figure 2F,G),while a PM>5.0 cm was not associated with any further improvement in DFS(P=0.419 and P=0.346,respectively)and OS(P=0.749 and P=0.357,respectively)vs.a PM of 2.1−5.0 cm.In patients with pTNM stage III disease,the PM distance was not significantly correlated with DFS and OS between patients with a PM of 2.1−5.0 cm and a PM≤2 cm,or between patients with a PM>5.0 cm and a PM of 2.1−5.0 cm,so there were no significant differences across the PM≤2.0 cmvs.PM of 2.1−5.0 cmvs.PM>5.0 cm(P=0.059 and P=0.064,respectively)(Figure 2D,H).
Patients in the subgroups of T1−2 stage and T3−4 stage showed close correlations with DFS(P<0.001 and P=0.003,respectively)(Figure 3Aa,b)and OS(P<0.001 and P=0.003,respectively)(Figure 3Ba,b)between a PM of 2.1−5.0 cm and a PM≤2.0 cm;however,there was no significant association with DFS(P=0.237 and P=0.149,respectively)(Figure 3Aa,b)and OS(P=0.435 and P=0.369,respectively)(Figure 3Ba,b)between PM>5.0 cm and a PM of 2.1−5.0 cm.In survival analysis of patients in the subgroup of N0 stage,there was a statistical difference in DFS(P<0.001)and OS(P<0.001)between a PM of 2.1−5.0 cm and PM≤2.0 cm(Figure 3Ac,Bc),and there were no statistically significant differences for DFS(P=0.090)and OS(P=0.155)between a PM>5.0 cm and a PM of 2.1−5.0 cm.In contrast,with regard to the correlation between long-term survival and N1 and N2−N3 stage,a statistical difference in DFS(P=0.149 and P=0.079,respectively)and OS(P=0.220 and P=0.092,respectively)was not observed(Figure 3Ad,e,3Bd,e).For the subgroups of tumor size and postoperative histology type,both patients with a tumor size≤5.0 cm and differentiated type disease showed a significant difference when the two subgroups were compared with a PM of 2.1−5.0 cm and PM≤2.0 cm in DFS(P<0.001 and P<0.001,respectively)and OS(P<0.001 and P<0.001,respectively)(Figure 3Af,h,3Bf,h);however,there remained a negative association between a PM>5.0 cm and a PM of 2.1−5.0 cm for DFS(P=0.075 and P=0.139,respectively)and OS(P=0.263 and 0.476,respectively).The subgroups with a tumor size>5.0 cm and undifferentiated type disease,which showed a similar survival curve,exhibited an insignificant difference between PM and long-term survival for DFS(P=0.167 and P=0.144,respectively)and OS(P=0.069 and P=0.588,respectively)(Figure 3Ag,i,3Bg,i).
Entire cohort cox regression analysis
Table 2documents the results of multivariate Cox regression analyses for DFS and OS.Age,pT,pN,tumor size and PM were correlated with significantly worse survival.The Cox proportional hazards model indicated that pT,pN and pM were independent prognostic factors for DFS and OS and that age was also a prognostic factor for OS.
Prediction model
After examination and transformation of the preoperative clinicopathologic variables to fit in the Cox regression model,variables were selected by the forward stepwise selection method(P<0.05).Table 3lists the selected variables with hazard ratios.The PM,cT,cN,CEA and CA199 are high hazard ratios of DFS,and except for these ratios,age is particular for OS.
Figure 4Ashows the nomogram predicting 1-year,3-year and 5-year DFS and OS was constructed based on the selected variables with hazard ratios.The nomogram can estimate the probability of survival by adding up the points identified on the points scale for each variable.The total points projected to the bottom scale indicate the probability of 1-year,3-year and 5-year survival.
In the training set,the C-indexes of DFS and OS were 0.721(95% CI,0.686−0.757)and 0.735(95% CI,0.698−0.773),respectively.In the validation set,the Cindexes of DFS and OS were 0.752(95% CI,0.703−0.801)and 0.751(95% CI,0.686−0.817),respectively.Figure 4Bshows the calibration curve of the nomogram,where the xaxis is the predicted survival calculated by the nomogram,and the y-axis is the actual survival analyzed by the Kaplan-Meier method.The solid line represents the ideal reference line where predicted survival corresponds with actual survival.The calibration of the model seemed to yield accurate and useful predictions of 1-year,3-year and 5-year survival in GC patients.
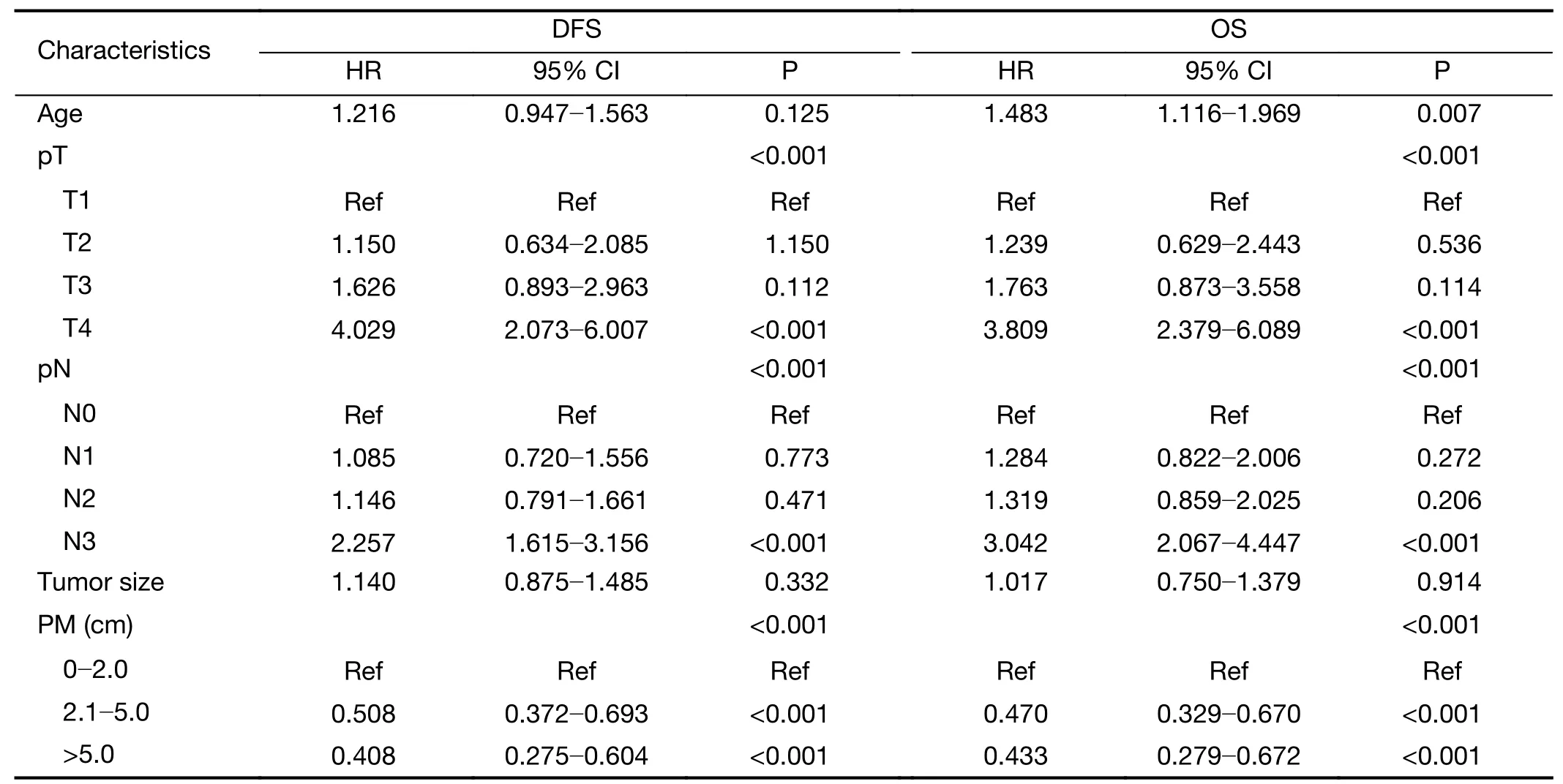
Table 2 Multivariate Cox regression analysis of risk factors associated with DFS and OS
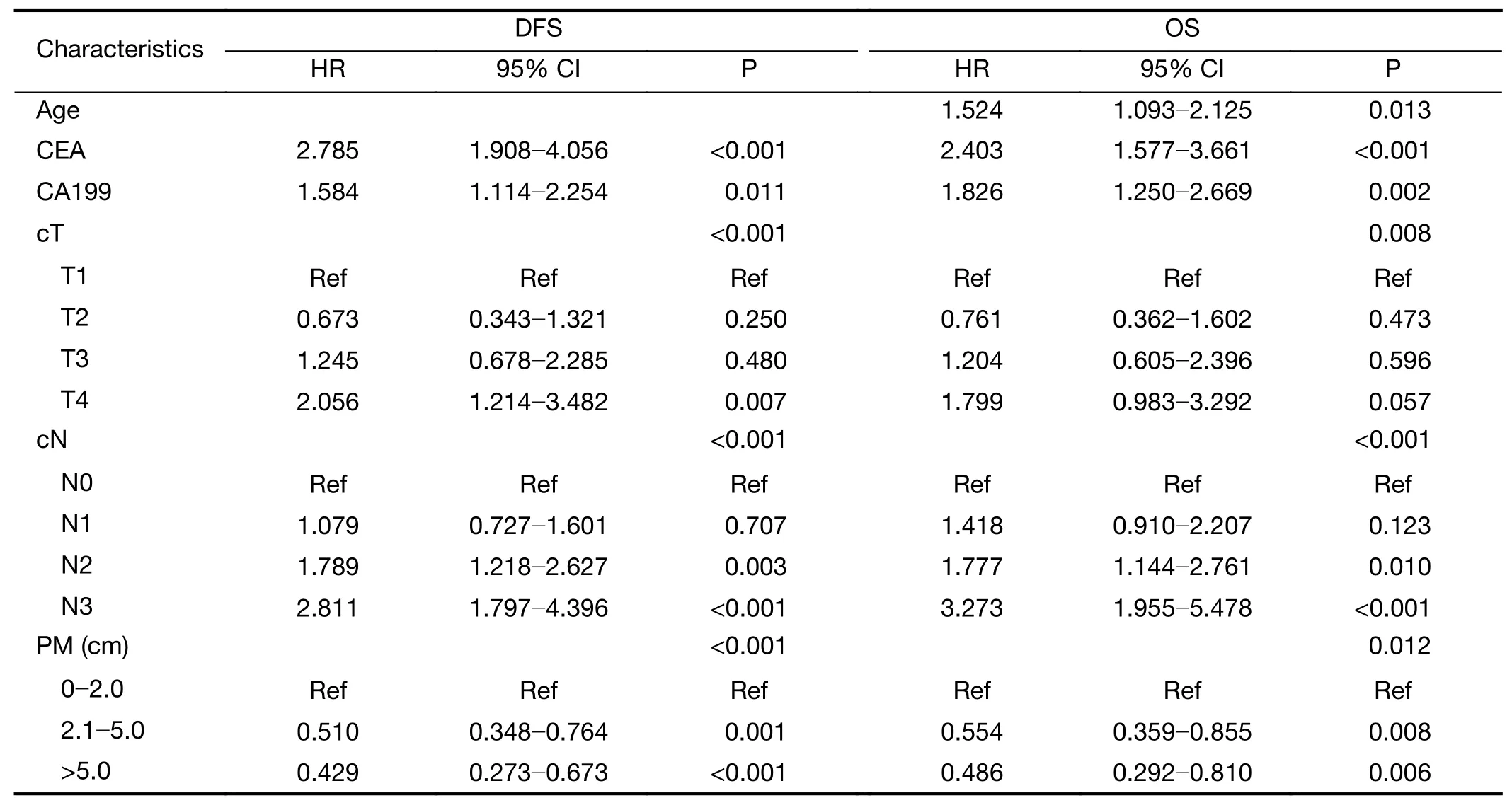
Table 3 Training set of multivariate Cox regression analysis
Discussion
For decades,scholars have advocated resection of greater than 6 cm to attain an adequate margin of resection in gastrectomy for cancer(9),which is in agreement with other reports advocating for wide gross resection to decrease the risk of positive margins and postoperative recurrence(12),especially for early-stage GC(13,14).For the resection margin of GC,the latest National Comprehensive Cancer Network(NCCN)practice guidelines dictate>4 cm for the gross tumor(15).The Japanese Gastric Cancer Treatment Guidelines advise that a>2 cm margin should be obtained for T1 tumors and 3−5 cm for T2−T4 tumors,depending on the growth pattern(16).To obtain an adequate PM,many surgeons insist on a total gastrectomy(TG)rather than a subtotal gastrectomy(SG)for GCs located in the middle-third of the stomach(17);however,surgeons may perform SG according to their own clinical experience and subjective judgment,taking into the account the results of intraoperative frozen-section examination that were established in order to achieve R0 resection.To determine the optimal surgical procedure for middle-third GC,TG or SG,previous studies have suggested that SG can be safely performed,resulting in a better nutritional status and quality of life(18-20),and both procedures have demonstrated similar long-term survival(20-23).Currently,some studies even suggest that the achievement of clear margins is sufficient for patients.All of these arguments result in inconsistencies of margin of resection in gastrectomy(24-26).In short,there are no established guidelines for the length of the resection margin.
This study provides a good reference for surgeons during gastrectomy,on a statistical basis,concerning decisions about the PM distance.In an analysis of the entire cohort,patients with a PM of 2.1−5.0 cm demonstrated significantly greater recurrence and long-term survival rates than patients with PM≤2.0 cm,while those with a PM>5.0 cm did not display significantly extended survival.Significant differences in recurrence and the long-term survival rate were found between patients with TNM stage I and II with a resection margin distance of less than or greater than 2.0 cm;however,the PM distance showed no significant correlation with the recurrence rate or the longterm survival rate in TNM stage III patients if the resection margin was negative.Significant differences were noted with a PM of 2.1−5.0 cmvs.PM≤2.0 cm in the survival curve,especially in the subgroups of T stage(T1−2,T3−4),N0 stage,differentiated type disease and tumor size≤5.0 cm.The remainder of the subgroups showed that a larger PM was not associated with additional survival benefits,as long as the surgeon ensured an R0 resection margin.
Our results are similar to those of Squireset al.,who proposed that a PM>3.0 cm was associated with improved survival in patients with TNM stage I disease,while the PM distance was not associated with the recurrence rate or the long-term survival rate in advanced GC(7).There was a little information in this study to support the role of PM length in gastrectomy because the PM distance was not related to survival in cases of more advanced TNM stage III.Several studies have reported that patients with a more aggressive stage may not necessarily benefit from a negative margin,as there is no significant difference in recurrence and survival between patients with R0 and R1 resection(26-29).We can deduce that a more advanced stage of GC is more than a local disease and that the tumor stage of patients is more strongly correlated with the prognosis than the margin of excision.In other words,more advancedstage tumors indicate the presence of systemic disease.
The intra-operative PM distance status should be determined by tumor characteristics,such as the subgroups of TNM stage,T stage,N stage,tumor size and histology type(26-29).There is no doubt that tumor size also affects the resection margin in SG,and to achieve negative margin status,tumor size should be considered as an important indicator for evaluation of the prognosis of GC(30).From the analysis of subgroups performed in the current study,it may be more meaningful to obtain a sufficient PM distance in early-stage disease,whereas PM distance was not associated with long-term survival in later-and aggressivestaged tumors.This result suggests that postoperative survival of such tumors may be associated with the particular biological characteristics of the tumor,rather than the surgical operation and margin of excision.Consequently,the surgeon should select patients based on the stage of the tumor and its biological characteristics,and then they can try their best to minimize the margin of excision as much as possible on the premise of ensuring an appropriate safety margin of excision,with the primary objective of improving the patient’s quality of life and postoperative nutritional status.Especially for some cases,SG rather than TG is perhaps the best option for tumors located in the middle-third of the stomach.In addition,intraoperative frozen sections should become a necessity when it is difficult to determine the status of the resection margin.
In this context,we created a simple but comprehensive survival prediction model for individual patients with different preoperative variables after radical DG.PM length was identified as an independent prognostic factor in the current study.However,other preoperative factors such as age,sex,CEA,CA199,CA724,cT,cN and biopsy histology type could be used for predicting individualized survival.Nomograms have been developed to quantify risk by combining prognostic factors in different diseases(31-35),which support the clinical reference value of this study.Preoperative examination data can be acquired using gastroscopy,endoscopic ultrasonography and computed tomography.Preoperative accurate diagnosis of the T and N stages and the collection of other preoperative clinical data in GC are important to permit tailored therapy to the extent of the excision margin during gastrectomy.Patients with GC should routinely have a set of serum tumor markers evaluated preoperatively,including CEA,CA199 and CA724,because elevated serum tumor markers are generally associated with recurrence and poor long-term survival(36,37).Among the three markers examined in this study,preoperative elevated CA199 and CEA were independent risk factors for reduced patient survival in a multivariate analysis when co-analyzed with CA724.
A recent study showed that for most colorectal cancer patients,“distant metastasis”originates from primary tumors,independent of any lymph node metastasis(38).A study led by Massachusetts General Hospital(MGH)investigators found that the traditional mode of spread of cancer cells—from the primary tumor,through nearby lymph nodes to other organs—may not apply in all cases(39).Thus,combined with the results regarding the appropriate margin of excision,I imagine that in some specific stages of GC,it may be possible to only perform local resection without(or with only limited)lymph node dissection in the future.
The conclusions of this analysis are limited by the singleinstitution and retrospective design of the study.The nomogram validation consisted of discrimination and calibration,but it lacked an additional external validation group.One key limitation of this analysis is that the number of patients with PM≤2.0 cm was relatively small,which weakened the conclusion of the study.Margin distance was measured intraoperative by surgeon within half an hour once after the stomach cancer specimen removed,which is maximally reflect the intraoperative measurement precisely.However,the decreased measurement of the margin length of the resected specimen fromin vivotissue should be taken into account(40-42).Therefore,the actual length of the PM distance is slightly longer than that reported in the results of this analysis.
Conclusions
According to the results of subgroup analysis,different types of patients require removal of individualized margin lengths.It is necessary to obtain not less than 2.0 cm of PM distance in early-stage disease,while PM distance was not associated with long-term survival in later and more aggressive stages of disease because more advanced GC is a systemic disease.We developed and validated a nomogram predicting 1-year,3-year and 5-year DFS and OS after radical DG for GC including PM length.We expect that our analysis of this cohort and our prediction model will provide a valuable reference tool for assessing prognosis in GC patients and may complement the existing clinical practice guidelines.
Acknowledgements
This study was supported by Grant of Wu Jieping Medical Funding(No.320.2710.1819).
Footnote
Conflicts of Interest:The authors have no conflicts of interest to declare.
 Chinese Journal of Cancer Research2020年2期
Chinese Journal of Cancer Research2020年2期
- Chinese Journal of Cancer Research的其它文章
- Cancer burden and trends in China:A review and comparison with Japan and South Korea
- The Bethesda System for Reporting Thyroid Cytopathology(TBSRTC):A report of 2,781 cases in a Chinese population
- Efficacy of platinum in advanced triple-negative breast cancer with germline BRCA mutation determined by next generation sequencing
- Clinicopathological analysis of early-stage breast cancer patients that meet indications for BRCA1/2 genetic testing
- Evaluation of human epidermal growth factor receptor 2 status of breast cancer using preoperative multidetector computed tomography with deep learning and handcrafted radiomics features
- Analysis and external validation of a nomogram to predict peritoneal dissemination in gastric cancer
