Clinicopathological analysis of early-stage breast cancer patients that meet indications for BRCA1/2 genetic testing
Hongyu Xiang,Ling Xin,Qian Liu,Hong Zhang,Shuang Zhang,Jingming Ye,Yuanjia Cheng,Ting Li,Yinhua Liu,Ling Xu
1Breast Disease Center,Peking University First Hospital,Beijing 100034,China;2Department of Pathology,Peking University First Hospital,Beijing 100034,China
Abstract Objective:To investigate the clinicopathological characteristics and prognostic factors of early-stage breast cancer patients with indications for breast cancer susceptibility genes 1/2(BRCA1/2)genetic testing in China.Methods:Based on the indication criteria for BRCA genetic testing specified in the National Comprehensive Cancer Network(NCCN)clinical practice guidelines in oncology,genetic/familial high-risk assessment:Breast and ovarian(Version 2.2019),a retrospective analysis was performed on patients with early-stage invasive breast cancer treated at Breast Disease Center,Peking University First Hospital between January 2008 and December 2016.Clinicopathological characteristics of all patients were analyzed,and prognoses were calculated using the Kaplan-Meier method and a Cox proportionate hazards model.Results:A total of 906 early-stage breast cancer patients who had indications for BRCA genetic testing and had complete clinicopathological data and follow-up information were included in the study group,accounting for 34.7% of all breast cancer patients treated in Breast Disease Center,Peking University First Hospital during the study period.Compared with breast cancer patients without indications for BRCA genetic testing,the overall survival(OS)and disease-free survival(DFS)of patients with indications were not significantly different.In the study group,patients with premenopausal status,high T stage,lymph node positive,estrogen receptor(ER)negative,Ki-67>20% and presence of a vascular tumor thrombus had worse prognosis.There were more family histories of gastrointestinal cancer in patients with related indications than in patients without such indications.Conclusions:Single-center data showed that more than 30% of patients with early-stage breast cancer had indications for BRCA genetic testing.There was no prognostic difference in patients with or without indications for BRCA genetic testing.Premenopausal status,high T stage,lymph node positive,ER negative,Ki-67>20%,and presence of a vascular tumor thrombus were associated with poor prognosis.
Keywords:Early-stage breast cancer;BRCA genetic testing;clinical pathology;prognosis
Introduction
The breast cancer susceptibility(BRCA)genes comprise an important group of tumor suppressor genes(1,2).GermlineBRCAmutations have been confirmed to be closely related to familial breast cancer and ovarian cancer(3,4).Studies have shown that the cumulative risk of breast cancer before the age of 70 in women withBRCA1andBRCA2mutations is 57%−60% and 49%−55%,respectively(5).The clinicopathological features and prognosis of patients withBRCAmutation-related breast cancer have received extensive attention in clinics.BRCAgenetic testing for patients with related indications in China has just started being implemented.To promote the standardized process of clinical detection,this study,referring to the indication criteria forBRCAgenetic testing specified in the National Comprehensive Cancer Network(NCCN)clinical practice guidelines in oncology,genetic/familial high-risk assessment:Breast and ovarian(Version 2.2019)(6),retrospectively analyzed patients with early-stage invasive breast cancer treated at the Breast Disease Center,Peking University First Hospital from January 1,2008 to December 31,2016.This study focused on investigating the clinicopathological features and prognosis of patients with indications forBRCAgenetic testing.
Materials and methods
Ethical approval
Ethical approval from the Institutional Ethics Examining Committee of Human Research of Peking University First Hospital(No.2018-19)was obtained prior to patient recruitment.All participants signed a written informed consent form after a full explanation of the study was provided,including the schematic procedures and potential benefits and complications.
Inclusion criteria
The study was performed to investigate early-stage invasive breast cancer patients with complete clinicopathological information and follow-up data from January 1,2008 to December 31,2016,seen in the Breast Disease Center,Peking University First Hospital.According to the indication criteria forBRCAgenetic testing specified in the NCCN clinical practice guidelines in oncology,genetic/familial high-risk assessment:Breast and ovarian(Version 2.2019),patients who had indications forBRCAtesting were included in the study group,while patients without such indications were included in the control group.The inclusion criteria were as follows:1)diagnosed at≤45 years old;2)diagnosed at 46−50 years old with an additional primary breast cancer at any age;3)diagnosed at≤60 years old with triple-negative breast cancer;4)had family history:(a)diagnosed at 46−50 years old,with one or more close relatives with breast cancer or prostate cancer(Gleason score≥7 points);(b)diagnosed at any age,with one or more close relatives with breast cancer(≤50 years old)or ovarian cancer,metastatic prostate cancer,male breast cancer,or pancreatic cancer;(c)diagnosed at any age,with≥2 additional diagnoses of breast cancer in close relatives;and(d)confirmed familialBRCA1/2mutation;5)male breast cancer patients;and 6)complicated with ovarian cancer and/or pancreatic cancer.The diagnosis and treatment of all included patients met the criterion of Chinese guidelines for diagnosis and treatment of breast cancer 2018(English version)(7).
Clinical staging and molecular subtyping
Clinical staging and prognostic staging of all patients were determined referring to the American Joint Committee on Cancer(AJCC)tumor-node-metastasis(TMN)staging for breast cancer(8th edition)(8),while molecular subtyping was confirmed based on the 2011 St.Gallen consensus criteria(9).
Histopathological immunohistochemistry and histological grades and interpretation criteria
Estrogen receptor(ER)/progesterone receptor(PR)status was determined according to the 2010 American Society of Clinical Oncology/American Society of Pathology(ASCO/CAP)recommended breast cancer ER/PR receptor immunohistochemical detection standards(10).Human epidermal growth factor receptor 2(HER2)expression status was determined according to the 2013 ASCO/CAP recommendations for the HER2(11).According to the 2013 St.Gallen consensus recommendations,we defined Ki-67≤20% as low expression and Ki-67>20% as high expression(12).Referring to the Nottingham Combined Histologic Grade grading standard(13),the invasive breast cancer histological grading indicators included tubule formation,nuclear pleomorphism and mitotic activity,which were divided into grades 1,2 and 3.
Follow-up
Follow-up was carried out by outpatient visit,telephone and mail.The follow-up was performed once each year,with a median follow-up time of 60 months.Disease-free survival(DFS)was defined as the time from the completion of radical surgery to the diagnosis of local or contralateral breast cancer recurrence or distant metastasis.Overall survival(OS)was defined as the time from the completion of radical surgery to death from any cause.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics(Version 20.0;IBM Corp.,New York,USA).Groups were compared by Student’st-test for continuous data,the Pearson χ2test and Fisher’s exact test for categorical variables and the Mann-WhitneyUtest for grading variables.The Kaplan-Meier method was used to calculate DFS and OS,and the log-rank test was used for comparisons between groups.Variables found to be significant in the univariate analysis were included in the Cox regression model using the forward method to exclude interfering factors among variables and to identify independent factors that affect survival.The significance level was 0.05.All analyses involved bilateral tests.
Results
General information
From January 1,2008 to December 31,2016,the Breast Disease Center at Peking University First Hospital admitted and treated a total of 2,611 patients with early invasive breast cancer with complete clinicopathological and follow-up data.The patients had a median age of 53(range:21−92)years old,and 2,594 patients were female,and 17 patients were male.
Clinicopathological characteristics
Among them,906 patients had indications forBRCAtesting and were included in the study group,accounting for 34.7% of all patients.Other patients were included in the control group.The clinicopathological features of the patients in the study and control groups were as follows(Table 1).Among patients with indications forBRCAtesting,656(72.4%)patients were equal or older than 45 years old,12(1.3%)patients were between 45−60 years old with secondary primary breast cancer,265(29.2%)patients were equal or younger than 60 years old with triple negative breast cancer,71(7.8%)patients had family history,17(1.9%)patients were male,6(0.7%)patients meanwhile had ovarian and or pancreatic cancer,and 116(12.8%)patients met two or more indications.Among the patients in the study group,17(1.9%)had other tumors,and 68(7.5%)had a family history of other cancers.Among patients in the control group,50(2.9%)had other tumors,and 110(6.5%)had a family history of other tumors(Table 2).
Prognostic analysis
All patients were followed up for 14−106 months(median follow-up duration,60 months).The 5-year OS was 92.3%,and the 5-year DFS was 89.3%.Patients in the study group were followed up for 16−106 months(median follow-up time,61 months);the 5-year OS was 92.6%,and the 5-year DFS was 89.6%.The patients in the control group were followed up for 14−106 months(median follow-up time,60 months);the 5-year OS was 92.3%,and the 5-year DFS was 89.2%.There were no significant differences in OS and DFS between the two groups(OS:P=0.808;DFS:P=0.396)(Table 3,Figure 1).
A total of 217 patients had recurrence and metastasis:82(9.1%)patients in the study group and 135(7.9%)patients in the control group.There was no significant difference in recurrence and metastasis rates between the two groups(P=0.645).Most common metastatic location was bone,accounted for 41.5% and 38.5% in the study group and control group,respectively.Other metastatic locations included liver,lung,lymph node,breast,brain and peritoneum.No statistically difference were observed in metastatic sites.
A total of 103 patients died of breast cancer:42(4.6%)in the study group and 61(3.6%)patients in the control group.There was no significant difference in breast cancerrelated mortality between the two groups(P=0.301).
Prognostic factors of patients in study group
Clinicopathological characteristics such as age,sex/menstruation status,T stage,N stage,TNM stage,ER,PR and HER2 expression,Ki-67 index,molecular subtype,prognostic stage,pathological type,histological grade,vascular tumor thrombus and neuroinvasion of the patients in the study group were included in a unilateral survival analysis(Table 4).Among them,sex/menstruation status,T stage,lymph node involvement,TNM stage,ER and PR expression,Ki-67 index,molecular subtype,prognostic stage,histological grade,and vascular tumor thrombus had significant effects on DFS and OS(P<0.05).TNM staging,molecular subtype and prognostic staging with confounding factors were excluded,and multivariate analysis was performed on the remaining clinicopathological characteristics that were significant in the univariate analysis.The results showed that high T stage,positive axillary lymph node,negative ER expression,Ki-67>20% and the presence of a vascular tumor thrombus were significantly correlated with a decrease in DFS;premenopausal,T stage 3−4,positive axillary lymph node,negative ER expression,Ki-67>20% and the presence of a vascular tumor thrombus were significantly associated with a reduction in OS(P<0.05)(Table 5).
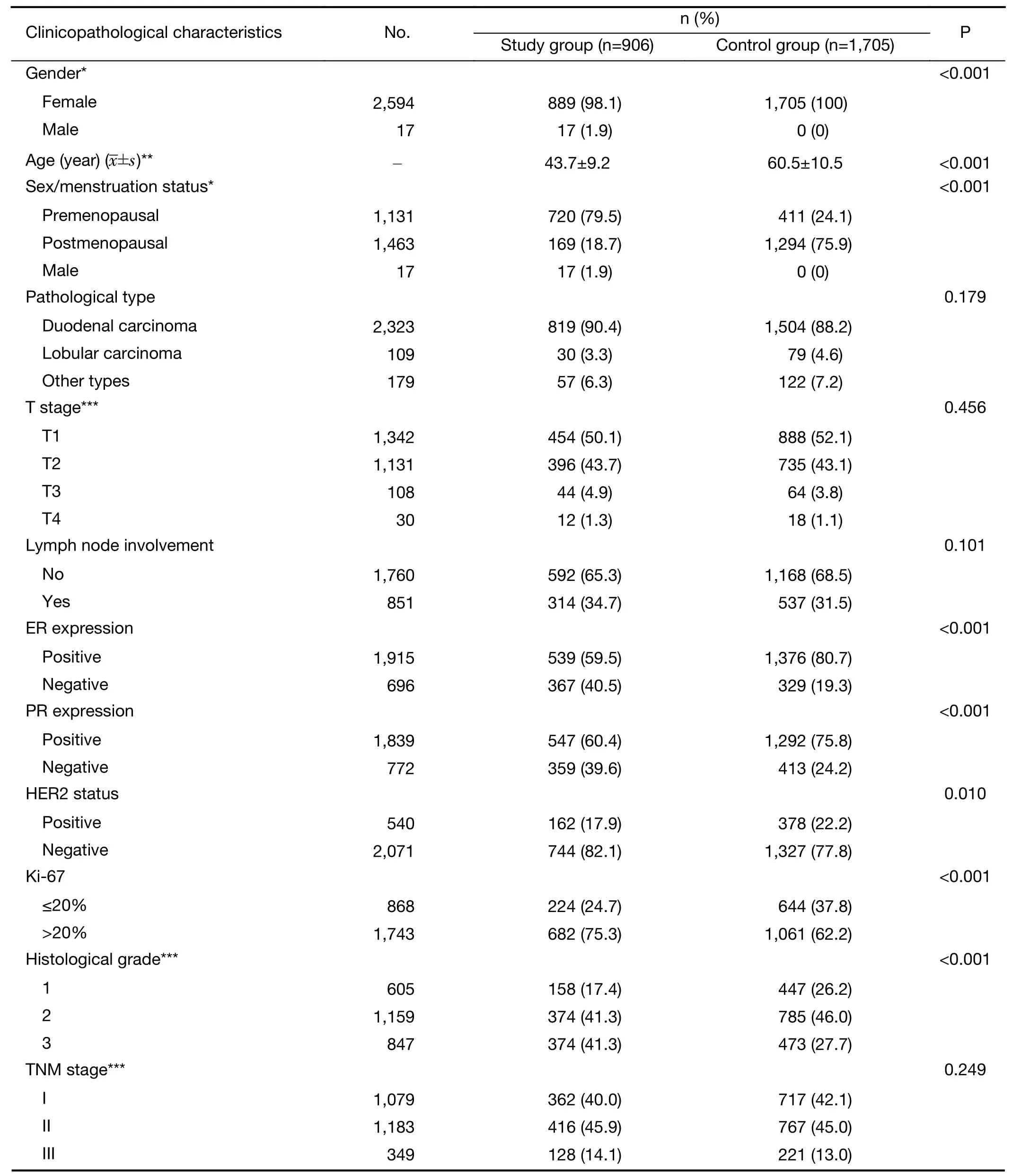
Table 1 Clinicopathological data of patients in study group and control group(N=2,611)
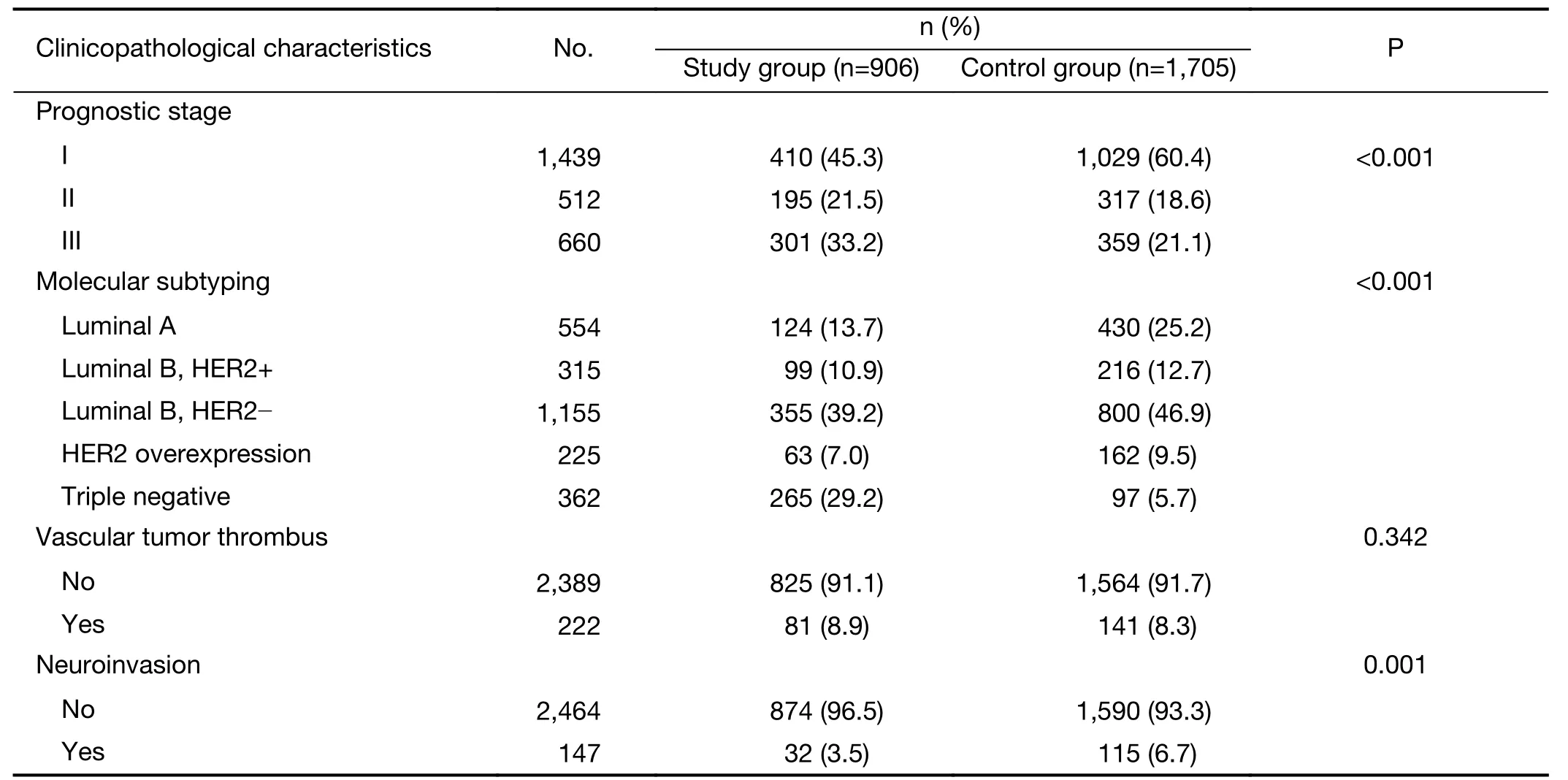
Table 1 (continued)
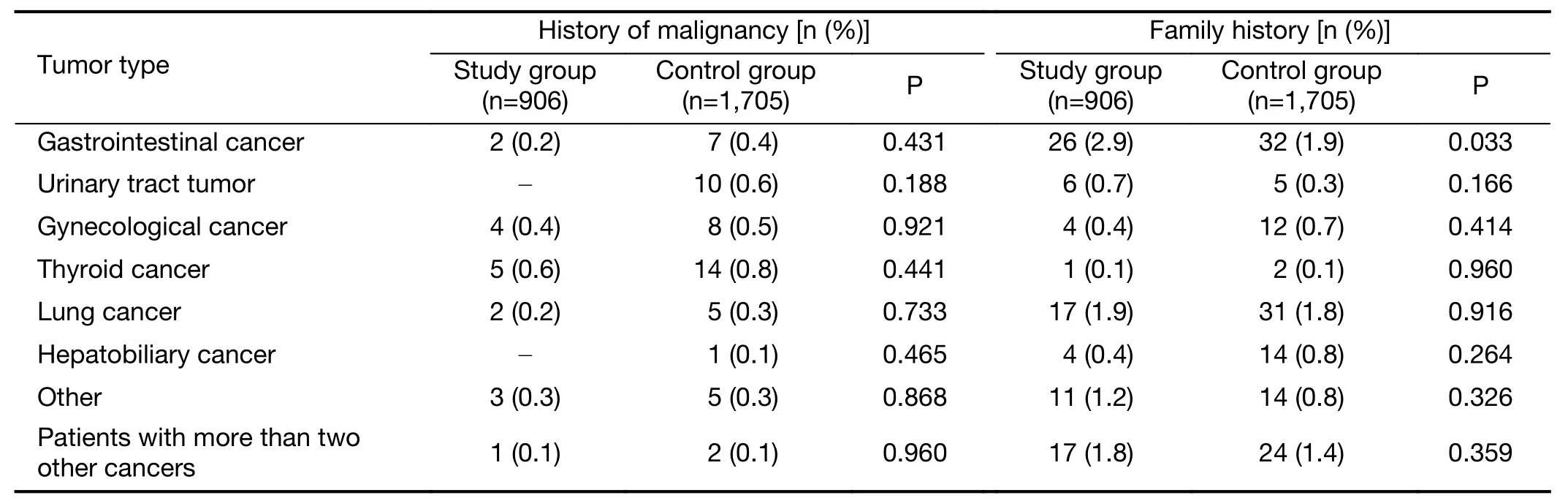
Table 2 Patients with medical history and family history of cancer in study group and control group(N=2,611)
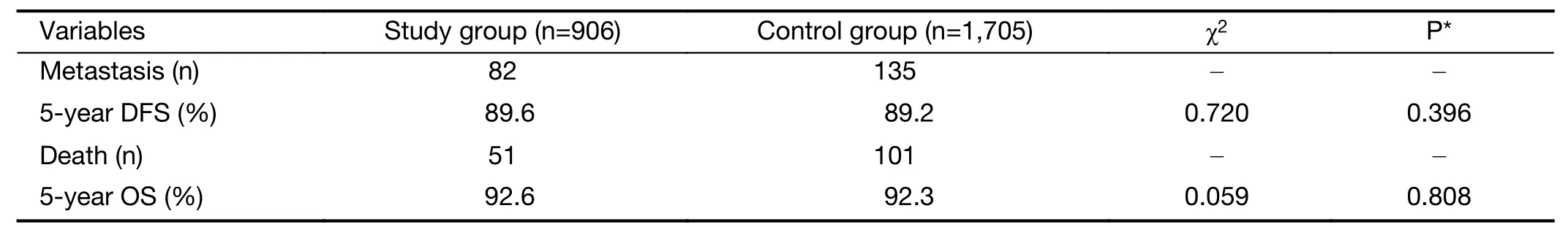
Table 3 Prognostic analysis of study group and control group
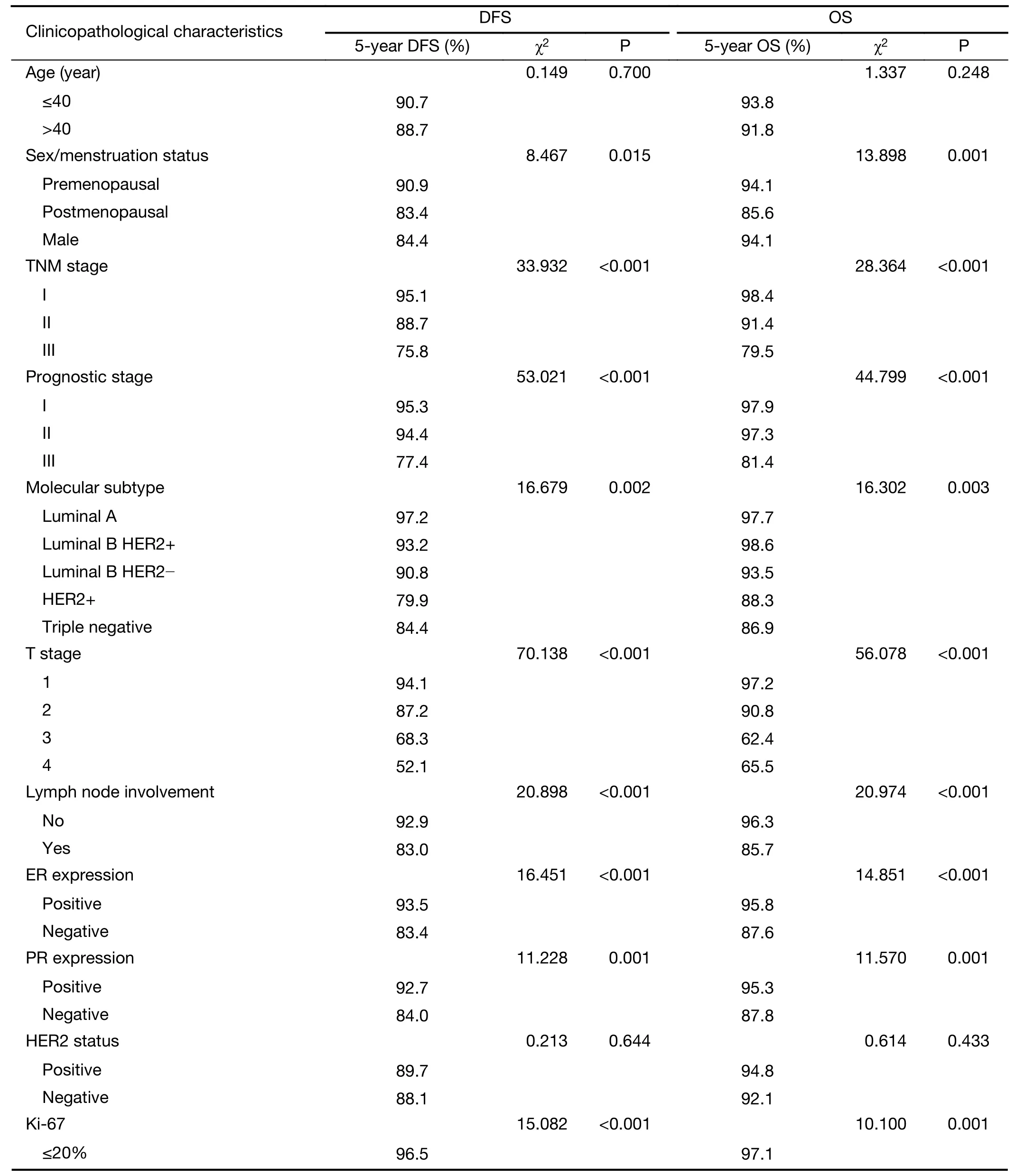
Table 4 Univariate analysis of clinicopathological characteristics and prognosis of patients in study group(n=906)
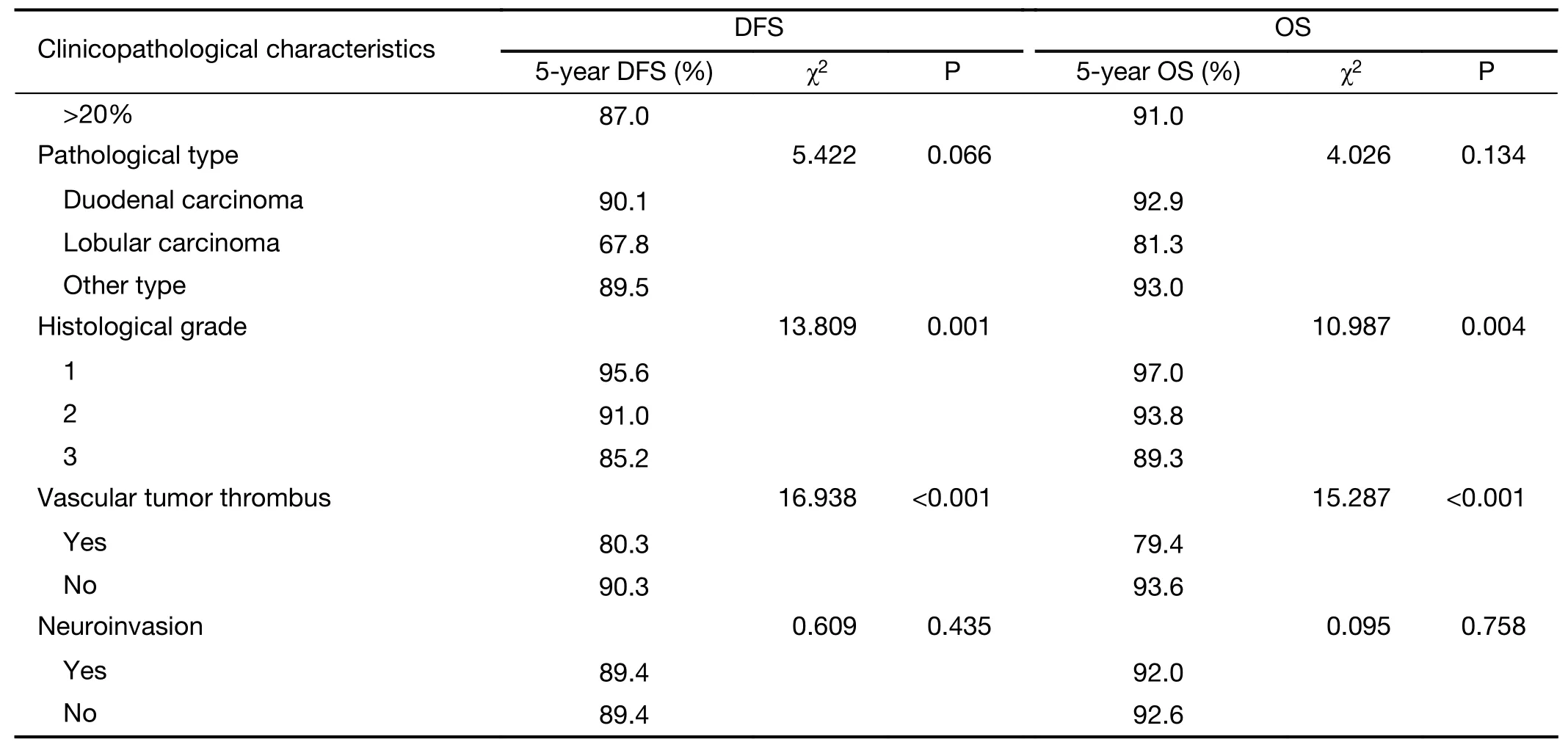
Table 4 (continued)
Discussion
Breast cancer is one of the most common malignant tumor in women.According to the data from National Central Cancer Registry of China,in 2014 approximately 278,900 breast cancer were diagnosed,ranking the first place of all cancer in Chinese women.The mortality of breast cancer in Chinese women was 9.90/100,000,which placed fifth in cancer mortality(14).Approximately 5%−10% of breast cancer is monogenic in origin,andBRCA1/2is the main gene related to hereditary breast cancer(15).Data in China showed that the detection rate ofBRCA1/2mutations in high-risk populations could reach over 10%(16-18).To study the basic situation ofBRCA1/2mutation-related breast cancer in China,this study,referring to NCCN guidelines(2019),performed a retrospective analysis of the data of patients with early-stage invasive breast cancer treated in the Breast Disease Center at Peking University First Hospital between January 2008 and December 2016.In this study,a total of 906 patients had indications forBRCAgenetic testing,accounting for 34.7% of all patients.This finding suggested that approximately 1/3 of breast cancer patients should receiveBRCA1/2testing.However,due to the lack of qualified institutions and authoritative test reports forBRCAtesting in China,none of the patients in this study received testing.Such a lack of testing indicates that a considerable number of patients withBRCAmutation-related breast cancer do not receive reasonable diagnosis and treatment in China.Therefore,the establishment of standardized laboratories to promote and regulateBRCA1/2testing for breast cancer patients in China should receive sufficient attention.
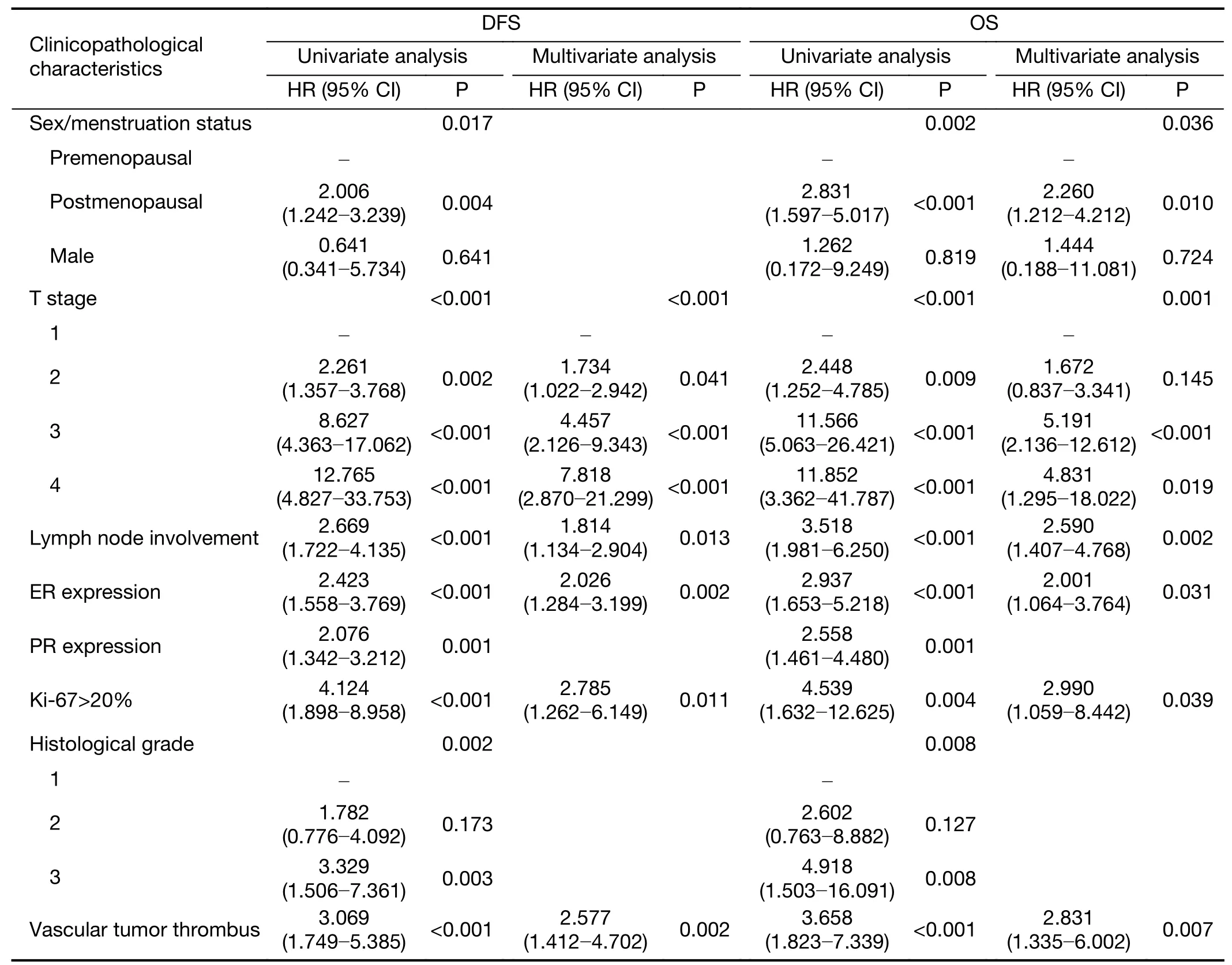
Table 5 Multivariate analysis of clinicopathological characteristics and prognosis of patients in study group
NCCN guidelines(2019)specify that,in addition to a family history of cancer(especially breast cancer,ovarian cancer and metastatic prostate cancer),age,sex,second primary breast cancer and triple-negative breast cancer are also indications forBRCAgenetic testing.In this study,the study group was selected according to the specified indications.Compared with the control group,patients in the study group were predominantly premenopausal,and the cancer exhibited poor differentiation and high prognostic staging,which was consistent with previous reports(19-21).This study also analyzed the family history of other tumors in the two groups and found that patients in the study group often had family histories of various cancers,for which a family history of gastrointestinal cancers was more prevalent than that in the control group(P=0.033).Similar findings have also been reported in other studies in China(22).Based on the above clinicopathological features,it is generally believed that the prognosis ofBRCA1/2mutation-related breast cancer patients is worse than that of patients with sporadic breast cancer.However,there is no evidence that the long-term survival ofBRCA1/2mutation breast cancer patients is worse(23).In this study,the recurrence rate and breast cancer-related mortality of the patients in the study group and the control group were not significantly different(recurrence and metastasis rate,P=0.645;breast cancerrelated mortality rate,P=0.301).In addition,there was no significant difference in OS and DFS between the two groups(OS:P=0.808;DFS:P=0.396),which is consistent with findings in other reports.
Due to the late start ofBRCAtesting in China,none of the patients in this study receivedBRCA1/2testing.Therefore,univariate and multivariate survival analyses were performed only for the prognosis of breast cancer patients with indications forBRCAtesting.Univariate analysis showed that menstrual status,T stage,positive axillary lymph node,TNM stage,ER expression,PR expression,Ki-67 index,molecular subtype,prognostic stage,histological grade,and vascular tumor thrombus were closely related to OS and DFS.After variables with confounding factors excluded,multivariate survival analysis was performed on variables of significance in univariate analyses and found that premenopausal status,T stage 3−4,positive axillary lymph node,negative ER expression,Ki-67>20% and vascular tumor thrombus were associated with a poor prognosis.These results suggested that patients with indications forBRCAgene testing,especially those who are also premenopausal or have high T stage,positive axillary lymph node,negative ER expression,Ki-67>20% and vascular tumor thrombus,should be selected for appropriate treatment.Further studies on whetherBRCA1/2gene testing indicated population with high risk of recurrence benefit from intensive treatment are expected.
The significance of cancer staging is to assess prognostic information and provide a scientific basis for the development of cancer treatment decisions.The AJCC Cancer Staging Manual,8th Edition(Breast Cancer chapter),first proposed the concept of prognosis staging.By adding biomarkers such as histologic grade and HER2,ER and PR expression as the basis for the evaluation of prognostic stage,the 8th edition significantly improved the tumor evaluation system.Our previous study found that the prognostic stage was inconsistent with anatomic staging in over 40% of breast cancer patients,wherein histological grade 2 or above and triple-negative type are important factors for a worse prognostic stage(24).In this study,there was no significant difference in TNM staging between the study group and the control group.However,the prognostic stage of the study group was worse than that of the control group(P<0.001).These results suggested that the population with indications forBRCAtesting had a high risk,and the prognostic stage may be a better reference for making clinical treatment decisions for patients with indications forBRCAtesting compared with anatomic stage.
The morbidity and clinicopathological features of breast cancer patients in China and in Western countries are different.The latest GLOBOCAN 2018 data show that the overall age-standardized incidence in East Asia is 39.2/100,000,lower than that in Western countries(25).The literature reports that in the United States,breast cancer patients with an onset age≤40 years only account for 4% while those≤50 years only account for 19% of all patients;luminal A type is the most common type(26).In this study,332(12.7%)patients had an onset age≤40 years,1,075(41.2%)had an onset age≤50 years,and 1,470(over 50%)had luminal B type breast cancer.Such differences in clinicopathological features will inevitably affect the diagnosis,treatment and outcomes of patients in China and in Western countries.
Studies abroad have shown that in addition to familial breast cancer,BRCA1/2mutations are also concentrated on patients with early-onset,triple-negative and second primary breast cancer and in male patients.Studies have shown that theBRCA1/2mutation rate in breast cancer patients with an onset age≤45 years is 12% and that the mutation rate in patients with an onset age>45 years is only 3%(27).The mutation rates ofBRCA1andBRCA2in patients with triple-negative breast cancer are 7%−28%and 1%−17%,respectively(28,29).The ratio ofBRCA2mutations in male breast cancer is 5%−16%(30,31).The risk of developing bilateral breast cancer in patients withBRCA1andBRCA2mutations within 20 years is 40% and 26%,respectively(32).Because the proportions and types ofBRCAmutations are ethnically and geographically specific,mutation proportions in Chinese populations are different from those in European and American populations.Chinese data show that theBRCAmutation rates in patients with familial breast cancer,early-onset breast cancer,triple-negative breast cancer,male breast cancer and bilateral breast cancer are 12.7%−16.9%,5.2%−8.9%,10%−12%,15.2% and 12.5%,respectively;furthermore,theBRCAmutation rate in patients with two or more indications is as high as 16.6%(16-18).The overall trend shows that the rate ofBRCA1/2gene mutations in patients with familial breast cancer is higher than that in patients in foreign countries,whereas those in patients with early-onset breast cancer and triple-negative breast cancer were the opposite.In this study,patients with an onset age≤45 years and triple-negative breast cancer patients≤60 years old were the two largest groups with indications for testing,accounting for more than 90% of the total population with indications.However,the detected mutation rates in these groups of patients were not high.Patients who had two or more indications had higher detected mutation rates but only accounted for 12.8% of the total population with indications.Therefore,we believe that the current NCCA indication guidelines forBRCA1/2genetic testing are not entirely applicable to Chinese patients.Big data of Chinese population are required to improve the indication ofBRCAgene testing in China.
This study has several limitations.First,this was a singlecenter study performed in a first-class hospital,and a selective bias was inevitable.Second,BRCA1/2gene testing developed late in China,so gene detection was not undertaken in this cohort,and the prognosis-related analysis was performed only for patients with indications forBRCA1/2genetic testing.Finally,the follow-up time was relatively short.Therefore,more detailed data with a longer follow-up time on a larger multicenter scale are encouraged.
The clinical diagnosis and treatment ofBRCAgene mutation-associated breast cancer in China has not been standardized.This study aimed to emphasize the necessity of implementingBRCAgenetic testing in clinics.The detectedBRCA1/2mutation rate in breast cancer patients with indications in China only accounted for approximately 10%.Researchers should carry out multicenter prospective studies to investigate standardizing and promotingBRCAgene testing.
Conclusions
Single-center data showed that more than 30% of patients with early-stage breast cancer had indications forBRCAgenetic testing.There was no prognostic difference in patients with or without indications forBRCAgenetic testing.Premenopausal status,high T stage,lymph node positive,ER negative,Ki-67>20% and presence of a vascular tumor thrombus were associated with poor prognosis.
Acknowledgements
None.
Footnote
Conflicts of Interest:The authors have no conflicts of interest to declare.
 Chinese Journal of Cancer Research2020年2期
Chinese Journal of Cancer Research2020年2期
- Chinese Journal of Cancer Research的其它文章
- Cancer burden and trends in China:A review and comparison with Japan and South Korea
- The Bethesda System for Reporting Thyroid Cytopathology(TBSRTC):A report of 2,781 cases in a Chinese population
- Efficacy of platinum in advanced triple-negative breast cancer with germline BRCA mutation determined by next generation sequencing
- Evaluation of human epidermal growth factor receptor 2 status of breast cancer using preoperative multidetector computed tomography with deep learning and handcrafted radiomics features
- Prognostic value and nomograms of proximal margin distance in gastric cancer with radical distal gastrectomy
- Analysis and external validation of a nomogram to predict peritoneal dissemination in gastric cancer
