Advances in Study of Natural Antibacterial Medicines
Ainuan LI, Xiao YANG, Zihao MA, Rui ZENG
College of Pharmacy, Southwest Minzu University, Chengdu 610041, China
Abstract This paper introduce the advances in study of antibacterial effects of natural medicines in the past ten years. The antibacterial components of natural medicines mainly include flavonoids, terpenoids, alkaloids, lignans, phenols, etc.. This paper is intended to provide data reference for the research and development of new natural antibacterial medicines.
Key words Natural antibacterial drug, Flavonoid, Terpenoid, Alkaloid, Lignans, Phenol
1 Introduction
Natural antibacterial medicines refer to effective active components derived from animals and plants. They have many types and complex structures. Antibacterial active components mainly include flavonoids, terpenoids , alkaloids, lignans, phenols,etc.. In recent years, antibiotics have been widely used, and drug-resistant bacteria have increased. The rate of discovery of new antibacterial medicines is slower than the rate of drug resistance. The development of new antibacterial medicines has become a global issue[1], while the development of lead compounds for antibacterial agents has attracted the attention of countries in the whole world.
2 Natural antibacterial medicines
2.1 FlavonoidsCrataeguspinnatifida(family Rosaceae, genusCrataegus) is a small deciduous tree, and it has various strong physiological activities such as blood lipid lowering, anti-atherosclerosis, antibacterial, antiviral, antitumor, and immune regulation. A new compound extracted fromC.pinnatifidathrough 70% ethanol: 2′-hydroxy-7-(3-hydroxypropyl)-6-methoxy-flavonoid (I). The biological activity test showed that the new compound had a MIC90value of (34.8±3) μg/mL against methicillin-resistantStaphylococcusaureus(MRSA) strains, and had a certain antibacterial activity[2]. Based on fingerprints, Zhang Jinjieetal.[3]analyzed antibacterial activity of 8 flavonoids of wild chrysanthemum: luteolin-7-O-β-glucoside (II), apigenin-7-O-β-glucoside (III), and locustin. -7-O-β-glucoside (IV), buddleoside (V), geraniol-7-O-β-D-glucoside (VI), luteolin (VII), kaempferol (VIII), tricin (IX), the results showed that the total flavones of wild chrysanthemum had good antibacterial activity againstCandidalusitaniae. Okoth DAetal.[4]extracted two new flavonoids: dihydrolanneaflavonol (XI) and lanneaflavonol (XII) fromLanneaalata(Engl.) Engl., they determined the antibacterial activity through disc diffusion experiment. The experimental results showed that dihydrolanneaflavonol and lanneaflavonol had inhibitory effect onPseudomonasaeruginosa(ATCC27853),Staphylococcusaureus(ATCC29213), andStaphylococcussciuri(29062). Xiao Lanqingetal.[5]isolated and extracted 3 flavonoids with clear activity fromScutellariabarbata. D. The isolated components had activity of resistingHelicobacterpylori, and the minimum inhibitory concentration (MIC): kaempferol-3-O-rhamnoside (XII) (15.63 μg/mL), kaempferol-3-O-glucoside (XIII) (15.63 μg/mL), kaempferol-3- 7-Dirhamnoside (XIV) (31.25 μg/mL). Edziri Hetal.[6]extracted and identified two flavonoids fromRetamaraetamflowers: Licoflavone C (XV) and Derrone (XVI). They used the paper sheet diffusion method to detect the antibacterial activity ofStaphylococcusaureus,E.coli,Enterococcusfaecalis,Pseudomonasaeruginosa,Candidaglabrata,Candidaalbicans,Candidaparapsilosis, andCandidakrusei; the experimental results show that the flavonoids XV and XVI have antibacterial activity against eight kinds of bacteria. The structural formula of compound I-XVI is shown in Fig.1.
2.2 TerpenoidsTerpenoids are metabolites of natural products, and they exist widely in nature. In recent years, the medicinal effects of terpenoids have attracted wider and wider attention. Extensive studies have shown that terpenoids have a variety of biological activities, including cytotoxicity, antifungal and antibacterial activity[7].
2.2.1Sesquiterpenes. Using column chromatography and other methods, AO Wu-Li-Jietal.[8]separated and purified the chloroform layer ofSyringapinnatifolia, and identified the compound structure by spectral analysis and physical and chemical analysis. They isolated and identified two new sesquiterpenoids from the extracts ofS.pinnatifolia, guai-9-en-4β-ol (XVII) and 14,15-dinorguai-1,11-dien-9, 10-dione (XVIII). Besides, they used the disc diffusion method to determine the antibacterial activity, and the experimental results showed that the two new compounds had good activity againstE.coliandS.aureus. Through extraction and purification ofHypneamusciformisby ethyl acetate extraction and column chromatography, and determination of antibacterial activity by plate diffusion method, Li XDetal.[9]separated 8 monoterpenoids, among which the new compound 7-hydroxylaurene acetate (XIX), allolaurinterol acetate (XX) have strong activity againstE.coliandS.aureus, and the remaining sesquiterpenoids such as: 10-bromo-7α, 8α-expoxychamigr-1-en-3-ol (XXI) 10-bromo-β-chamigren-8-ol (XXII), 10-bromo-3-chlorocupar-5-en-2-ol (XXIII), laurene (XXIV), C12-acetogenin, and desepilaurallene (XXV) have weak bacterial activity.
2.2.2Monoterpenoids.PolygonummultijiorumThunb. has anti-aging, lipid-lowering and liver-protecting effects, improving immunity, and treating osteoporosis[10]. Kouam SF[11]separated crude extracts of ground part ofCanthiummultiflorum(Rubiaceae) and obtained 13 compounds, including 6-oxo-genipin (XXVI), macrophy lloside (XXVII), garjasmine (XXVIII), gardenine (XXIX), gardenamide (XXX), galioside (XXXI), deacethyl asperulosidic acid (XXXII), and determined the antibacterial activity of five different strains ofS.aureus,Actinomycete,Serratia,Pseudomonasschneiderii, andE.coliusing agar diffusion method. The results indicate that XXVIII showed significant inhibitory activity against all tested microbial strains, especiallyS.aureus. Some scholars have isolated a new terpene glycoside Hoplanoside A (XXXIII) and known monotezrpene glycoside (XXXIV) fromHostaplantaginea. TakingStreptomycinas a positive control, they had antibacterial activity againstE.coli,S.aureus, andBacilluscereus,Yersiniaenterocolitica[12].

Fig.1 Chemical structural formula of flavonoids
2.2.3Diterpenoids. Some scholars have isolated three or two triterpenoids and three abietane diterpenoids fromEuphorbiapseudocactusBerger, and used the plate diffusion method to determine the antibacterial activity againstS.aureus,B.subtilis,P.aeruginosa,E.coli,MoniliaalbicanandAspergillusflavus, and the results indicate that three diterpenoids 3-hydoxy-19-Cyclopropenoyloxy-abietane (XXXV), 12,19-dihydroxy-abieta-5-ene (XXXVI), ent-abieta-9, 12, 14-triene-12, 16-olide (XXXVII) have moderate antibacterial activity and no activity against fungi and yeast[13]. The structural formula of XVII to XXXVII compounds is shown in Fig.2.

Fig.2 Chemical structural formula of terpenoids
2.3 AlkaloidsThe alkaloids were extracted fromNelumbonuciferaGaerth. Six alkaloids were identified by column chromatography and spectrometry. Six alkaloids were Nuciferin (XXXVIII), 2-hydroxy-1-methoxyapophine (XXIX), Liriodine (XL), lysicamine (XLI), N-Nornuciferine (XLII), and Armepavine (XLIII). The microbiological dilution method was used to determine the antibacterial activity against 10 pathogenic bacteria. TheMICsof XLIII againstE.coli,B.subtilisandS.aureuswere 32 μg/mL, and theMICsof Trichophyton rubrum and gypsum microspores by XXIX were 16 μg/mL[14]. Xu Chanetal.[15]separated seven monomer compounds from methanol extract ofBerberissoulieanaSchneid, among which 5 are alkaloids, isordinine (XLIV), berberine (XLV), jatrorrhizine (XLVI), palmatine (XLVII), berberine (XLVIII), respectively. Their antibacterial activity againstS.aureus,Staphylococcusepidermidis,E.coli,Bacillussubtilis,Klebsiellapneumoniaeand five fungal strainsC.albicans,Candidakrusei,Candidaparapsilosis,Torulopsisglabrata, andCryptococcusneoformans. The results showed that XLV-XLVIII Gram-positive bacteriaS.aureusandS.epidermidisshowed good antibacterial activity, and theMICvalue had strong antibacterial activity in the range of 12.5-50.0 μg/mL. Through extraction with methanol and separation of active metabolites by column chromatography, Deng Yechengetal.[16]isolated stephanine (XLIX) and crebanine (L) from tubers ofStephaniadielsiana, 4 negative bacteria (E.coli,P.aeruginosa,Proteus,Salmonella) withMICvalue of 0.625-7.5 g/L, 5 positive bacteria (Micrococcus,B.cereus,B.megaterium,B.subtilis,S.aureus) withMICvalue of 0.078-0.321 g/L, and all have high antibacterial activity. The structural formula of compounds XXXVII to L is shown in Fig.3.
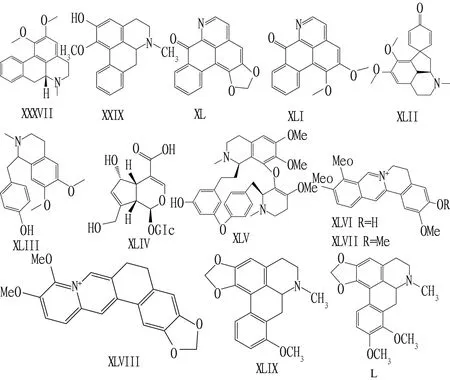
Fig.3 Chemical structural formula of alkaloids
2.4 LignansThe lignan component has anti-cancer, antibacterial, antioxidant, anti-inflammatory and immunosuppressive activities. Li Yinghongetal.[17]isolated and identified n-butanol layer lignans from extract ofCinnamanumcamphoralleaves, (+) pinoresin-β-D-glucoside (LI) and sesamin (LII), which were first isolated fromC.camphoralleaves. The antibacterial activity of lignans fromC.camphoralleaves against four test bacteria was determined by agar diffusion method and double dilution method. The experimental results show that LI and LII have different degrees of inhibition on the four common test bacteria in food, and the inhibition of bacteria is better than that of fungi. Wang Xiaowenetal.[18]extractedGynostemmapentaphyllum(Thunb.) Makino with 95% ethanol to obtain a crude extract ofG.pentaphyllum. Through identification after isolation, the three compounds were all lignans. Studies on theinvitroantioxidant effects of LIII, LIV, and LV have shown that compound LIV has a strong scavenging effect on DPPH free radicals, and compound LIII has a weak scavenging ability, while compound LIV does not show a DPPH free radical scavenging effect in the experimental concentration range. Antibacterial research onStreptococcusmutans,S.aureus,SalmonellaandE.colishowed that the compound LIV has a certain inhibitory effect onS.mutans, a strong inhibitory effect onS.aureus, and weak inhibition onE.coliandSalmonella; the compound LV shows only a weak inhibitory effect onS.aureus,E.coli, andSalmonella, and the compound LIV also shows a similar bacteriostatic trend as the compound LVII, but the effect is weaker. The structural formula of compounds LI to LV is shown in Fig.4.
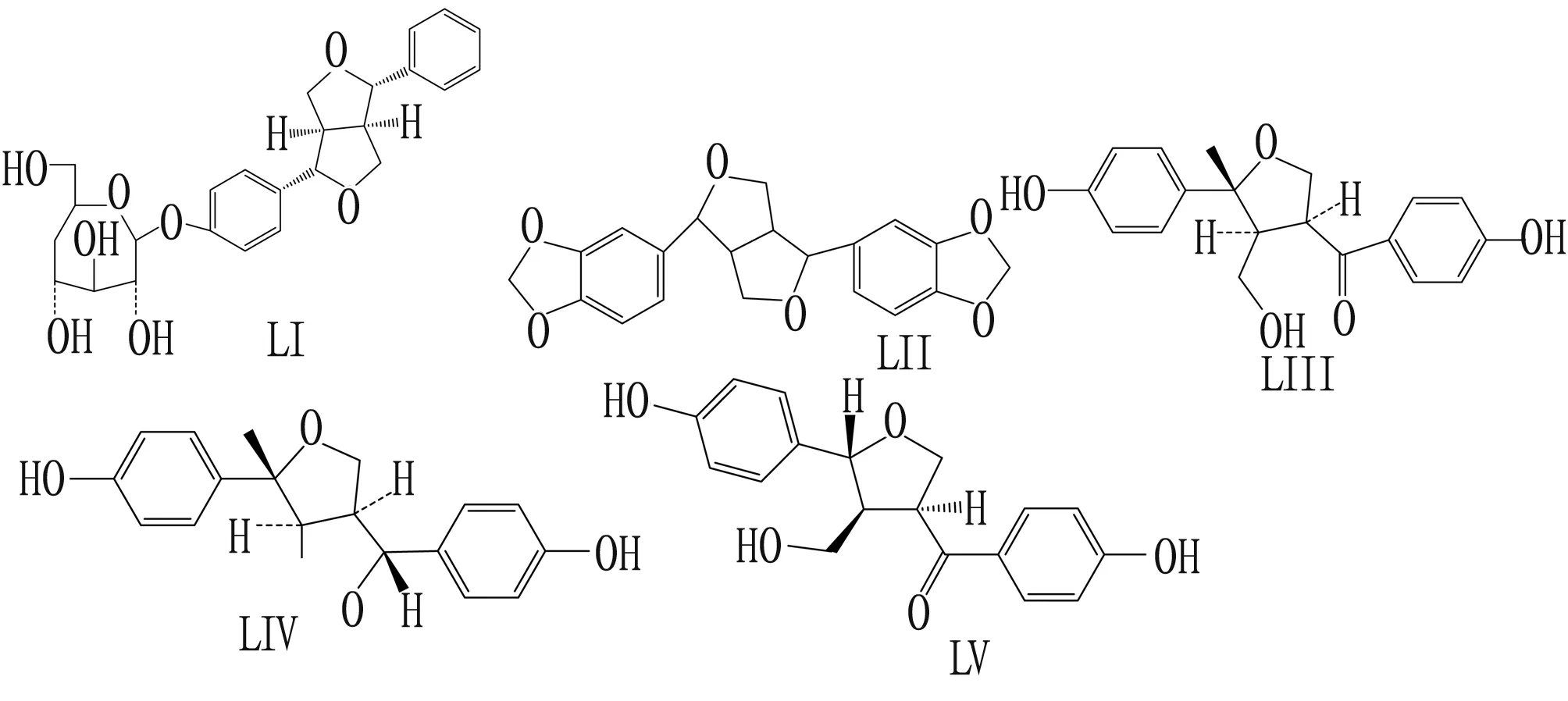
Fig.4 Structural formula of lignans
2.5 PhenolsXanthiumsibiricumPatrinex Widder is a plant of the genus Xanthium. Tao Xin[19]took driedX.sibiricumextract with ethyl acetate by ethanol percolation method, and separated by macroporous adsorption resin column chromatography, and used NMR, MS and other spectroscopic methods to identify the structure of the compound, and isolated 15 phenolic compounds fromX.sibiricum, and seven of them had good inhibitory effects onS.aureus,E.coli,Bacillusparadysenteriae,P.aeruginosa, andShigelladysenteriae. Seven phenol compounds are chlorogenic acid (LVI), 1-O-caffeoylquinic acid (LVII), 4-O-caffeoylquinic acid (LVIII), 5-O-caffeoylquinic acid (LIX), 1 , 4-O-dicaffeoylquinic acid (LX), 1,3-O-dicaffeoylquinic acid (LXI), and quercetin-3-O-β-D-glucoside (LXII). Peng Yushuaietal.[20]identified a phenolic acid compound fromTrolliuschinensisBunge by1H NMR,13C NMR and two-dimensional spectrum, and identified the compound as glucosinolate (LXIII), studied antibacterial activity againstS.aureus,P.aeruginosa,Proteus, andK.pneumoniaeby the broth microdilution method, and measured the minimum inhibitory concentration (MIC) of drugs to these four kinds of bacteria. TheMICvalues of LXIV againstS.aureusandP.aeruginosawere 256 and 128 μg/mL, respectively. Wu Yanpingetal.[21]isolated a new phenolic compound 3-p-trans-coumaroyl-2-hydroxyquinic acid (LXVI) fromCedrusdeodara, and they studied the activity of the compound againstE.coli,Salmonella,Vibrioparahaemolyticus,B.cereus,Clostridiumperfringens, andS.aureus. The determination of the minimum inhibitory concentration (MIC) showed that theMICvalue was 2.5-10.0 mg/mL. The structural formula of compounds LVI-LXI is shown in Fig.5.

Fig.5 Structural formula of phenols
2.6 OthersHe Fetal.[22]isolated a new polysaccharide from the fermentation solution ofStreptomycesH03, and determined the antibacterial activity of the compound by the paper diffusion method. The results showed that it was effective againstS.aureus,B.subtilis, andL.monocytogenes,E.coli,C.albicansandCandidahave strong antibacterial activity. Two new anthraquinone dimers isolated from marine fungi have selective antibacterial activity against Gram-positiveS.aureus[23].
3 Conclusions and prospects
At present, drug resistance of antibiotics has become a global issue. Natural medicines contain many antibacterial components. It is able to develop new antibacterial agents through the extraction, separation and structural identification of natural products. Although some progress has been made in the development of antibacterial agents, because the rate of discovery of antibacterial agents is slower than the development of antibiotic resistance, it is necessary to constantly develop new antibacterial agents.
- Medicinal Plant的其它文章
- Application of Chaihu plus Longgu Muli Decoction in Treatment of Physical and Mental Diseases
- Study on Pharmacological Effects of Calycosin in Astragali Radix
- Advances in Research on Treatment of Heart Failure with Yangxinshi Tablet
- Advances in Chemical Constituents and Pharmacological Activity of Pholidota spp.
- Treatment of Arthralgia Syndrome from Zang and Fu
- Effects of Zingber mioga Aqueous Extract on Hepatic Anti-alcoholism in Mice

