Lignans from Seeds of Herpetospermum caudigerum Wall. Protect Against Alcoholic Liver Injury via TGF-β/Smads Pathway in Rats
Qi MA, Jian GU, Puyang GONG, Maojia LI, Ni MA, Yanxi LI
College of Pharmacy, Southwest Minzu University, Chengdu 610041, China
Abstract [Objectives] This study aimed to investigate the effects of lignans from seeds of Herpetospermum caudigerum Wall. (SHC) on expression of TGF-β/Smads in liver tissue of hepatic alcohol-injuried rats, and to explore its protective mechanism. [Methods] A total of 60 SD rats were randomly divided into six groups. The rats in all the groups except those in the normal group were given with white spirit by gavage for 8 weeks to establish alcoholic liver injury models. After rat models were established successfully, they were administered intragastrically with 400, 200 and 100 mg/kg of lignans, respectively. The rats in the normal group were administered intragastrically with 50 mg/kg of silymarin. The administration lasted for 8 weeks, once a day. The changes in the general state, liver tissue pathology and collagen deposition of the rats were observed. The expression of TGF-β, TGF-β1 receptor 1 (TβR-I), TGF-β1 receptor 2 (TβR-II), Smad2/p-Smad2 and Smad3/p-Smad3 in the hepatic tissue was detected. [Results] The expression levels of TGF-β1, Smad2, p-Smad2, and p-Smad3 significantly declined, and the expression levels of TβR-I, TβR-II and Smad3 did not change significantly in the liver tissue of rats in the lignans groups and the expression levels of TGF-β, Smad2, and p-Smad3 significantly declined. Meanwhile, the expression levels of TβR-I, TβR-II, p-Smad2 and Smad3 did not change significantly in the silymarin group. [Conclusions] The lignans from SHC have significant intervention effects on alcoholic livery injury. The mechanism may be related to the inhibition of hepatic stellate cell(HSC) activation, TGF-β secretion and p-Smad2, p-Smad3 expression in the signaling pathway.
Key words SHC, Lignan, Alcoholic liver injury, TGF-β/Smads pathway
1 Introduction
Dried mature seeds ofHerpetospermumcaudigerumWall. (Cucurbitaceae) (SHC) are a commonly used Tibetan medicine, which has been documented in the Drug Standards of the Ministry of Health of the People’s Republic of China: Tibetan Medicine (Vol.1)[1]. SHC are bitter in taste and cold in nature, with effects of clearing interior heat. They are used to treat interior heat (e.g. hepatitis, cholecystitis) and indigestion[2]. SHC have a long history of use, mainly for Tibetan and Mongolian medicine. A lot of researches showed that lignans are the main active ingredients in SHC for protecting liver. Lignans have certain protective effects on liver fibrosis and liver injury.
Alcoholic liver disease (ALD) mainly refers to liver toxicity caused by long-term heavy drinking, such as alcoholic fatty liver, alcoholic liver fibrosis, alcoholic hepatitis and alcoholic cirrhosis[3]. Modern medical treatment methods for ALD include alcohol abstinence and symptomatic treatment, and other therapies have been rarely mentioned. In recent years, traditional Chinese medicine has created more achievements in aspects of extracts of single herbs and clinical prescriptions. According to literature, lignans from SHC have certain protective effect on acute alcoholic liver injury and they can reduce serum indicators, and the protective effect of lignans in SHC on liver morphology and liver functions of rats with acute alcoholic liver injury may be related to down-regulation of TGF-β expression and decline in serum HA and HYP levels. Therefore, the action mechanism of lignans from SHC in the TGF-β/Smads and CTGF signaling pathways was studied in this paper, so as to provide new ideas for the treatment of alcoholic liver injury.
2 Materials and methods
2.1Materials
2.1.1Animal. Healthy adult male SD rats of SPF grade, weighing (200 ± 15.63) g, were provided by Chengdu Dashuo Experimental Animal Research Center[license No.SCXK (Sichuan) 2015-030].
2.1.2Drugs and reagents. Dried mature SHC were identified by Professor Jian Gu from the College of Pharmacy, Southwest Minzu University. After extraction with ethanol, concentration, degreasing with petroleum ether, extraction with ethyl acetate and concentration, total lignans fraction were obtained. The other drugs and reagents used included white spirit (56%vol, Beijing Hongxing Limited by Share Ltd); silymarin (Chengdu Hengli High-tech Zone Weilan Chemical Reagent Business Department); RIPA lysate, 50*ccoktail, PMSF (100 mM), phosphorylated protease inhibitor, BCA protein quantitative detection kit, SDS-PAGE gel preparation kit, ECL, color developing reagent, β-actin, GAPDH, Histone H3, HRP-labeled goat anti-rabbit IgG, HRP-labeled donkey anti-goat IgG, HRP-labeled goat anti-mouse IgG, HRP-labeled goat anti-rat IgG, transfer buffer, electrophoresis buffer and TBS buffer (Wuhan Google Biotechnology Co., Ltd.); protein marker[Therm (Fermentas), USA]; TRIS, glycine, SDS, TWEEN20 (Solarbio, USA); PVDF membrane (0.22 μm, 0.45 μm) (Millipore, USA); BSA (Roche, USA).
2.1.3Instruments. PL-203 electronic balance[Mettler Toledo Instruments (Shanghai) Co., Ltd.]; TGL-16c desktop centrifuge (Shanghai Anting Scientific Instrument Factory); Neofuge 15R refrigerated centrifuge (Heal Force Bio-Meditech Holdings Limited); AJC-0501-P water purifier (Chongqing Aikepu Company); TYXH-II vortexer, (Guangzhou Tianyue Electronics Technology Co., Ltd.); 79-1 magnetic stirrer, (Changzhou Aohua Instrument Co., Ltd.); WD-9405A decolorization shaker, DYY-6C electrophoresis instrument (Beijing Liuyi Instrument Factory); AX-II cassette (Guangdong Yuehua Medical Devices Co., Ltd.); dark room lights (Longkou Shuangying Medical Devices Co., Ltd.); PF-S-200 sealing machine PF (Wenzhou Jiangnan Machinery Factory); BCD-186 refrigerator (Siemens, Japan); V300 scanner (Epson, Japan); alphaEaseFC grayscale analysis software (Alpha Innotech, USA); KZ-II homogenizer (Kangtao Technology Co., Ltd.).
2.2Methods
2.2.1Animal grouping and treatment. After one week of adaptive feeding, the 60 male rats were randomly divided into normal group, model group, silymarin group (50 mg/kg), high-dose lignans group (400 mg/kg), medium-dose lignans group (200 mg/kg) and low-dose lignans group (100 mg/kg) according to body weight. The rats in each administration group were given with corresponding drug, respectively, and those in the normal group and model group were given with equal volume of distilled water. After 0.5 h of administration, the rats in all the groups except those in the normal group were given with white spirit (56%vol) by gavage, and the rats in the normal group were given with distilled water. In the first week, the administration dose was 8 mL/kg; Then, the administration dose was increased by 0.1 mL every week (until increased to 15 mL/kg). The administration of white spirit lasted for 8 weeks to establish rat model of chronic alcoholic liver injury. At the end of the 8th week, all the rats were fasted for 12 h but provided with free access to drinking water and then slaughtered after taking blood from the femoral artery.
2.2.2Sample collection. The rats were anesthetized, and their liver tissues were sampled by laparotomy. The sampled liver tissues were subjected to routine fixation, embedding, sectioning and HE staining.
2.2.3HE and Sirius red staining microscopy of rat liver. The right anterior lobe of each liver tissue was placed in paraformaldehyde solution. After being fixed for 24 h, the sampled liver tissues were transferred into PBS containing 0.02% sodium azide, cut into tissue pieces, marked, and subjected to dehydration, paraffin embedding, dewaxing, sectioning and staining in success. The liver fibrosis semi-quantitative integration system was used to score the degree of liver injury in the rats of each group.
2.2.4Western blotting. The expression of liver tissue indicators of the TGF-β1/Smad signaling pathway, including TGF-β, TGF-β1 Receptor 1 (TβR-I), TGF-β1 receptor 2 (TβR-II), Smad2/p-Smad2 and Smad3/p-Smad3 were detected by Western blotting.
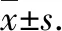
3 Results and analysis
3.1EffectsongeneralstateofratswithchronicalcoholicliverinjurySix rats dead during the experiment, including three from the model group, one from the low-dose lignans group, one from the medium-dose lignans group and one from the high-dose lignans group. The rats in the normal groups were in good condition without death.
3.2HEstainingIn the normal group, the hepatic lobule structure was clear, the hepatic cord was arranged neatly, the hepatocyte cytoplasm was abundant, the morphological structure was normal, the hepatic sinus was not obviously dilated or squeezed, and no obvious inflammation was found. In the model group, hepatic sinusoidal dilatation was seen around the central vein, hepatocyte punctate necrosis was seen around the local central vein, nuclear fragmentation was associated with lymphocyte punctate infiltration, and neutrophil punctate infiltration was seen around the bile duct in the manifold region. In the lignans groups, the hepatic lobule structure was clear, the hepatic sinus was not dilated or squeezed, and a small amount of hepatocyte necrosis was seen locally, accompanied by lymphocyte punctate infiltration. In the control group of silymarin, the hepatic lobule had clear structure and normal morphology, and bile duct hyperplasia was seen in the region with lymphocyte infiltration (Fig.1).
3.3SiriusredstainingThe effects of lignans from SHC on the liver tissue Sirius red staining results of hepatic alcohol-injuried rats are shown in Fig.2. There was no obvious collagen fibrosis in all the groups except the model group, indicating that the modeling was successful and lignans have a certain inhibitory effect on the generation of hepatic collagen fibers. Combined with the HE staining results, compared with the normal group, the model group showed obvious lesions; compared with the model group, the symptoms of the high, medium and low-dose lignans groups were alleviated, and with the increase of lignans dose, the degree of lesions gradually reduced. It indicates that the lignans from SHC have the effect of inhibiting the generation of collagen fibers, and with the increase of the dose, the inhibitory effect is enhanced.
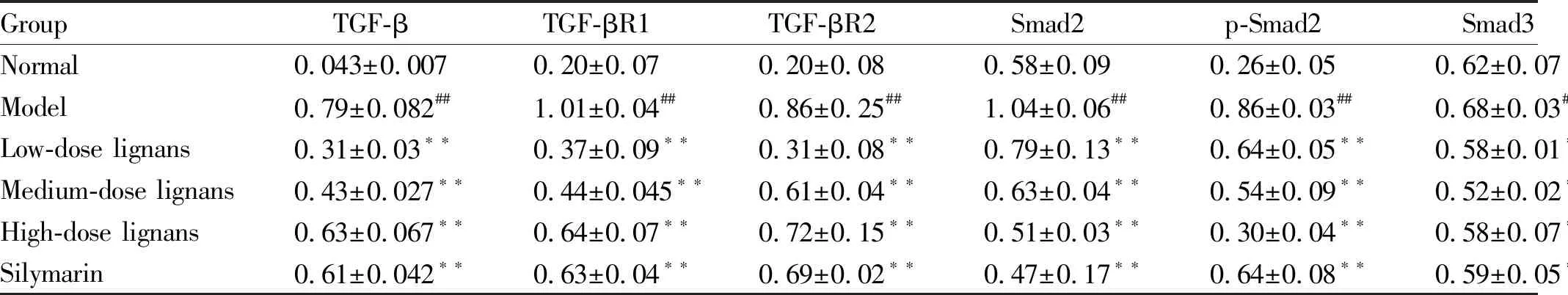
3.4WesternblottingThe detection results show that comped with the normal group, the expression levels of TGF-β, TβR-I, TβR-II, Smad2, p-Smad2, Smad3 and p-Smad3 in the liver tissues of the model group all increased significantly (P<0.05). Compared with the model group, the expression levels of TGF-β, Smad2, p-Smad2 and p-Smad3 declined significantly (P<0.05), and the expression levels of TβR-I, TβR-II and Smad3 did not change significantly (P>0.05) in the high, medium and low-dose lignans groups. In the silymarin group, the expression levels of TGF-β, Smad2, p-Smad2 and p-Smad3 down-regulated significantly (P<0.05), and the expression levels of TβR-I, TβR-II and Smad3 did not change significantly (Table 1).
Fig.1 HE staining of the liver tissues of the rats
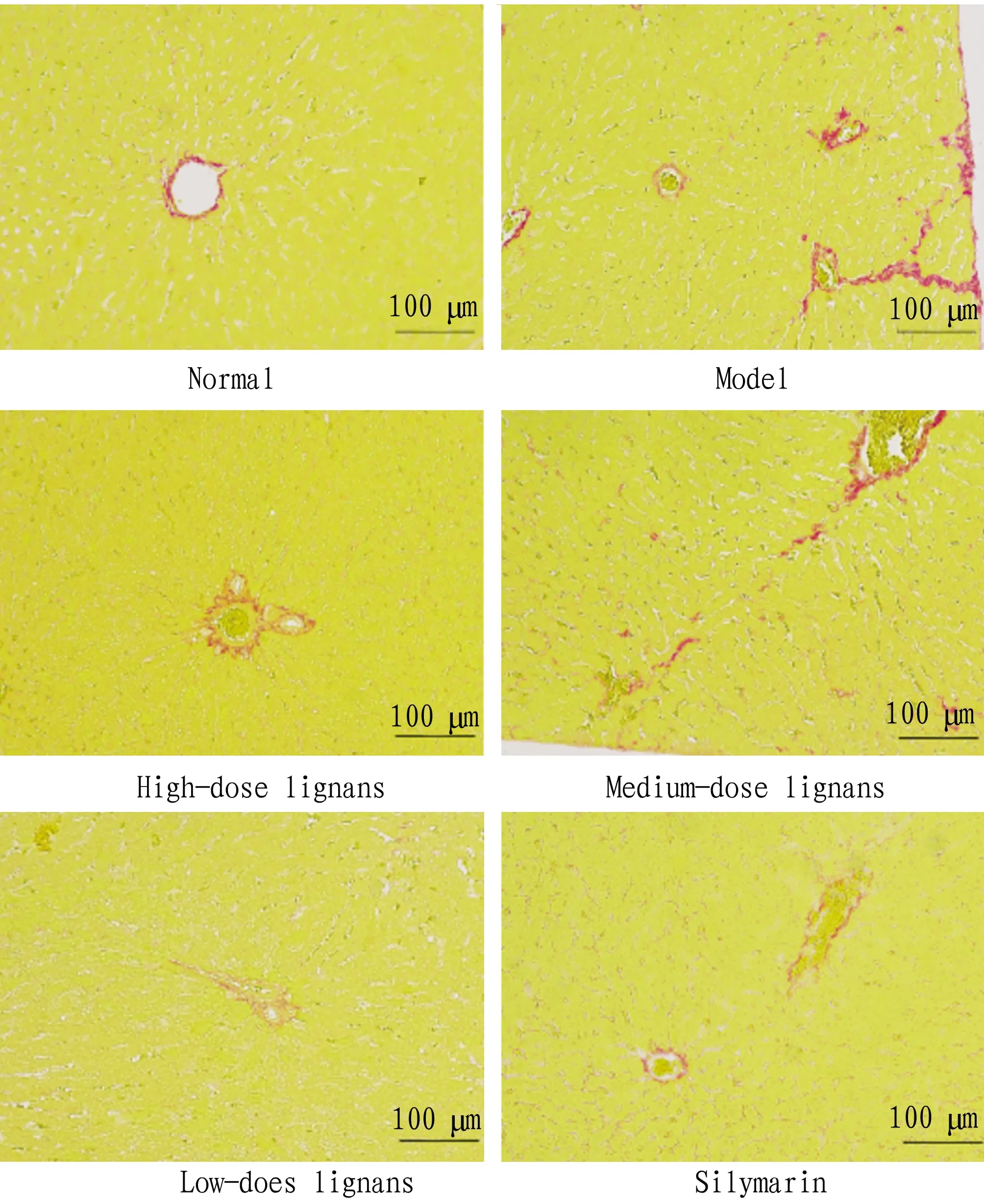
Fig.2 Sirius red Staining of the liver tissues of the rats
Table 1 The ratio of gray value of target band to gray value of internal reference band
GroupTGF-βTGF-βR1TGF-βR2Smad2p-Smad2Smad3p-Smad3Normal0.043±0.007 0.20±0.07 0.20±0.08 0.58±0.09 0.26±0.05 0.62±0.07 0.15±0.05Model0.79±0.082##1.01±0.04##0.86±0.25##1.04±0.06##0.86±0.03##0.68±0.03##0.90±0.01##Low-dose lignans0.31±0.03∗∗0.37±0.09∗∗0.31±0.08∗∗0.79±0.13∗∗0.64±0.05∗∗0.58±0.01∗∗0.65±0.09∗∗Medium-dose lignans0.43±0.027∗∗0.44±0.045∗∗0.61±0.04∗∗0.63±0.04∗∗0.54±0.09∗∗0.52±0.02∗∗0.54±0.04∗∗High-dose lignans0.63±0.067∗∗0.64±0.07∗∗0.72±0.15∗∗0.51±0.03∗∗0.30±0.04∗∗0.58±0.07∗∗0.30±0.02∗∗Silymarin0.61±0.042∗∗0.63±0.04∗∗0.69±0.02∗∗0.47±0.17∗∗0.64±0.08∗∗0.59±0.05∗∗0.29±0.01∗∗
Note: Compared with the normal group,#P<0.05,##P<0.01; compared with the model group,*P<0.05,**P<0.01.
There was no significant difference in the expression of Smad3 in the liver tissues of rats in different groups. The expression of p-Smad3 in the liver tissues of rats in the model group up-regulated compared with that in the normal group (P<0.01). Compared with the model group, the expression of p-Smad3 in the liver tissues of rats in the high, medium and low-dose lignans groups and silymarin group down-regulated significantly (P<0.05,P<0.01) (Fig.3).
4 Discussions
SHC are mainly used in the clinical treatment of liver and gallbladder diseases. Chen Xingetal.[4]studied the protective effect of ethyl acetate fraction of extracts from SHC on acute liver injury (ALI) induced by CCl4 in mice. The results showed that difference doses of ethyl acetate faction of extracts from SHC reduced the serum ALT and AST levels and liver MDA content in animal models to varying degrees, indicating that the ethyl acetate fraction of extracts from SHC have obvious anti-hepatic injury effect.
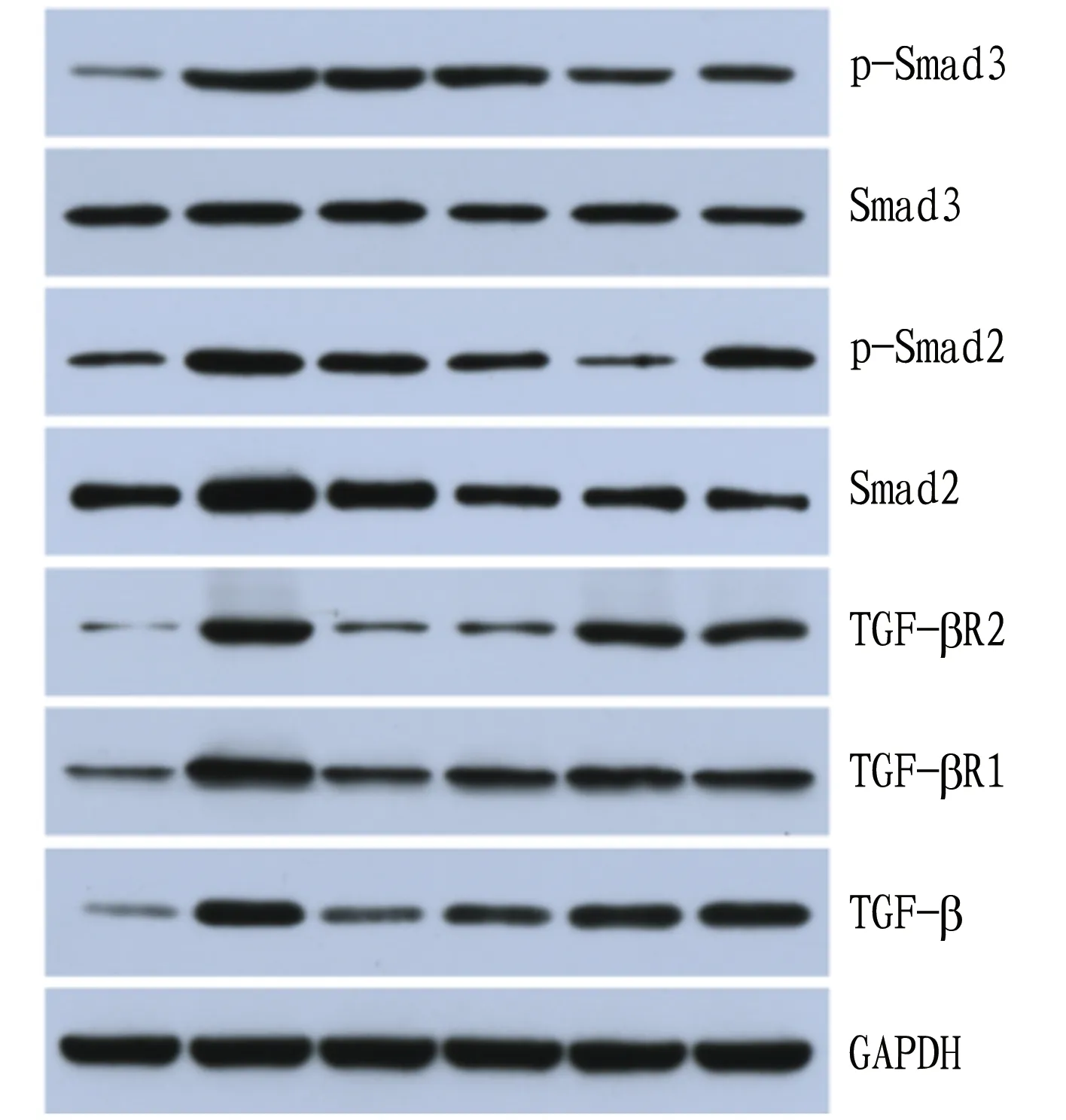
Note: From left to right: normal group, model group, high-dose lignans group, medium-dose lignans group, low-dose lignans group and silymarin group.
Fig.3 Expression of various proteins in the liver tissues of the rats
Lv Junlanetal.[5]studied the protective effect of herpetrione nanosuspension on D-GaIN-induced ALI in mice. The results showed that different doses of herpetrione all had protective effect on D-GaIN-induced ALI in mice. The protective effect of lignans from SHC on CCl4-induced liver injury in mice was achieved by enhancing the activity of SOD and GSH-Px and reducing the amount of MDA in the liver homogenate. Han Yumeietal.[6]studied the herpetrione, extract from SHC on the expression of hepatitis B virus (HBV). The results showed that herpetrione could inhibited HBV, which was achieved by significantly reducing HBV-DNA replication level and inhibiting HBsAg and HBeAg replication.
The results of this study showed that among the indicators of alcoholic liver injury detected, some liver fibrosis indicators improved. Therefore, this article mainly discusses the therapeutic mechanism of lignans on liver injury in rats from the aspect of anti-fibrosis. The expression of TGF-βinvivois mainly related to the TGF-β/Smads signal transduction pathway[7-8]. Smad is a messenger protein in the TGF-β superfamily. TGF-β is the promoter of this pathway. This pathway mediates the TGF-β signal from the cell membrane into the nucleus to play an intermediary function. It activates hepatic stellate cells with cytokines such as platelet-derived factors and reactive oxygen species to make them proliferate and transform into myofibroblasts[9]. As a result, a large amount of ECM is synthesized, secreted, and deposited between liver cells, and eventually liver damage occurs. Smad proteins are divided into three categories by function: receptor-activated or pathway-restricted Smad proteins (R-Smads), Smad sharing the same pathway (Co-Smad) and inhibitory Smad proteins (I-Smads). R-Smads can be activated by type I receptor and form a transient complex with the receptor. They are divided into two categories, namely AR-Smads activated by activin TGF-β, including Smad2 and Smad3, and BR-Smads activated by bone morphogenetic proteins,etc., including Smad1, Smad5, Smad8 and Smad9[10]. According to other related studies, Smads play a key role in transmitting TGF-β signals from cell surface receptors to the nucleus, and different Smads mediate the signal transduction of different members of the TGF-β family. In this study, by administering lignans from SHC to the rats with alcoholic liver injury, the expression of various proteins in the liver tissues of rats showed obvious differences. The expression levels of TGF-β, Smad2 and Smad3 significantly reduced. This was mainly because that the drug could effectively repair damaged liver cells, alleviate the symptoms of liver injury in different stages, mediates the positive effect of TGF-β and slows down the onset of liver injury towards liver fibrosis. Therefore, further elucidating the mechanism of interaction between TGF-β and Smad can bring new opportunities for the treatment of liver injury.
In summary, the lignans from SHC have therapeutic effects on rats with alcoholic liver injury, and the mechanism of action is related to the inhibition of HSC activation, TGF-β secretion and p-Smad2 and p-Smad3 expression in the signaling pathway.
- Medicinal Plant的其它文章
- Application of Chaihu plus Longgu Muli Decoction in Treatment of Physical and Mental Diseases
- Study on Pharmacological Effects of Calycosin in Astragali Radix
- Advances in Research on Treatment of Heart Failure with Yangxinshi Tablet
- Advances in Chemical Constituents and Pharmacological Activity of Pholidota spp.
- Treatment of Arthralgia Syndrome from Zang and Fu
- Effects of Zingber mioga Aqueous Extract on Hepatic Anti-alcoholism in Mice

