Carbon dioxide-angiography for patients with peripheral arterial disease at risk of contrast-induced nephropathy
Amol Gupta,Akinsansoye K Dosekun,Vinod Kumar
Abstract Patients with peripheral arterial disease(PAD)and critical limb ischemia are at risk for limb amputation and require urgent management to restore blood flow.Patients with PAD often have several comorbidities,including chronic kidney disease,diabetes mellitus,and hypertension.Diagnostic and interventional angiography using iodinated contrast agents provides excellent image resolution but can be associated with contrast-induced nephropathy(CIN).The use of carbon dioxide(CO2)as a contrast agent reduces the volume of iodine contrast required for angiography and reduces the incidence of CIN.However,CO2 angiography has been underutilized due to concerns regarding safety and image quality.Modern CO2 delivery systems with advanced digital subtraction angiography techniques and hybrid angiography have improved imaging accuracy and reduced the incidence of CIN.Awareness of the need for optimal imaging conditions,contraindications,and potential complications have improved the safety of CO2 angiography.This review aims to highlight current technological advances in the delivery of CO2 in vascular angiography for patients with PAD and critical limb ischemia,which result in limb preservation while preventing kidney damage.
Key words: Endovascular procedures;Angiography;Digital subtraction;Chronic kidney disease;Peripheral artery disease;Carbon dioxide
INTRODUCTION
Approximately 8.5 million Americans have peripheral arterial disease(PAD)[1],and these patients can have several associated comorbidities,including diabetes mellitus,chronic kidney disease(CKD),and hypertension.Patients with diabetes mellitus are reported to account for the majority of lower limb amputation with a rate of 1.5-5.0 events per 1000 persons per year[2,3].When medication and exercise do not reduce the symptoms of claudication,and in cases of critical limb ischemia(CLI)when the patient is at risk of lower limb amputation,endovascular revascularization is the standard of care[4].
Diagnostic and therapeutic angiography procedures used to salvage the limb have traditionally used contrast agents to identify regions of stenosis in the target artery.Diagnostic angiography routinely uses iodinated contrast material,but for patients with impaired renal function or contrast allergy,alternatives include the use of gadolinium and dilute iodinated contrast material[5].Iodinated contrast agents provide excellent resolution but carry the risk of kidney damage due to immediate effects that damage the nephron,including vasoconstriction,induction of cell apoptosis,and oxidative stress associated with the generation of reactive oxygen species[6-9].Contrast-induced nephropathy(CIN)typically presents within 24-72 h following injection of contrast and can be harmful to patients with pre-existing renal impairment[10].A recently published review has highlighted the associations between PAD and CKD that lead to increased morbidity associated with interventional vascular procedures[11].Therefore,to avoid further kidney damage,there may be a reluctance to perform interventional angiography to salvage the limb in patients with PAD and CKD.
Carbon dioxide(CO2)has been used as a safe diagnostic contrast agent since 1914[12].CO2has been used in angiography since the 1950s and 1960s,and has been used to detect pericardial effusion[13,14].In the 1980s,digital subtraction angiography(DSA)was developed with CO2as a contrast agent for vascular imaging[15].Currently,CO2is used as an alternative to contrast medium in lower extremity vascular diagnosis and intervention,particularly in patients who are at risk from CIN[16].
With advances in gas delivery systems and imaging technology,the use of CO2is rapidly increasing.In the United States,many centers have adopted a combination of CO2with iodine contrast to reduce the total amount of iodine injected[17-20].The use of CO2angiography has significantly reduced the incidence of CIN and is opening the way for patients with CKD to benefit from endovascular procedures[17-20].
This review aims to highlight the current technological advances in the delivery of CO2in vascular angiography,resulting in reduced volumes of iodinated contrast medium,which may improve clinical outcome for patients with PAD and CLI that preserves both renal function and the lower limb.
CONTRAST-INDUCED NEPHROPATHY
CIN is an iatrogenic renal injury that follows the intravascular administration of radio-opaque contrast media and results in the impairment of renal function[6,10,21,22].Meta-analysis data have shown a 6% incidence of CIN following contrast-enhanced computed tomography(CT)[23],and CIN following peripheral angiography has been reported to occur in 9% of patients[24].The administration of contrast medium can immediately affect the nephron by causing vasoconstriction,cell apoptosis,and oxidative stress resulting in impaired renal function[6-9].The level of cytotoxicity of contrast medium may be associated with the ionic strength,osmolarity,or viscosity[6,25].CIN occurs in patients with and without CKD;however,patients with CKD have reduced nephron capacity,which increases their risk of developing CIN[6,22,26].As a result,these patients are at a heightened risk of long-term renal function loss,cardiovascular events(i.e.,myocardial infarction),and death[27].
CIN-associated renal impairment is defined as an increase in serum creatinine by≥25%(relative)or≥0.5 mg/dL(absolute)within three days of injection of contrast medium[28].Serum creatinine levels will peak at 3-5 d after administration of contrast medium but typically return to or near baseline levels within 1-3 wk[10,21].Mortality rates have been reported to rise significantly when creatinine levels increased by more than 25%(P<0.0001)[28].
CONTRAST-INDUCED NEPHROPATHY PATHOGENESIS
The pathomechanisms of renal injury in CIN are poorly understood.Several different contributing factors,such as direct contrast media noxious effects,oxidative stress,vasoconstriction,and medullary ischemia are likely involved[29-31].The physiochemical properties of contrast media,along with vasoconstrictive and cytotoxic effects of contrast media contribute to the pathogenesis of CIN[6-8].Upon administration of contrast media,a transient release of nitric oxide is triggered,which induces endothelium-dependent vasodilation,leading to temporary renovascular dilation and increased renal blood flow within the renal vascular arcades.The contrast media is filtered through the fenestrated endothelium of the glomerulus and enters Bowman’s capsule and the urinary space with exposure to the tubular epithelial cells,where it is re-absorbed.The contrast medium is cytotoxic to the tubular epithelial cells.As a result of a contrast medium osmotic diuresis,there is increased sodium delivery to the thick ascending limb and the macula densa resulting in adenosine is released from the macula densa,and increased tubule-glomerular feedback.Increased tubuleglomerular feedback is one mechanism of increased vasoconstriction and hypoxia.There is also a disturbance of the balance of vasoactive mediators-vasoconstrictive(adenosine,endothelin)vsvasodilatory(nitric oxide,prostacyclin)further resulting in vasoconstriction and hypoxia.The outer medulla is particularly vulnerable to hypoxia while increased sodium delivery to the thick ascending limb increases its metabolic requirement for oxygen further aggravating relative hypoxia in the region.Decreased renal blood flow also causes contrast media to accumulate in the renal medulla.As a result,the renal tubular cells are exposed to contrast media for a prolonged interval time period,resulting in more cellular injury and triggering the release of noxious vasoactive mediators,such as reactive oxygen species,that lead to prolonged vasoconstriction,apoptosis,and oxidative damage[6,32].Previous studies have illustrated contrast media to be trapped for several days,further propagating renal cell damage and death[33].
INCIDENCE OF CONTRAST-INDUCED NEPHROPATHY AND ITS HEALTHCARE BURDEN
CIN is the third most common cause of acquired acute renal failure[22],and the most common cause of acute kidney injury in hospitalized patients[23],In the general population,the prevalence of susceptibility to CIN is >2%[22,24];however,in several patient subsets,the prevalence becomes significantly higher,ranging from between >20% and >30%[22,24].Patients considered to be at increased risk for the development of CIN typically have one or more pre-existing conditions,such as diabetes,congestive heart failure,chronic renal impairment,age≥75 years,hypotension,the presence of an intra-aortic balloon pump,and anemia[6,21,22,34].Pre-existing renal insufficiency or underlying CKD is the most important risk factors for the development of CIN,with the incidence of CIN in patients with CKD ranges from 14.8%-55%[35-37].
Previous studies have indicated decreased renal function and increased dose of contrast media are both risk factors for CIN,and,therefore,have been implicated as a prediction tool for the development CIN.In fact,the ratio of contrast volume to creatinine clearance(V/CrCl)is used as an independent predictor of CIN following angiography in patients with and without impaired renal function[38-42].A recent study consisting of 2308 patients that underwent coronary angiography and/or percutaneous coronary intervention evaluated the predictive power of the V/CrCl ratio.Overall incidence of CIN was 12.2%(281/2308)and a V/CrCl ratio >6.15 was independently associated with an elevated risk of CIN.CIN occurred in 25.1% and 9.7% of patients with V/CrCl≥6.15 and <6.15,respectively.This evaluation parameter was confirmed for diabetic and non-diabetic patients[43].
CIN is independently associated with increased length of hospital stay,increased medical care costs,and unfavorable in-hospital outcome at one year[10].In the United States,more than one million radiocontrast procedures are performed annually,and there are approximately 150000 episodes of CIN per year[44].At least 1% of these cases of CIN require dialysis and are associated with an average stay in hospital of 17 d and an additional annual cost of $32 million,compared with two days for patients that do not require dialysis[44].For patients requiring post-discharge dialysis,the cost has been estimated to be $128000 per quality-adjusted life-year and $51000 for dialysis treatment[45].The resulting annual cost of CIN is approximately $180 million in the United States[10,44].Also,the results from a one-year outcome analysis showed that patients with CIN experienced higher rates of hospital death,out-of-hospital death,and major adverse cardiac events[10,46].
RISK FACTORS FOR CONTRAST-INDUCED NEPHROPATHY
Several factors that increase the risk for the development of CIN are patient-related and can be identified from the clinical history,physical examination,and laboratory tests(Table 1).However,some risk factors are procedure-related and are modifiable.Previous studies have developed clinically useful multivariate predictive tools for assessing the risk of developing CIN and a list of contributing or risk factors have been developed[21,47].The risk factors for CIN include hypotension,diabetes,chronic heart failure,age >75 years,anemia,increased baseline estimated GFR(eGFR),increased red cell distribution width,increased serum triglycerides,creatinine,total cholesterol,high-density lipoprotein,low-density lipoprotein,blood urea nitrogen(BUN),and sodium levels[21,47].Other risk factors include increased platelet count,international normalized ratio,blood glucose,the volume of contrast medium,and the requirement for intra-arterial balloon pump therapy before imaging[21,47].
CLINICAL GUIDELINES FOR THE PREVENTION OF CONTRAST-INDUCED NEPHROPATHY
In 2014,the European Society of Cardiology established preventive guidelines for CIN and provided an outline of care for low,moderate,and high-risk patients[48].All patients undergoing a procedure involving the use of contrast medium should first be assessed for their risk of CIN,which includes the measurement of baseline serum creatinine levels and calculation of the eGFR[48].Patients are considered to be at increased risk of CIN if the serum creatinine level is >1.4 mg/dL and the eGFR is <60 mL/min/1.73m[2,6,48].CIN has been defined as an increase from baseline in the serum creatinine of 0.5 mg/dL or 25% relative increase within 48 h following the administration of contrast medium[47,48].Current recommendations are that patients should be adequately hydrated before the procedure,and patients who are at risk should be given intravenous hydration with isotonic saline before,during,and after the procedure[6,48].Increased hydration works to counteract renal vasoconstriction[30,49,50],prevents renal medullary hypoxia by increasing renal blood flow through the nephrons[49,51,52],dilutes contrast media[29,51,53],and reduces nephrotoxic effects on the tubular epithelium by decreasing how long contrast remains in contract with the kidney[29,51,53].Results from numerous experimental and clinical studies have supported the use of hydration to prevent CIN[54-64];however,no prospective trials have been conducted to determine the minimum effective treatment and optimal fluid composition of IV hydration[65].Patients who have diabetes are advised to discontinue the use of any nephrotoxic medication before the procedure and physicians should use the lowest possible volume of low-osmolar contrast medium(LOCM)or iso-osmolar contrast medium(IOCM)[6,48].Withholding nephrotoxic medications helps to minimize nephron injury by preventing nephritis[53,66]and contrast accumulation in the proximal tubule cells[66];however,these medications should only be discontinued when clinical feasible,which is not the case for all patients[52].LOCM and IOCM are preferred because high-osmolarity elevate the viscosity of the agent and flow resistance in renal tubules[31].LOCM and IOCM have been found to reduce the incidence of CIN by 50%[67];however,they can still cause CIN[68,69].

Table 1 Risk factors for the development of contrast-induced nephropathy
NEW APPROACHES FOR THE PREVENTION OF CONTRASTINDUCED NEPHROPATHY
Additional preventive treatments outlined by the European Society of Cardiology guidelines include the use of N-acetylcysteine(NAC)and high-dose statins[48].Nacetylcysteine has antioxidant and vasodilatory properties,which help to prevent the formation of free radicals in the renal tubules[25],reducing the oxidative stress;however,it does not provide protection against CIN[70].In several clinical studies,high-dose statin therapy has shown to be effective in preventing CIN in statin-naïve patients[6,48,71,72].In 2015,Marenziet al[73]reported the findings from a meta-analysis of the effects of short-term and intensive treatment with statins before imaging on the reduction of CIN in patients with and without acute coronary syndrome undergoing coronary angiography and percutaneous coronary intervention.A systematic review of the literature identified nine clinical trials that included 5212 patients(age 65±5 years),and meta-analysis supported the use of pre-treatment with statins in this patient population[73].Statin therapy is contraindicated for patients with decompensated cirrhosis or acute liver failure[74].
CO2 AS A CONTRAST AGENT
CO2is a negative contrast agent due to its low density and its ability to absorb X-rays to a lesser degree than the surrounding blood vessels and the vessel wall.Gaseous CO2is extremely buoyant,which allows it to float in the blood,and when using selective injection with reflux,the visceral arteries can be visualized[75].Because CO2does not mix with blood,it moves through the blood as an isolated bubble or bubbles and remains undiluted,which is significantly different from iodine contrast.The bubble of CO2has a characteristic parabolic flow pattern along the anterior part of the vessel,while the posterior portion remains filled with blood and free of CO2.As a result,the vessels running anterior to the injection site produce more enhanced images[75].High-quality CO2DSA images of the lower extremity arteries can be obtained by overcoming the buoyancy limitation,which is done by positioning the patient in the Trendelenburg position,administering nitroglycerin by intra-arterial injection,and gas reflux by giving a distal injection[16,75].
High-resolution DSA is required for CO2imaging.However,bowel gas,peristalsis,and movement due to respiration may interfere with DSA negative contrast image quality.These problems can be overcome by the use of rapid exposure with masked images,which eliminate these artefacts.The masked images allow for enhanced subtraction,pixel shifting,and image stacking[75].Several studies have shown that glucagon or hyoscine butylbromide(Buscopan)can reduce the effects of movement of the bowel to improve image quality[19,76-78].
MISCONCEPTIONS OF CO2 ANGIOGRAPHY THAT HAVE BEEN BARRIERS TO ITS USE
The use of CO2as a contrast agent has been documented over the decades but is not routinely used,as the use of iodinated contrast medium remains the gold standard for imaging techniques[48].Several misconceptions have resulted in the limited use of CO2angiography,including the belief that there will be poor image quality,fear of air embolism,lack of available CO2delivery systems or difficulties in their use,fear of explosive gas delivery,insufficient filling of arteries,and also lack of experience in this technique.The development of new technology overcomes some of these limitations and has enabled CO2angiography to become increasingly used.
NEW SYSTEMS FOR CO2 DELIVERY AND IMPROVED SAFETY
Older CO2delivery systems were typically homemade and consisted of large bulky tanks,CO2transport containers,and multiple valves[19,79-81].These older delivery systems caused concern regarding the development of an air embolism,intrapulmonary vapor lock due to air contamination,neurotoxicity resulting from injection of CO2into the cerebral circulation,hypoxia,or hypotension due to the use of an excessive volume of CO2[75].The application of a CO2delivery system has been approved by the United States Food and Drug Administration[82].The delivery system holds 10000 mL of CO2and has valves to prevent mixing of CO2with room air,a 60 mL reservoir syringe,and a 30 mL injection syringe that delivers CO2into the angiography catheter[82].
In our institution,recently developed pre-assembled portable medical CO2delivery systems are currently used and include the CO2mmander®and the AngiAssist®Delivery System(AngioAdvancements Inc.,Fort Myers,FL,United States)(Figure 1)[83].The new portable CO2delivery systems have been designed to remove the limitations and disadvantages of previous equipment and have the advantage of being user-friendly,safe,compact,and easy to move.The use of modified portable CO2delivery systems has overcome the reluctance of use of experienced and nonexperienced operators and may increase the use of CO2as a contrast agent that benefits patients[83].The system consists of three main components that include a compact CO2medical gas cylinder,a portable,hand-held regulator,and a preassembled delivery system.The compact 10000 mL laboratory-grade cylinder is portable and disposable,making it easy to store in a convenient location.Also,there is unique threading that matches the regulator,which prevents the insertion of the wrong gas.Previous systems relied on large,bulky tanks that were never fully depleted of gas,allowing for the accumulation of contaminants,including bacteria,mold,carbonic acid,and rust[83].
The CO2mmander®has a low-pressure regulator that converts medical grade liquid CO2in the cylinder to gas,which is then transferred to the delivery system.The lowpressure system can be regulated from 1-30 pounds-force per square inch,which prevents explosive delivery of the CO2gas[83].All components of the device,including the gauges and valves,are internalized and contained in a protective and durable aluminium shell[83].The AngiAssist®is a pre-assembled delivery system that consists of two one-way valves,a reservoir syringe,a delivery syringe,a K-valve,and afferent and efferent tubing[83].The design of each component prevents inappropriate connections and the direct transfer of CO2from the regulator to the patient[83].The included syringes(60 mL reservoir syringe and 30 mL delivery syringe)can be easily exchanged for other sizes,if required.A one-way valve is located at the end of the efferent tubing to prevent retrograde flow of blood into the system[83].The delivery catheter is attached to a three-way stopcock,which can be used to equalize the pressure of the delivery syringe to prevent explosive CO2delivery and excessive volume.Saline and contrast can also be delivered through the stopcock[83].
Figure 2 shows the CO2angiography imaging findings from a recent case from our institution of a 71-year-old man with PAD that demonstrates the effective use of CO2in angiography-guided endovascular therapy.
SAFETY OF CO2 IN PATIENTS UNDERGOING PERIPHERAL ANGIOGRAPHY
A recent meta-analysis investigated the safety of CO2 in patients undergoing peripheral angiography with CO2and iodinated contrast medium[84].The metaanalysis included seven observational studies and one randomized controlled trial with 754 peripheral angiographic procedures for 677 patients.Patients undergoing CO2angiography experienced a higher incidence of minor,non-renal complications that included limb and abdominal pain(11vs0;P=0.001),nausea and vomiting(9vs1;P=0.006).Major adverse events attributable to CO2were rare[84].

Figure 1 The pre-assembled portable medical AngiAssist® delivery system consisting of two one-way valves,a reservoir syringe,a delivery syringe,proprietary K-valve,and afferent and efferent tubing.
IMAGE QUALITY
In 2016,Palenaet al[80]reported the findings from a prospective,single-center study that evaluated the diagnostic accuracy of automated CO2angiography(ACDA)during the endovascular treatment of patients with diabetes and CLI with CKD of stage 3 or more.The study included 36 patients with a mean age of 74.8±5.8 years.All patients underwent lower limb ACDA,followed by angiography with iodinated contrast medium and subsequent balloon angioplasty.Angiography was performed through the femoral artery using antegrade access.There were two experienced operators,and interobserver variability following independently reviewed DSA images was found to be very low(κ=0.89).The diagnostic accuracy of ACDA was 89.9%,the sensitivity was 92.3%,the specificity was 75%,the positive predictive value was 95.5%,and negative predictive value was 63.1%.The difference in qualitative diagnostic accuracy between CO2angiography and the use of iodinated contrast medium was not statistically significant(P=0.197),which supports the use of isolated CO2angiography for therapeutic evaluation and intervention of PAD in patients with CKD[80].
New developments in interventional CO2angiography of the lower limb include digital variance angiography(DVA).A recent study compared the qualitative and quantitative performance of DVA with standard DSA[85].The findings from this recent study showed that for CO2angiography of the lower limb,the use of DVA resulted in a higher signal-to-noise ratio and a significantly better image quality when compared with DSA[85].The development of lower limb CO2angiography using the new DVA image processing technique may increase the use of CO2angiography in clinical practice for patients with PAD and CLI who are at risk from CIN.
HYBRID ANGIOGRAPHY IS AN IMPROVED TECHNIQUE THAT REDUCES CONTRAST-INDUCED NEPHROPATHY
In conventional angiography,iodinated contrast medium is used to visualize smaller arteries and to confirm stenotic segments,particularly in vessels below the knee[17,20].Imaging of the aorta and large arteries,such as the iliac and femoral arteries,would require increased volumes of contrast.During conventional angiography,the use of iodinated contrast medium results in high-resolution images,but the patient is at increased risk for kidney damage[19].The image quality of CO2angiography alone has reduced resolution,but CO2is non-nephrotoxic and non-allergenic.However,hybrid angiography uses a combination of iodinated contrast medium and CO2to reduce the required volume of iodine and overcomes some of the problems associated with the use of iodinated contrast medium or CO2alone.Hybrid angiography reduces the required volume of iodinated contrast medium while providing the necessary image quality[19].

Figure 2 Carbon dioxide angiography imaging findings. A-C:Carbon dioxide angiography imaging findings of lower extremity vessels of a 71-year-old male with peripheral arterial disease.AO:Aorta;CIA:Common iliac artery;EIA:External iliac artery;SFA:Superficial femoral artery;TP Trunk:Tibioperoneal trunk;ATA:Anterior tibial artery;PTA:Posterior tibial artery.
Stegemanet al[20]demonstrated the efficacy of hybrid CO2angiography in 191 patients with lower extremity claudication and CLI,Interventions were performed with iodinated contrast medium alone,or CO2combined with iodinated contrast medium(hybrid angiography),or CO2alone.All the patients underwent an extensive pre-interventional ultrasound exam to localize the flow-limiting stenosis and stent sizing.There were no severe complications reported and no significant differences between the technical success rate,total irradiation,and intervention time for the iodinated contrast medium and CO2groups.Patients in the CO2group exhibited significantly lower baseline renal function compared with the iodinated contrast medium group.The required volume of contrast and the incidence of CIN was significantly lower with CO2angiography for patients with both claudication and CLI(P<0.05).Patients in the iodinated contrast medium group experienced a higher rate of CIN(19%;29/154)compared with the patients in the CO2group(5%;2/37;P=0.044).Sub-group analysis that compared diabetic and non-diabetic patients in each therapy group further demonstrated the safety of CO2as a contrast agent.The incidence of CIN in diabetic(5%)and non-diabetic(6%)patients did not significantly differ in the CO2group,while diabetic patients in the iodinated contrast medium group experienced a markedly higher rate of CIN(25%)compared with the nondiabetic patients(13%)[20].
COMPLICATIONS OF THE USE OF CO2 ANGIOGRAPHY
Complications associated with the use of CO2are uncommon and are usually minor and transient.However,the clinician should be aware of two main complications that can arise from the use of CO2angiography,which are gas embolism and explosive gas delivery(Table 2).Gas embolism is the inadvertent mixing of air with CO2during delivery.CO2is highly soluble,which allows it to be safely and effectively injected into the arteries and veins below the diaphragm[79].The blood carries dissolved CO2to the lungs,where the gas is expired in a single pass before it reaches the coronary or cerebral circulation,which eliminates the possibility of gas embolism[79].
The compressibility of CO2can significantly impact the patient experience and image quality of a CO2angiography.A physical characteristic of gases is that pressure is inversely related to the volume at a constant temperature.Therefore,as a force is exerted on CO2during its administration,its density and pressure increase,and the volume decreases.When CO2exits the catheter,the gas will expand,which explains the phenomenon known as explosive delivery.This effect may cause transient discomfort for the patient and poor image quality.This limitation of CO2as a contrast agent can be overcome by injecting 3-5 mL of CO2into the catheter to remove any fluid or blood,which decreases gas compression and explosive delivery[75].Movement can affect image quality,but this limitation can also be overcome.Palenaet al[80]reported treating patients with intra-arterial lidocaine before injection of CO2,which completely resolved the patient’s pain,preventing involuntary movement during repeat procedures.
There are minor complications associated with CO2angiography that are outweighed by the benefits.In 2015,in a multi-center prospective study,Fujiharaet al[17]showed that the technical success rate of CO2angiography-guided angioplasty of renal and iliofemoral artery disease was 97.9%.The incidence of CIN was 5.1%(5/98),but the prevalence of CO2angiography-associated complications was 17.3%(17/98).The complications were mainly minor from diarrhea,leg and abdominal pain(15/17),and a rare major complication was non-occlusive mesenteric ischemia(2/17)[17].

Table 2 Overcoming complications associated with the use of carbon dioxide angiography
CONTRAINDICATIONS TO THE USE OF CO2 ANGIOGRAPHY
When considering the use of CO2in interventional angiography of the lower limb in patients PAD and CLI,the clinician should be aware of the contraindications to its use and how they may be avoided(Table 3)[48].
CO2arteriography performed above the diaphragm may result in cerebral air embolism if the patient has a left-to-right cardiopulmonary shunt.Intra-arterial supradiaphragmatic injections,which include thoracic aortography,coronary arteriography,and cerebral arteriography,risk developing a cerebral gas embolism due to an air block phenomenon[75,81,86].The findings from small animal studies showed that carotid injections of CO2were neurotoxic and associated with multifocal ischemic infarctions associated with disruption of the blood-brain barrier[87,88].In 2010,a clinical study reported by Kariyaet al[89]showed that CO2could reflux into the thoracic aorta and cerebral circulation following CO2injection into the brachial artery,and this resulted in seizures and loss of consciousness(Table 3).
The concurrent use of nitrous oxide anesthesia and CO2should be avoided because the nitrous oxide may diffuse into the CO2bubbles,causing them to expand,which could lead to a pulmonary artery vapor lock(Table 3)[75].CO2should be avoided for use in the venous circulation of patients that have a right-to-left intracardiac shunt.If there is a critical requirement for CO2venography,the patient should be placed in the left lateral decubitus position to allow CO2to trap and dissolve in the right atrium(Table 3)[90].
The relative contraindications to the intravenous use of CO2as a contrast agent include chronic obstructive pulmonary disease and pulmonary hypertension.Both of these conditions are sensitive to pulmonary arterial pressure changes,and because CO2increases pulmonary artery pressure,its use can exacerbate pulmonary hypertension[75].Therefore,the volume of intravenous injections of CO2should be reduced,while the interval between multiple injections should be increased to 3-5 minutes to avoid CO2accumulation and pulmonary artery vapor lock(Table 3)[75].
IMPACT OF THE USE OF HYBRID CO2 ANGIOGRAPHY IN OUR VASCULAR LAB
Real-world findings(unpublished)from the office-based laboratory service of our vascular lab illustrate the clinical impact following the use of hybrid CO2angiography in the management of patients with PAD and CLI who are at risk of CIN.We reviewed a random sample of 50 peripheral interventional procedures performed in patients with PAD who were Rutherford Class 3-6.The patients were between 57-92 years of age,and 98% had a history of hypertension,67% had diabetes mellitus,45%had coronary artery disease,and 21% had congestive heart failure.During interventional angiography,patients were given CO2in doses ranging from 35-500 mL,with the dose of iodinated contrast agents ranging from 8-80 mL.None of the patients developed acute renal failure or CIN after the procedure.The average amount of iodinated contrast medium used per procedure was 41 mL as compared to an average of 102 mL used before hybrid CO2angiography was used(Figure 3).This has had a significant impact on outcomes for our patients with CLI with concomitant CKD.
CONCLUSION
Recent advances in the delivery of CO2and imaging technology have increased the use of CO2angiography in patients with PAD who have CLI as a safe alternative tothe use of iodinated contrast medium,resulting in the preservation of renal function and preventing limb amputations.Hybrid angiography,using combined CO2and reduced amounts of iodine,has reduced the incidence of CIN.The use of modern CO2delivery systems with advanced DSA techniques have improved diagnostic accuracy and have reduced morbidity and mortality rates.
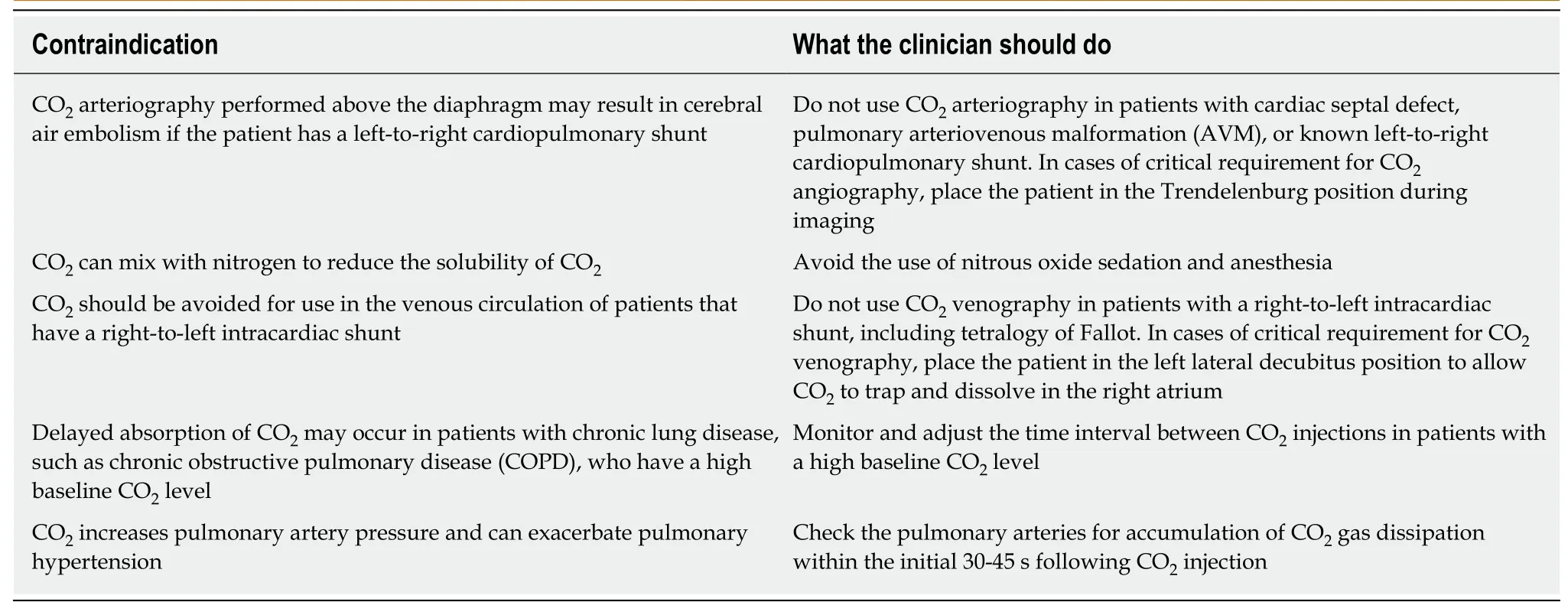
Table 3 Contraindications for the use of carbon dioxide angiography

Figure 3 Average contrast used per endovascular procedure.Data from our Office Based Lab shows a reduction in Total Iodine Contrast used per procedure after adopting Hybrid CO2 Angiography.
ACKNOWLEDGEMENTS
The authors are grateful to the clinical staff of the Heart,Vascular,and Leg Center for helpful discussions,and to the Radiology Department,who provided the images used in this article.We would also like to thank Superior Medical Experts for assistance with editing.

