Rapid generation advance(RGA)in chickpea to produce up to seven generations per year and enable speed breeding
Srinivsn Smineni, Mdhuprni Sen, Sobhn B. Sjj, Poorn M. Gur,b,*
aInternational Crops Research Institute for the Semi-Arid Tropics (ICRISAT), Patancheru, Telangana 502324, India
bThe UWA Institute of Agriculture, University of Western Australia, Perth, WA 6009, Australia
Keywords:Photoperiod Immature seeds Flowering Rapid generation turnover Speed breeding
ABSTRACT This study was aimed at developing a protocol for increasing the number of generation cycles per year in chickpea(Cicer arietinum L.).Six accessions,two each from early(JG 11 and JG 14),medium(ICCV 10 and JG 16),and late(CDC-Frontier and C 235)maturity groups,were used. The experiment was conducted for two years under glasshouse conditions. The photoperiod was extended to induce early flowering and immature seeds were germinated to further reduce generation cycle time. Compared to control, artificial light caused a reduction in flowering time by respectively 8-19, 7-16, and 11-27 days in early-, medium-,and late-maturing accessions. The earliest stage of immature seed able to germinate was 20-23 days after anthesis in accessions of different maturity groups. The time period between germination and the earliest stage of immature seed suitable for germination was considered one generation cycle and spanned respectively 43-60, 44-64, and 52-79 days in early-,medium-,and late-maturing accessions.However,the late-maturing accession CDCFrontier could not be advanced further after three generation cycles owing to the strong influence of photoperiod and temperature.The mean total number of generations produced per year were respectively 7, 6.2, and 6 in early-, medium-, and late-maturing accessions.These results have encouraging implications for breeding programs: rapid progression toward homozygosity, development of mapping populations, and reduction in time, space and resources in cultivar development (speed breeding).
1. Introduction
The time required to develop a crop cultivar depends largely on the number of years needed to develop homozygous lines following hybridization. Generally, it takes 7-9 years to develop homozygous lines after hybridization if only one crop generation is produced per year. For this reason,development of doubled haploids (DH) or rapid generation advance (RGA) are used to reduce the number of years required to reach homozygosity and develop a cultivar. The RGA technology has been successfully used to accelerate breeding cycles and breeding progress(in a process referred to as speed breeding) in many crops [1-5]. This technology involves the use of immature seeds to produce miniature plants in artificial medium under controlled conditions and allowing them to produce a few flowers that bear seeds that are harvested before normal seed maturity,thus reducing the duration of breeding cycles.The length of breeding cycle from seed to seed often becomes a limiting factor for developing RILs and for exploiting molecular marker technology [6,7].RGA methods increase the rate of genetic gain by reducing the generation interval and accelerating selection cycles.Thus,to rapidly fix genes in breeding lines, breeders are successfully using various methods of RGA, as lately reported for rice [8]and wheat[9].
Breeding strategies in legumes such as chickpea,lentil,and field pea generally involve hybridization among cultivars or between cultivars, landraces, or primitive forms, followed by combinations of pedigree, bulk, backcross or single-seed descent methods of selection [10]. In most cases, breeders can produce one field-based generation per year, or three generations if plants are grown intensively under controlled glasshouse conditions.In cereals and oilseeds,plant breeders have access to DH technology, which permits the development of homozygous plants from gametes in a single generation. However, in grain legumes this technology has not yet been successful, and to date no robust protocol has been proposed [11-13]. Chickpea is a quantitative long-day plant, and breeding programs for this crop successfully produce two generations per year, one in the field during the regular crop season and the other during the off season in either the glasshouse or off-season nurseries. In south Asia,chickpea is grown as a winter-season crop and generally sown between October and November. The length of the crop season varies from 90 to 160 days depending on cultivar and growing conditions. The International Crops Research Institute for the Semi-Arid Tropics(ICRISAT)has been successfully accelerating generation turnover of chickpea using three generations per year at Patancheru in southern India (a short-season environment) for mainly short- and mediumduration crosses [4]. Two approaches are being used to produce three generations per year: (1) one crop in the field during crop season (Oct.-Feb.) and two in the glasshouse during the off season (Feb.-Sep.), and (2) the first crop in the field during the crop season(Oct.-Feb.),the second crop in the field under late-sown conditions with irrigation(Feb-Apr)and the third crop in an off-season nursery (May-Jul.) at Hiriyur,Karnataka state,India.
Changes in environmental conditions strongly influence plant morphological growth and reproductive behavior. The effect of temperature and photoperiod on flowering time in grain legumes has been well documented[14-16].Photoperiod altered developmental activities such as germination, shoot growth, leaf expansion, and flowering [17,18]. In most coolseason legume crops including chickpea, long days promote flowering. A study using different light sources found that incandescent lamps encourage elongation of stems and suppresses lateral branching, whereas fluorescent lighting had the opposite effects [17]. Despite the long photoperiod,light quality also plays a greater role in reducing flowering time in late-maturing than in early-maturing genotypes of lentil,chickpea,field pea,and lupine[19].
Reducing generation cycle time using RGA will enable speed breeding and accelerate genetic gain. RGA will also accelerate development of recombinant inbred lines (RILs)and near-isogenic lines [20,21]. The objective of the present study was to develop an RGA protocol to accelerate generation cycling among chickpea genotypes that have different maturity durations.
2. Materials and methods
Experiment I was conducted to identify the earliest stage at which seeds can germinate after flower formation in six chickpea accessions. The six accessions belong to three maturity groups (Table 1). CDC-Frontier is a kabuli-type chickpea variety released by Crop Development Centre,University of Saskatchewan, for cultivation in western Canada [22] and the remaining accessions are popular Indian cultivars. The experiment was conducted under glasshouse conditions at ICRISAT during 2014. Three plants of each accession were used in the experiment. Flowers were tagged every day after first flower formation,continuing for 10 days.Immature green seeds from all plants were extracted from pods 16 days after flower formation and germinated artificially in Petri plates(with wet blotting paper in the bottom)to identify the earliest stage of seed development suitable for germination. No growth medium or plant hormone was applied to seeds to induce germination. Pods 16-24 days oldwere selected to test germinations.The Petri plates were kept in the dark.
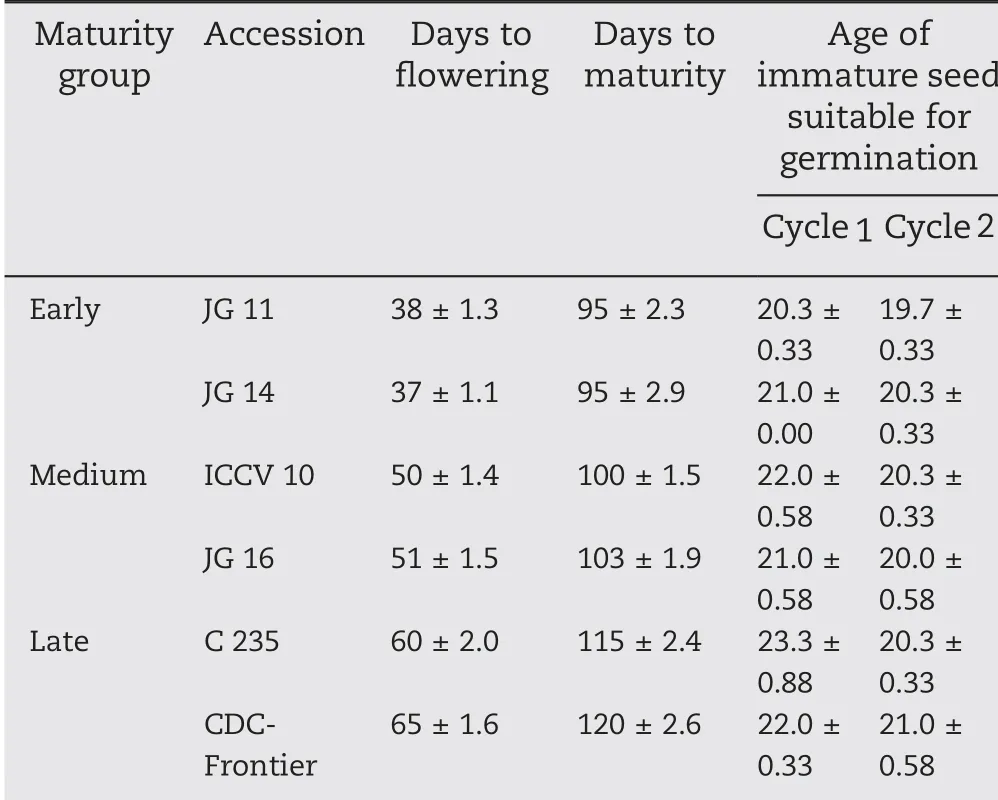
Table 1-Stage of immature seed suitable for germination in six accessions of different maturity groups(day).
Experiment II was conducted to estimate the length of each generation cycle and the number of generations that can be advanced in a year under glasshouse conditions at ICRISAT, for two consecutive years: 2014-2015 and 2015-2016(initiated in November). The maximum temperature in the glasshouse was maintained at (25 ± 1) °C throughout the experimental period. Weather data such as temperature and photoperiod were obtained from the weather station at ICRISAT (Table 2). These values were averaged for a given generation cycle.
Four seeds of each accession were planted in 30.5-cm deep pots filled with potting mixture composed of vertisols,vermicompost, and sand in a 6:2:1 ratio. Four pots were planted for each accession. Set 1 consisted of 24 pots(including all six accessions) arranged in a completely randomized design. Set 2 consisted of 12 pots (two pots of each accession,including all six accessions) as control.Seeds of each accession were sown in separate pots and allowed to germinate. After germination two plants were maintained in each pot, and at the 10-day-old seedling stage, artificial light(AL)treatment was applied to set 1,whereas set 2 was grown normally without any artificial light treatment.Plants in set 1 and set 2 were grown under the same glasshouse conditions.AL was provided using four standard incandescent bulbs of 60 W each with a light intensity of 870 lm for 12 h from 18:00 to the following 6:00. As soon as plants under AL started flowering,light treatment was discontinued for the respective accessions and the first flower on each plant was tagged.Based on the results obtained from experiment I, immature seeds at the earliest stage at which they could germinate in each variety were harvested. Soon after harvesting, the immature seeds were directly sown in pots filled with potting mixture to start the next generation cycle.The length of each generation cycle was calculated as the number of days from sowing to harvest of immature seeds suitable for germination.
3. Results
In experiment I, the earliest age of immature seeds found suitable for germination in the genotypes studied ranged from 20 to 23 days after tagging of flowers in cycle 1(Table 3).Seeds germinated in cycle 1 were advanced to the next generation(cycle 2). Seed aged 21 days after tagging of flowers was theearliest that could germinate across all genotypes in cycle 2.From both cycles it was clear that irrespective of the large variation in phenology among these accessions, immature seeds (younger than 23 days) germinated successfully for generation advance(Fig.1).
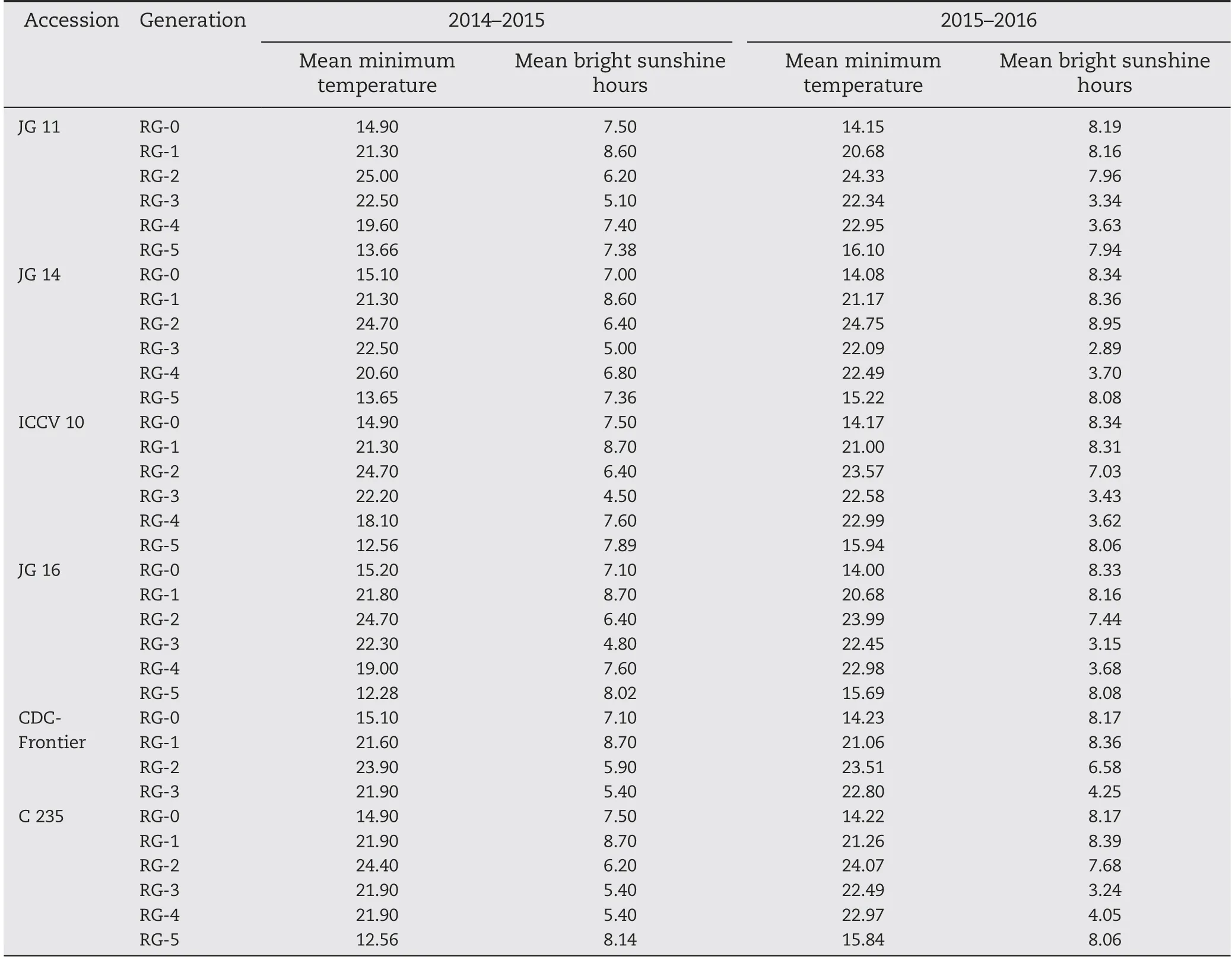
Table 2-Mean minimum temperature and bright sunshine hours prevailing during each rapid generation cycle.

Table 3-Response of six chickpea accessions to altered photoperiod in the glasshouse.
In experiment II, plants that received AL treatment flowered earlier than the control (Table 3). In early-maturing accessions, light reduced flowering time by 10-19 days in JG 11 and 8-13 days in JG 14.In medium-maturing accessions,AL reduced flowering time by 9-16 days in ICCV 10 and 7-8 days in JG 16. A similar trend was observed in late-maturing accessions, with flowering time reduced by a maximum of 27 days in CDC-Frontier and 11 days in C 235. CDC-Frontier responded to the conditions imposed for early flowering positively up to the rapid generation (RG)-2 cycle; however,after RG-2,this accession did not respond to light and did not flower earlier than the control. Flowering initiated at 90 days after sowing in the light and control treatments and pod formation continued up to 125 days(data not shown).Most of the pods produced were aborted or had underdeveloped seeds and were accordingly not considered as the RG cycle. When fresh seed of CDC-Frontier along with C 235 was grown in RG-5,the accession stopped responding to light after RG-2 in both years and most of the pods formed were also aborted.
The mean age of immature seed suitable for germination was 20 days in early-maturing, 20-22 days in mediummaturing, and 21-25 days in late-maturing accessions in the two growing seasons(Table 3).
The duration from seed germination to immature seed of the next generation suitable for germination varied across accessions.Interestingly,the RG cycle showed some relationship with maturity group. In early-maturing accessions, the RG cycles were 43-58 days for JG 11 and 47-58 days for JG 14.In medium-maturing accessions, the RG cycles were 44-64 days for ICCV 10 and 51-67 days for JG 16. In latematuring accessions, the maximum days from seed to seed were much longer than for the other two maturity groups,and the RG cycles were 53-112 days for CDC-Frontier and 52-79 days for C235.CDC-Frontier took 112 days to complete a cycle in the RG-2 generation and further cycles were not completed owing to lack of response to supplemental artificial lighting(Table 3).
The overall mean generation times were 50.0 (2014-2015)to 52.7 days(2015-2016)in early-maturing accessions and 58.6(2014-2015) to 55.4 days (2015-2016) in medium-maturing accessions. The late-maturing line C 235 completed six generations and the mean was 61 days in both years. In the conventional method where the seed is left to mature on the plant, 95-120 days elapsed between sowing and harvest(Table 1). After three successive rapid generation cycles, the CDC-Frontier did not set pods. CDC-Frontier took 88 days in 2014-2015 and 55 days in 2015-2016 for flowering under AL conditions(Table 3).
4. Discussion
Accessions were selected from different maturity groups to represent different agro-ecologies to enhance our knowledge about the response of accessions with different maturity durations to the current RGA methodologies used. All accessions (except CDC-Frontier) responded positively and the earliest stage of seed capable of germination varied between 1 and 4 days among accessions, irrespective of maturity type. This finding indicates that the method would work with a wide range of chickpea accessions in diverse maturity groups.
The experiments were performed in the glasshouse to measure flowering time and immature seed germination time without adding growth regulator. In previous studies in monocot [23] and dicot plant species [24], early flowering was induced by spraying growth regulators.In addition,in vitro and in vivo approaches were reported for shortening the flowering time and increasing generation cycles in lupine [19], and pea and bambara groundnut [6]. Similarly, RGA was found promising in crops such as lentil[25],pea(P.sativum)[26],amaranth(Amaranthus spp.)[27]and peanut(Arachis hypogaea)[28].
Multiple generations per year can be produced in several crops,such as 3.7 generations in canola,4.5 in chickpea,5.3 in barley, and 5.6 in wheat [29]. Watson et al. [29] used a temperature-controlled glasshouse with supplementary light followed by five days of drying(35 °C),one day soaking of seed(room temperature) and four days of chilling treatment at 4 °C.The average total generation time of chickpea accessions(late-maturing) was 82.1 days. Our results suggest that respectively 7.0,6.2,and 6.0 generations per year are possible for early-, medium-, and late-maturing chickpea accessions. Our protocol does not require application of chemicals or hormone treatment of seeds or growing plants at different stages.It offers a cost-effective methodology to achieve homozygosity within a year to accelerate genetic gain in chickpea crop improvement.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
This work was undertaken as part of the CGIAR Research Program on Grain Legumes and Dryland Cereals. We would like to thank Dr. Mahesh D. Mahendrakar, Research Fellow,chickpea breeding and Dr. Yashoda, Research Fellow at ICRISAT,for their help during data compilation and technical inputs in manuscript preparation.
- The Crop Journal的其它文章
- Brief Guide for Authors
- Transcriptomic responses in resistant and susceptible maize infected with Fusarium graminearum
- Relay-intercropping soybean with maize maintains soil fertility and increases nitrogen recovery efficiency by reducing nitrogen input
- Genetic analysis and QTL mapping of stalk cell wall components and digestibility in maize recombinant inbred lines from B73 × By804
- Genetic bases of source-, sink-, and yield-related traits revealed by genome-wide association study in Xian rice
- Performance and yield stability of maize hybrids in stress-prone environments in eastern Africa

