Localized primary gastric amyloidosis:Three case reports
Xue-Mei Liu,Lian-Jun Di,Jia-Xing Zhu,Xing-Long Wu,Hong-Ping Li,Hui-Chao Wu,Bi-Guang Tuo
Xue-Mei Liu,Lian-Jun Di,Jia-Xing Zhu,Hong-Ping Li,Hui-Chao Wu,Bi-Guang Tuo,Department of Gastroenterology,Affiliated Hospital of Zunyi Medical University,Zunyi 563003,Guizhou Province,China
Xing-Long Wu,Department of Pathology,Affiliated Hospital of Zunyi Medical University,Zunyi 563003,Guizhou Province,China
Abstract BACKGROUND Localized primary gastric amyloidosis is a rare disorder characterized by the extracellular deposition of insoluble fibrillary protein in the stomach and can mimic various diseases on endoscopic examination,including gastrointestinal stromal tumors,gastric cancer and ulcers.CASE SUMMARIES Here,we report a series of three cases of localized gastric amyloidosis mimicking gastric mucosa-associated lymphoid tissue(MALT)lymphoma on endoscopic examination that were evaluated over the past ten years in our hospital.The different detection times of this rare disease resulted in three completely different outcomes,indicating the strong importance of early detection,diagnosis and treatment.The difficulties encountered in making an accurate diagnosis and differential diagnosis are highlighted,and this report provides clinical experience for the diagnosis of localized primary gastric amyloidosis.CONCLUSION Localized gastric amyloidosis is a rare metabolic disease that resembles MALT lymphoma.Early detection,diagnosis and treatment of localized gastric amyloidosis result in an excellent prognosis.
Key Words:Localized gastric amyloidosis;Mucosa-associated lymphoid tissue lymphoma;Different outcomes;Rare disease;A case series study;Case report
INTRODUCTION
Localized gastric amyloidosis is a rare disorder characterized by extracellular deposition of insoluble fibrillary protein in the stomach[1].The clinical manifestations of localized gastric amyloidosis are often uncharacteristic and subclinical[2].However,the gastric endoscopic findings include redness,erosion,ulcers,submucosal tumorlike features,and scirrhous-like features,among others[3].Although imaging studies such as computed tomography(CT),contrast-enhanced radiography,endoscopic ultrasound(EUS),and upper endoscopy are helpful in the initial assessment of patients,determining the correct clinical diagnosis remains difficult.Previous reports have indicated that in the differential diagnosis,the main diseases considered alongside localized gastric amyloidosis include gastrointestinal stromal tumors[4,5],gastric cancer[3]and healing gastric ulcers[6].Herein,we report three cases of localized gastric amyloidosis with different outcomes over the past ten years at our hospital.In these cases,the disease mimicked gastric mucosa-associated lymphoid tissue(MALT)lymphoma on upper endoscopy,suggesting the importance of early detection,clinical and pathological diagnosis and treatment.
CASE PRESENTATION
Case 1
Chief complaints and history of present illness:A 36-year-old woman visited our hospital due to epigastric pain for approximately 1 mo.There was no obvious cause of the paroxysmal dull abdominal pain.
History of past illness:She had no specific history of past illness.
Personal and family history:She had no specific personal or family history.
Physical examination upon admission:There were no obvious abnormalities during the physical examination.
Laboratory examinations:Routine laboratory investigations were unremarkable.Urine immunoelectrophoresis was negative for the Bence-Jones protein,and serum immunoglobulin levels were normal.
Imaging examinations:Esophagogastroduodenoscopy revealed a faint reddish flat elevated lesion,15 mm x 16 mm in size,with multiple nodules on the surface in the great curvature of the antrum adjacent to the corpus(Figure 1A).The lesion showed a defined green area on narrow-band imaging(NBI)(Figure 1B).Magnifying endoscopy with NBI(ME-NBI)revealed expanded normal glands with changed polarity as well as dilated and tortuous vessels,suggesting that something other than an epithelial tumor was located in the lamina propria mucosae or deeper tissue(Figure 1C).Based on morphology,Helicobacter pylori(H.pylori)-negative MALT lymphoma was considered first,but further examination by hematoxylin-eosin(H&E)staining and Giemsa staining of multiple gastric biopsies showed that the samples were negative for the bacterium and that the histology of the lesion indicated only nonspecific inflammation.Therefore,after informed consent was obtained from the patient,diagnostic endoscopic submucosal dissection(ESD)was successfully performed.Histologically,massive and cord-like pink substances were deposited in the mesenchyme and inside the blood vessel wall at the bottom of the lamina propria mucosa,muscularis mucosae and superficial submucosal layer,with cystic dilated gastric glands suggesting cystic gastritis(Figure 1D).Additionally,Congo red staining with potassium permanganate pretreatment confirmed the light-chain amyloid(AL)type,which was reconfirmedviapolarized light microscopy,with the observation of apple-green birefringence in the lesions(Figure 1E and F).Ultrasonography and CT revealed a normally functioning heart and normal-sized liver and kidneys,with no amyloid deposition histologically observed anywhere in the duodenum,colon or rectum.
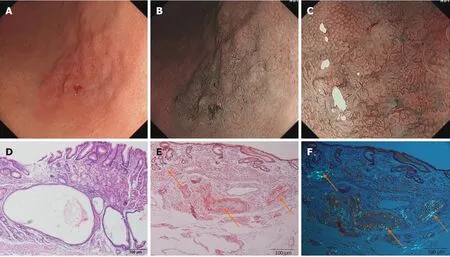
Figure 1 Endoscopic and histological data of patient 1.A:Esophagogastroduodenoscopy revealed a faint reddish flat elevated lesion,15 × 16 mm in size,with multiple nodules on the surface in the great curvature of the antrum adjacent to the corpus.B:Narrow-band imaging(NBI)indicated a defined green area.C:Magnifying endoscopy with NBI showed expanded normal glands with changed polarity as well as dilated and tortuous vessels.D:Hematoxylin-eosin staining showed cystic dilated gastric glands.E:Massive and cord-like pink substances were deposited in the mesenchyme and inside the blood vessel wall at the bottom of the lamina propria mucosa,muscularis mucosae and superficial submucosal layer.F:Polarized light microscopy revealed apple-green birefringence in the lesions.Orange arrows indicate amyloid depositions.
Case 2
Chief complaints and history of present illness:A 28-year-old woman was admitted to our hospital due to epigastric pain for 6 mo.There was no obvious cause of the burning pain in the upper abdomen.
History of past illness:She had no specific history of past illness.
Personal and family history:She had no specific personal or family history.
Physical examination upon admission:There were no obvious abnormalities during the physical examination.
Laboratory examinations:Routine laboratory investigations were unremarkable.Urine immunoelectrophoresis was negative for the Bence-Jones protein,and serum immunoglobulin levels were normal.
Imaging examinations:Conventional endoscopy identified red and white areas in the gastric antrum,mainly white lesions,accompanied by active gastritis as well as multiple areas of atrophy,nodules and erosion lesions(Figure 2A).NBI showed a defined light brownish area(Figure 2B),and expanded normal glands with changed polarity and tree-like vessels were seen by ME-NBI.The first suspected diagnosis wasH.pylori-positive MALT lymphoma(Figure 2C);however,pathological examination demonstrated gastric amyloidosis in the mucosa,and no malignant tumor was found(Figure 2D and E).Moreover,CT indicated no abnormalities.
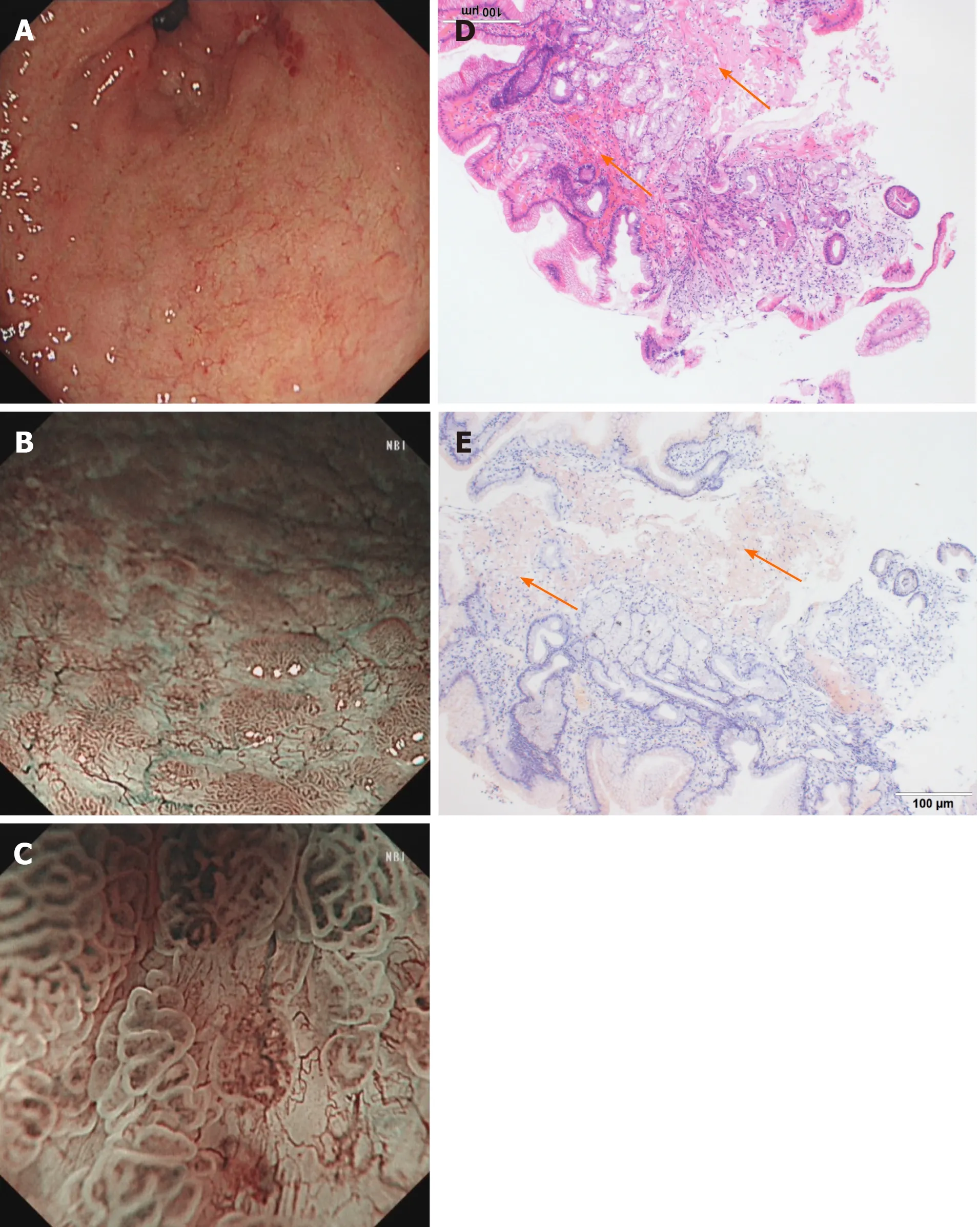
Figure 2 Endoscopic and histological data of patient 2.A:Conventional endoscopy showed a red and white area in the atrophic gastric antrum with active gastritis.B:Narrow-band imaging(NBI)showed a defined light brownish area.C:Magnifying endoscopy with NBI revealed expanded normal glands with changed polarity and tree-like vessels.D:Hematoxylin-eosin staining showed abundant cord-like red substances in the mucosal layers.E:These tissues were positive for Congo red staining.Orange arrows indicate amyloid depositions.
Case 3
Chief complaints and history of present illness:A 72-year-old man was referred to our hospital due to intermittent epigastric pain and vomiting for 2 years.His symptoms had worsened;he could not eat at all and had severe vomiting.Before this visit,he had never undergone any examinations at the hospital.
History of past illness:He had no specific history of past illness.
Personal and family history:He had an extensive smoking history of 30 years,with an average of 10 cigarettes/day.This patient had no familial history of genetic diseases.
Physical examination upon admission:Physical examination revealed an emaciated body shape,anemic appearance and edema of both lower limbs.Tenderness in the upper abdomen was detected.There were no other obvious abnormalities during the physical examination.
Laboratory examinations:Routine laboratory investigations showed that his hemoglobin was 89 g/L,K+was 3.31 mmol/L,albumin was 24 g/L,and prealbumin was 64 g/L.The other routine blood parameters were unremarkable.Immunoelectrophoresis was negative for the Bence-Jones protein,and serum immunoglobulin levels were normal.
Imaging examinations:Conventional endoscopy demonstrated that the entire gastric mucosa was congestive,edematous and mainly red.Enlarged and thickened irregular gastric folds were also detected in the entire stomach with sporadic and large sheet erosions.Moreover,gastric antrum peristalsis was absent,and gastric outlet obstruction occurred due to narrowing of the antrum(Figure 3A).On EUS,the mucosa and submucosa layer were thickened,although the muscle and serosal layers were intact(Figure 3B).MALT lymphoma and gastric cancer were highly suspected.CT showed that the pyloric wall was thickened,but submucosal enhancement was not obvious.There were no signs of amyloidosis in the liver,spleen,or heart,and no amyloid deposition was histologically observed elsewhere in the entire gastrointestinal(GI)tract.Finally,H&E staining showed abundant amyloid deposition in the mucosal and submucosal layers(Figure 3D and E)that were positive for Congo red staining.No bone destruction was found in the lumbar spine,pelvis or skull by CT examination.
FINAL DIAGNOSIS
According to the endoscopic examination,histopathologic assessment and CT examination,all three patients were diagnosed with primary localized gastric amyloidosis,respectively.
TREATMENT
After informed consent was obtained from the first patient,diagnostic ESD was successfully performed.In contrast,due to the large lesion sizes and the lack of gastric outlet obstruction,ESD was not performed in the second patient,who was just treatedH.pylorieradication for active gastritis.In the third case,to relieve the patient’s discomfort,we only dissected some tissue to remove the obstruction,and a gastric tube was placed into the antrum to provide nutritional support(Figure 3C).No other endoscopic or surgery treatment was performed.
OUTCOME AND FOLLOW-UP
The first patient was discharged uneventfully after ESD,and no local or systemic recurrence was seen after 2 mo,6 mo or 1 year of follow-up.This case presents the endoscopic features and associated histology of confirmed gastric amyloidosis with cystic gastritis based on an ESD sample of the lesion,contributing to the endoscopic detection of a primary localized gastric amyloidosis.The second patient was followed up for 4 years,and there were no changes in the relevant lesions.Unfortunately,due to a lack of financial assistance,the third patient did not undergo further treatment and died 2 wk later due to severe malnutrition.
DISCUSSION
Amyloidosis is characterized by the extracellular deposition of abnormal proteins in organs and includes six types:Primary,secondary,hemodialysis-related,hereditary,senile,and localized[1].Primary AL amyloidosis is associated with monoclonal light chains in the serum and/or urine,with 15% of patients having multiple myeloma.Secondary(AA)amyloidosis is associated with inflammatory,infectious,and neoplastic diseases,and these two types of amyloidosis are the most common types in clinical practice.In general,amyloid deposits are distributed along the GI tract,liver,kidney,and spleen and are sometimes associated with the onset of inflammatory bowel disease[1].The duodenum and stomach are the most common sites of such protein deposition.Indeed,GI involvement is common in cases of systemic amyloidosis,and the majority of cases of gastric amyloidosis are related to systemic involvement of amyloidosis.The symptoms include nausea,vomiting,hematemesis,and epigastric pain;furthermore,purpura,macroglossia,joint swelling,congestive heart failure and hepatomegaly are the typical characteristics of systemic amyloidosis on physiological examination.
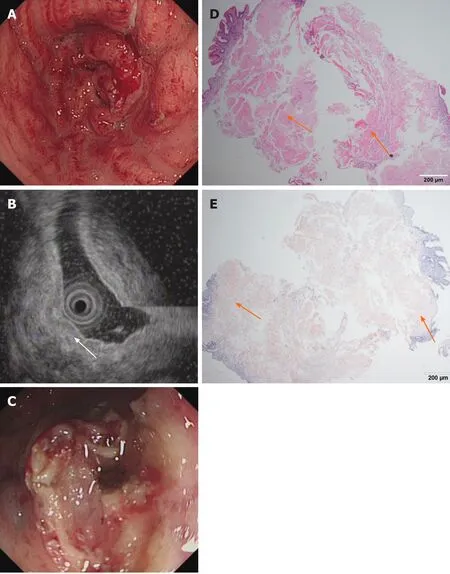
Figure 3 Endoscopic and histological data of patient 3.A:Conventional endoscopy demonstrated that the whole gastric mucosa was congestive,edematous and mainly red.B:Endoscopic ultrasound showed that the mucosa and submucosa layer were thickened(white arrow)but that the muscle and serosal layers were intact.C:A gastric tube was placed into the antrum to provide nutritional support.D:Hematoxylin-eosin staining revealed an abundant brick-red substance in the mucosal and submucosal layers.E:These tissues were positive for Congo red staining.Orange arrows indicate amyloid depositions.
For localized primary amyloidosis in the GI tract,the most common site is the stomach[7],followed by the esophagus[8],small bowel[9],and colon[10].Localized gastric amyloidosis is a rare disorder characterized by the extracellular deposition of insoluble fibrillary protein in the stomach[4].The clinical presentation of localized gastric amyloidosis ranges from no symptoms to nausea,vomiting hematemesis,melena,abdominal pain,a gastric mass or tumor,inflammation,erosions,healing ulcers,and even perforation[11,12].Epigastric pain is one of the most common symptoms,and gastric outlet obstruction may be due to submucosal tumors[13],polyps,or antral narrowing[14].In our study,all lesions were located in the gastric antrum;one lesion spread to the entire stomach,and the background gastric mucosa displayed active gastritis.In these three cases,epigastric pain was a prominent symptom,with no typical signs of systemic amyloidosis on physiological examination,strongly suggesting local gastric amyloidosis.
The conventional endoscopic findings of gastric amyloidosis,including thickened irregular gastric folds[15],gastric outlet obstruction[14],loss of rugal folds[1,14],gastric ulcers with clean bases or irregular edges[14,16,17],arteriovenous malformations[18],granular-appearing mucosa[19],plaque-like lesions[20],ulcerative gastritis[21],submucosal tumor-like features[4],healing gastric ulcer[6]and gastroparesis[22],have been reported.The three patients in this series had different lesions:A faint reddish flat elevated lesion with multiple nodules on the surface;a white-yellowish circular area with the appearance of multiple nodules and active gastritis;and redness,sporadic erosion,thickened irregular gastric folds and gastric outlet obstruction.Therefore,no specific feature of localized primary amyloidosis was detected during white light endoscopic examination.With the development of endoscopic technology,NBI and ME-NBI are becoming increasingly useful in detecting early gastric cancer.Thus,it is important to exclude cancers such as undifferentiated adenocarcinoma or MALT lymphoma as localized gastric amyloidosis commonly appears as a cancerous lesion or mass.Based on our observations,it was difficult to distinguish MALT lymphoma from gastric amyloidosis by NBI and ME-NBI because expanded normal glands with changed polarity,mucosal irregularities,and round small vascular changes,such as dilated vessels without variable caliber on the surface and tree-like vessels,which are considered characteristics of MALT lymphoma[23],were observed in our cases.Furthermore,despite the reported utility of EUS in diagnosing gastric amyloidosis[24],specific EUS features have not been well defined.In our three cases,the EUS images of localized primary amyloidosis showed thickening of the gastric wall with homogenous lesions in the first and second layers only.Therefore,it remains difficult to distinguish gastric amyloidosis from other lesions,such as those of gastric cancer and MALT lymphoma,by EUS.Regardless,it is very important for endoscopists to consider this rare disease when performing endoscopic diagnoses.
Pathological analysis is the gold standard for the diagnosis of gastric amyloidosis,and biopsy with pathology assessment and staining is very helpful for determining the correct diagnosis.In AL amyloidosis,there is greater amyloid deposition in the muscularis mucosa,submucosa,and muscularis propria than in AA amyloidosis,and this deposition does not involve deeper layers of the gastric wall[3]but instead manifests as mucosal lesions that can be visualized by endoscopy and biopsy.Accordingly,it is necessary to perform a biopsy to reach the muscularis mucosa.In these three cases,H&E staining showed abundant amyloid deposition in the mucosal or submucosal layers.Congo red staining with potassium permanganate pretreatment confirmed the AL type,which was reconfirmed by polarized light microscopy with the observation of apple-green birefringence in the lesions.Additionally,serum and urine immunoelectrophoresis showed no monoclonal immunoglobulin or free light chain,and the κ and λ light chains in the serum and urine were all in the normal range.These are typical pathological findings in primary amyloidosis.
For patients diagnosed with amyloidosis,it is important to determine whether they have systemic or localized disease as the treatment and prognosis are different for each disease entity.For instance,the treatment for systemic AL amyloidosis is chemotherapy and stem cell transplantation[1],and localized GI amyloidosis without evidence of systemic involvement has an excellent prognosis.
In the first case,because the lesion was less than 2 cm,ESD was recommended not only for diagnosis but also for treatment and resulted in a good prognosis and minimal recovery time.Such treatment is also recommended when the mucosal biopsies are negative[6].In the second case,due to the circular area in the gastric antrum and the absence of a gastric outlet obstruction due to narrowing of the antrum,we performedH.pylorieradication to reduce her pain,and the treatment was successful.She was followed up for 4 years,and there were no changes in the relevant lesions.At this stage,no other treatment options other than close follow-up were recommended.These clinical observations suggest that patients with localized primary amyloidosis should be monitored and treated symptomatically because they rarely experience progression to systemic disease,and their survival outcomes are excellent.The outcome of the third patient was unfortunate;due to late detection,amyloid was deposited in the entire stomach,particularly in the gastric antrum,resulting in low gastric motility,obstruction and gastric retention.There was no opportunity for effective treatment.
CONCLUSION
In conclusion,localized gastric amyloidosis is a rare metabolic disease that can resemble MALT lymphoma.Early detection,diagnosis and treatment of this rare disease result in an excellent prognosis.In this study,the difficulties in making an accurate diagnosis and differential diagnosis were highlighted,providing more clinical experience for the diagnosis and treatment of localized primary gastric amyloidosis.
ACKNOWLEDGEMENTS
We obtained consent from the patients when they were hospitalized again for a postoperative check-up for the publication of these case reports in print and electronically.
 World Journal of Clinical Cases2020年19期
World Journal of Clinical Cases2020年19期
- World Journal of Clinical Cases的其它文章
- Role of monoclonal antibody drugs in the treatment of COVID-19
- Review of simulation model for education of point-of-care ultrasound using easy-to-make tools
- Liver injury in COVID-19:A minireview
- Transanal minimally invasive surgery vs endoscopic mucosal resection for rectal benign tumors and rectal carcinoids:A retrospective analysis
- Impact of mTOR gene polymorphisms and gene-tea interaction on susceptibility to tuberculosis
- Establishment and validation of a nomogram to predict the risk of ovarian metastasis in gastric cancer:Based on a large cohort
