Spermatozoa morphometry and ultrastructure in estuarine crocodile (Crocodylus porosus)
Wan-Nor Fitri, Haron Wahid, Putra Tengku Rinalfi, Dana Raj, Yawah Donny, Latip Qayyum,Ab Aziz Abdul Malek
1Department of Veterinary Clinical Studies, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia
2Research Centre for Wildlife, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia
3Department of Veterinary Pre-Clinical Studies, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia
4Sarang Buaya Pasir Gudang, Aras 2 Menara Aqabah, Jalan Bandar, 81700 Pasir Gudang, Johor, Malaysia
5Wildlife Veterinary Section, Ex-Situ Conservation Division, Department of Wildlife and National Parks (PERHILITAN) Peninsular Malaysia
ABSTRACT Objective: To evaluate normal spermatozoa morphometry and ultrastructure in estuarine crocodile (Crocodylus porosus).Methods: Four adult male crocodiles aged between 12-15 years,with an average snout to tail length of (3.15±0.01) m were selected for this study. Manipulation of the phallus digitally from the base of the penis was performed on four adult male crocodiles to facilitate the flow of semen to the sulcus. Semen was collected from all individuals for spermatozoa morphometry and ultrastructure study.Morphometry analysis was performed from eosin-nigrosin stained spermatozoa sample. Scanning electron microscopy was conducted to observe the surface ultrastructure of spermatozoa.Results: The morphology of crocodile spermatozoa was made up of acrosome, head, and tail which corresponded to (5.55±1.20) µm,(12.74±1.57) µm, and (70.67±4.40) µm, respectively. The total length of spermatozoa in estuarine crocodile was measured at(88.96±0.52) µm. The most common spermatozoa abnormalities found in the samples were cytoplasmic droplets.Conclusions: Normal morphology, morphometry of spermatozoa in estuarine crocodile which are vermiform shaped-head with a long tail along with questionably high cytoplasmic droplets count are described in this study.
KEYWORDS: Spermatozoa; Scanning electron microscopy;Morphology; Crocodile; Cytoplasmic droplets
1. Introduction
Spermatozoa morphology is important in the development of standards in breeding soundness evaluation. Before an appraisal of a particular breeder is made, it is important to distinguish between normal and abnormal spermatozoa. Spermatozoa morphology is becoming more important recently in discovering fertility potential complementing conventional breeding soundness assay[1].Spermatozoa morphology was found to be more superior by using ultrastructural means for diagnosis of abnormal spermatozoa[2,3].Besides that, the potential to relate spermatozoa morphology and functions such as the motility, storage ability and freezing ability should also be the future direction of the study[4].
The study of spermatozoa morphology is imperative to understand the spermatozoa function. The success of cryopreservation had opened the opportunity of assisted reproductive technology in crocodile[5]. Size and length of spermatozoa is an evolutionary adaptation to spermatozoa competition in a population[6]. However,the information and relationship in reptile are scarce especially in crocodilians, contributing to the sluggish development of assisted reproductive technology in the species[7]. The literature in crocodilian spermatozoa morphology and characteristics is still limited and has only been explored recently[5,8-10]. Due to the rapid selection and evolution, species-specific variation in spermatozoa dimension is expected. Therefore, it is important to build speciesspecific knowledge in crocodile for the establishment of assisted reproductive technology in the industry.
2. Materials and methods
2.1. Animals
The crocodile sanctuary was located in Southern Peninsular Malaysia;Sarang Buaya, Pasir Gudang, Johor (latitude 1.44249 °N, longitude 103.99933 °E). Four adult male estuarine crocodile [Crocodylus (C.)porosus] aged between 12-15 years, with an average snout to tail length of (3.15±0.01) m were selected for this study. The four male crocodiles were kept together with 7 other females in an enclosure with pond and land for basking and space for nesting. The crocodiles were fed with a whole chicken, at the frequency of six times monthly in a total of approximately 7 kg of chicken for each crocodile.
2.2. Semen collection
Semen collection were performed in all four crocodiles following physical restraint. The procedure of semen collection was slightly modified from the Australian saltwater crocodile[11]. The procedure involved restraining the crocodile on an elevated platform with the cloaca region exposed. Lubrication was applied generously to the hand of the operator especially the digits, and the crocodile’s penis was exteriorized from the cloaca. Digital manipulation of the phallus was performed at the base of the penis in order to facilitate the flow of semen to the sulcus[12]. Semen was collected in a graduated test tube and was analysed immediately for morphometric analysis and stored in 2.5% glutaraldehyde for electron microscopy.
2.3. Spermatozoa morphometry analysis
Morphology of spermatozoa was performed from stained semen samples. The morphological analysis was founded based on normal spermatozoa standard in domestic animals and crocodiles[4,11].The glass slide was prepared from a mixture of semen with eosinnigrosin stain (1:3 ratio). Thin semen film was smeared on a prewarmed glass slide and left to air-dry. Individual spermatozoa were measured from various fields for this morphometric analysis. The morphology study was performed with a light microscope attached to a high-resolution camera and manually measured with an image analyzer (NIS Elements D, Nikon Instruments Inc., America) at 1 000×magnification. Regions measured were the acrosome, head, and tail of the spermatozoa. A total of 200 spermatozoa were selected and measured from the different fields.
2.4. Scanning electron microscopy
The sample was fixed with 2.5% glutaraldehyde immediately in the field, stored at 4 ℃ and transported to the laboratory. The sample was processed by using a slightly modified scanning electron microscopy protocol[2,13]. In brief, the spermatozoa were washed with 0.1 M sodium cacodylate buffer, post-fixed with 1% osmium tetroxide, dehydrated with series of acetone concentration 35%,50%, 75%, 95%, and 100%. The sample was also subjected to critical point drying, mounted onto the stub and finally gold coated in a sputter coater. The viewing of scanning electron microscopy was performed by using a scanning electron microscope at magnification between 1 500× to 8 500× (Leo 1455 VP, Cambridge, England).
2.5. Statistical analysis
Ejaculates collected were subjected to analysis by using the statistical package IBM SPSS Statistics 20. Descriptive statistics were applied with values reported as mean±standard deviation(mean±SD).
2.6. Ethics statement
The study was conducted with the approval of Institutional Animal Care and Use Committee, Universiti Putra Malaysia (approval No.FYP- 2014/FPV.039) and a special permit from the Department of Wildlife and National Parks Peninsular Malaysia (B-00450-16-14).
3. Results
3.1. Spermatozoa morphometry analysis
The spermatozoa were made up of acrosome, head and tail which corresponded to (5.55±1.20) µm, (12.74±1.57) µm, and(70.67±4.40) µm, respectively. The percentage of length matched to acrosome, head and tail were 6.24%, 14.32% and 79.44% of the total length. The total length ofC. porosusspermatozoa was(88.96±4.60) µm. The consistency of semen and normal spermatozoa was described in Figure 1. Summary of normal spermatozoa morphometry was represented in Table 1.
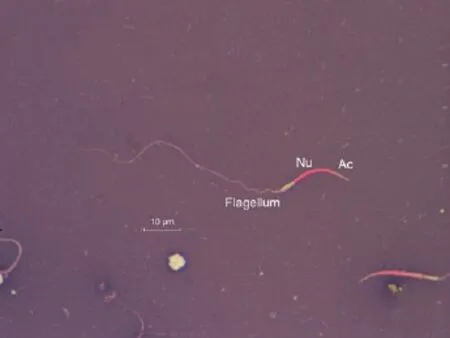
Figure 1. The normal morphology of estuarine crocodile spermatozoa stained with eosin-nigrosin is vermiform shaped with elongated nucleus part (stained in red). However, in live spermatozoa, the nucleus component will not take up the red stain.

Table 1. Spermatozoa morphometry of the acrosome, head, tail and total length of Crocodylus porosus as viewed from the electron microscope.
3.2. Scanning electron microscopy
Electron microscopy separated detailed distinction of the regions of the spermatozoa: acrosome, nucleus, midpiece, principal piece and end piece (Figure 2). During a closer examination, it was found that there was an indentation between the nucleus and midpiece which was not apparent under the light microscope (Figure 3). The most common spermatozoa abnormalities found in the samples was the cytoplasmic droplets. The surface structure of cytoplasmic droplets was smooth and lobulated with the majority of droplets localizing in the midpiece (Figure 4).
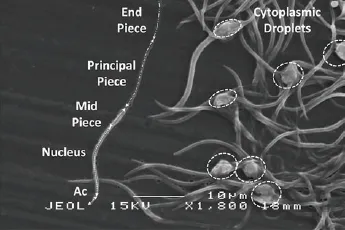
Figure 2. Detailed distinction of spermatozoa region. Acrosome (Ac),nucleus, midpiece, principal piece, and end piece can be distinguished from electron microscopy. Cytoplasmic droplets are indicated by the dotted circle..

Figure 3. Normal spermatozoa of C. porosus is divided into various segments.a: acrosome, n: nucleus, m: midpiece, p: principle piece and e: end piece.Note the indentation labeled ‘b’ visible between the nucleus and midpiece.

Figure 4. Cytoplasmic droplets appeared as an irregular and lobulated clump mostly located at the midpiece of spermatozoa.
4. Discussion
The length and morphology of spermatozoa have different functions reflecting the species-specificity and potential fertilizing ability of a breeder. The total length of the spermatozoa in crocodile is longer than mammals due to its vermiform shape and long tail.The length of the crocodile’s spermatozoa found in this study is tenfold longer than mammals[14]. This study also reported the total length of spermatozoa ofC. porosus[(88.96±4.60) µm] was longer than that of other crocodilians (20 µm)[6]. Length of spermatozoa is longer in polyandrous species due to high spermatozoa competition. Long flagella and bigger midpiece render to the swimming velocity of the spermatozoa. However, the length of flagella may not have an absolute effect on the swimming velocity,as another study suggests the importance of head to the tail ratio in determining the swimming velocity[15]. In addition, the shape of the crocodile’s spermatozoa head is also strikingly different from mammals that are paddle-shaped. The vermiform shape, however,is similar to other reptiles, amphibians, and birds[16]. In strong sperm competition, the lengths of the midpiece and flagellum are also expected to increase[10]. Thus, the longer length inC. porosusspermatozoa may suggest strong sperm competition in the species.In crocodile, other factors to take into consideration in spermatozoa competition aside from morphometry are the long spermatozoa storage ability and longevity in the female reproductive tract[17].
The main role of fertilization in crocodile includes the consideration of spermatozoa storage abilityin vivoandin vitro.The elongated shape of the crocodile spermatozoa head may result from strong sperm competition. The vermiform shape may aid in improving swimming efficiency and increasing the lifespan of the spermatozoa by using the energy reserve well[18]. Crocodile,like many reptiles, has an extended storage ability in the female reproductive tract. Study inC. porosusin Malaysia has found that even while chilled, the lifespan of spermatozoa could extend as long as 11 days[12]. Compared to mammals, the longevity in chilled extender is longer in crocodile when compared to mammals in similar egg yolk concentrations of the extender[19]. In the alligator, spermatozoa were found to survive up to seven months in the oviduct[17]. This is a significant temporal difference when compared to mammals which only survived for five days in the oviduct[20].
Spermatozoa abnormalities, especially the cytoplasmic droplets need to be investigated further in terms of fertility. High cytoplasmic droplets counts were consistent in all four crocodiles.There has been no study in the function of cytoplasmic droplets to the fertility of crocodile to date. Yet similar finding on the high count of cytoplasmic droplets was found in the spermatozoa of Australian crocodile[11]. In mammals, cytoplasmic droplets are associated with spermatozoa immaturity and infertility[21].However, the function in reptiles is still unknown. In the testudines,cytoplasmic droplets were a presence in the male reproductive tract but not after ejaculation in the female, suggesting a maturational event for the spermatozoa in the female reproductive tract[22]. It is also important to establish the presence of cytoplasmic droplets in crocodile and its relationship with fertility. Cytoplasmic droplets do not travel down the flagellum in turtle[22,23]. However, cytoplasmic droplets found in spermatozoa ofC. porosusin this study were consistent in both light and electron microscopy.
There is an apparent limitation in observing the spermatozoa under an electron microscope than to a light microscope. The obvious difference was in the indentation between the nucleus and the midpiece which was not apparent under light microscopy.The ultrastructural morphometry in this study shed light into the potential species-specificity difference in crocodilian especially on the high counts of cytoplasmic droplets. Transmission scanning electron microscopy was conducted in boa however had no mention of the presence of cytoplasmic droplets[24]. High cytoplasmic droplets counts are a unique and consistent finding in this study,which warrants further investigation in relation to fertility.
In conclusion, the total length of normal spermatozoa inC.porosusmeasured at (88.96±0.52) µm. The ultrastructural study suggests that cytoplasmic droplets are evident beyond midpiece in the flagellum. Spermatozoa length may be a good indicator of storage ability in the female reproductive tract.
Conflict of interest statement
None of the authors had a conflict of interest with respect to this manuscript.
Authors’ contributions
Wan-Nor Fitri and Dana Raj were involved with manuscript preparation. Haron Wahid designed and was mainly responsible for the main content of the manuscript. Putra Tengku Rinalfi was inlvolved in statistical analysis and discussion of the manuscript.Yawah Donny, Latip Qayyum and Ab Aziz Abdul Malek were involved in field logistics, planning and execution of the experiment.
 Asian Pacific Journal of Reproduction2020年2期
Asian Pacific Journal of Reproduction2020年2期
- Asian Pacific Journal of Reproduction的其它文章
- Erectile dysfunction and statins: The assorted view of preponderance
- Effect of routine iron supplementation on copper level and oxidative stress status in pregnant women
- Effect of short-term gavage of ethanolic extract of cogon grass (Imperata cylindrica L)root on the ovarian activity and estrus behavior of female mice
- Effect of aqueous seed extract of Mucuna pruriens on arsenic-induced testicular toxicity in mice
- Effects of ciprofloxacin on testicular tissue and sperm quality in rabbits
- Influence of N-acetylcysteine on pituitary-gonadal axis hormones and protamine expression level in streptozotocin-induced diabetic male rats
