Influence of butylated hydroxytoluene addition to cryodiluents on freezability and DNA integrity of Boer and Zaraibi buck spermatozoa
Ahmed R. M. El-Khawagah, Zaher M. Rawash, Diya A. El-Badry, Mohamed M. M. Kandiel✉
1Theriogenology Department, Faculty of Veterinary Medicine, Benha University, Egypt
2Artificial Insemination and Embryo Transfer Department, Animal Reproduction Research Institute, Agriculture Research Center, Giza, Egypt
ABSTRACT Objective: To evaluate the cryoprotective effect of butylated hydroxytoluene on buck frozen semen.Methods: Semen was collected from Boer (n=6) and Zaraibi(n=6) bucks by electroejaculator for 5 weeks. Semen aliquots were diluted at 38 ℃ in Tris-buffer with egg yolk 15.0% (vol/vol) (Trisegg yolk extender) or soya lecithin 2.5% (weight/vol) (Tris-soya lecithin extender) supplemented with butylated hydroxytoluene at 0.0 (as the control), 0.5, 1.0, 2.0 and 4.0 mM. Post-thawing motility (at 400× magnification), plasma (hypo-osmotic swelling test), acrosome (Trypan blue/Giemsa dual staining) membranes,DNA (comet assay), and lipid peroxidation (by malondialdehyde concentration) were assessed.Results: Spermatozoa motility was enhanced by butylated hydroxytoluene in Tris-soya lecithin extender at 0.5 mM in the two breeds, and in Tris-egg yolk extender at 1.0 mM in Boer and at 2.0 mM in Zaraibi bucks for up to 3 h post-thawing. Plasma and acrosome membranes and DNA integrity of the two breeds were maximally high with butylated hydroxytoluene at 1.0-2.0 mM in Tris-egg yolk extender and at 0.5-1.0 mM in Tris-soya lecithin extender. Lipid peroxidation was minimal with butylated hydroxytoluene at 1.0-2.0 mM in Tris-egg yolk and soya lecithin extenders in the two breeds. Butylated hydroxytoluene at 4.0 mM deteriorated spermatozoa motility, and plasma and acrosome membranes.Conclusions: The consequence of butylated hydroxytoluene on buck frozen-thawed spermatozoa varies with the levels of supplementation, buck breed, and phospholipid source in the extender. Semen parameters of Boer buck are better in their response to butylated hydroxytoluene than Zaraibi buck. Butylated hydroxytoluene at 1.0 and 2.0 mM in Tris-egg yolk extender,and at 0.5 mM in Tris-soya lecithin extender represents the best concentrations and profitably improves the semen quality of buck semen.
KEYWORDS: Butylated hydroxytoluene; Buck; DNA; Frozen semen; Lipid peroxidation
1. Introduction
Artificial insemination is an important achievement of the modern animal husbandry technology as it allows the use of excellent male resources, improves farm animal’s genetic characteristics and controls diseases transmission[1,2]. Semen cryopreservation of the domestic species is challenging. At least 50% of sperm viability and fertility is decreased by cryopreservation[3]. Buck sperm has been reported to be sensitive to the temperature changes due to its high level of polyunsaturated fatty acids[4]. Reactive oxygen species(ROS) concentrations increases with temperature change and these exacerbate membrane lipid peroxidation during the freezing process. The ROS are formed as a natural byproduct of normal metabolism of oxygen and have important roles in fertilization.ROS, highly reactive molecules containing free oxygen radicals,“steal” electrons from the lipids in sperm membranes, resulting in an irreversible damage to the structure and functions of the sperm cells[5]. Therefore, a suitable antioxidant is mandatory at an appropriate dose for regulation of the amount of ROS.
Egg yolk has been established as the most common component of diluents for cryopreservation purposes due to its protective effect against freezing-thawing damages[6]. However, egg yolk can reduce the fertilizing ability of sperm by increasing the risk of microbial contamination and endotoxin production[7]. In addition, the problem of using egg yolk extenders in buck semen is attributed to egg yolk coagulating enzyme which has a harmful effect to the sperm cells[8,9]. An alternative for replacement of egg yolk in semen extenders is the soybean lecithin, a natural mixture of phosphatidylcholine and several fatty acids[10]. The addition of soybean lecithin to semen extender has been reported to improve the post thawing sperm viability, motility, acrosome and plasma membrane integrities in small ruminants[6,10].
Butylated hydroxytoluene (BHT), a synthetic analogue of vitamin E, checks the auto-oxidation reaction by converting peroxy radicals to hydroperoxides. BHT penetrates sperm membrane and increases its fluidity to prevent ice crystal formation within the cell[11]. In addition, lipid solubility of BHT influences its preference over other antioxidants due to its ability to function as an antioxidant inside and outside the sperm membrane. BHT scavenges ROS from surroundings of the sperm and converts these molecules into hydroperoxides[12], thereby reducing the harmful effects of ROS on sperm cells during freeze-thaw procedures. BHT has been tested successfully to minimize cold shock damage in buck[13,14], ram[15]and bull[16] spermatozoa. In a recent study, an inclusion of BHT reduced the motility of the spermatozoa extended in diluents with no egg yolk when compared to those extended in diluents with egg yolk in buffalo bull semen[17].
The present study aimed to determine the effect of different concentrations of BHT (0.5, 1.0, 2.0 and 4.0 mM) on Boer and Zaraibi buck frozen semen extended with Tris-egg yolk or soya lecithin extenders.
2. Materials and methods
2.1. Animals
The bucks under the present study were kept under natural photoperiod and maintained using conventional feeding, housing and lighting conditions. Twelve mature, clinically healthy Zaraibi(n=6; aged 2.5-3.0 years and weighting 50-60 kg) and Boer (n=6;aged 1.5-2.5 years and weighting 80-90 kg) bucks were assigned to the study. Zaraibi bucks were kept in the experimental farm of the Animal Reproduction Research Institute while Boer bucks were reared in Jazerat Al-Shaer production farm of the Animal Production Research Institute.
2.2. Semen collection and evaluation
On a weekly collection schedule, five ejaculates were obtained from each buck. Semen collection was performed early in the morning by means of electro-ejaculator (Electrojac III®, Ideal instruments Co., USA) with small ruminant probe (diameter and length were 3.2 and 35.0 cm, respectively). Males were secured,then the rectum was cleaned of faces and the preputial area was shaved and washed with physiologic saline. The electroejaculation regime consisted of consecutive 4 series of five pulses of 13 volt,each separated by five breaks. Immediately after collection, samples were evaluated for volume, mass motility and individual motility by using a pre-warmed stage of phase contrast microscope (×400). The sperm concentration was determined by means of a hemocytometer.Only ejaculates of more than 1 mL volume, spermatozoa with >80%progressive motility, and a concentration higher than 2.0×109sperm cells/mL were used for the freezing protocol. A total number of 60 ejaculates was used in this study.
2.3. Processing of semen for cryopreservation
Each ejaculate was divided into two equal parts in two 15 mLFalcon tubes and diluted with Tris buffer at ratio of 1:5 at 38 ℃[18].Semen samples were centrifuged at 1 000×gfor 10 min to remove the seminal plasma. After centrifugation, sperm pellets were diluted in a Tris-based extender [Tris 254 mM, citric acid 78 mM, fructose 70 mM, glycerol 6% (vol/vol), pH 6.8] either containing 15% (vol/vol) egg yolk or 2.5% (weight/vol) soya lecithin in a proper dilution rate to obtain sperm cell concentration of 100×106spermatozoa per mini-straw[18]. Each diluted semen samples in the above-mentioned extenders was re-divided into five equal parts supplemented with BHT at concentrations of 0.0 mM (the control), 0.5 mM, 1.0 mM,2.0 mM and 4.0 mM and were cooled throughout 90 min to 5 ℃ in a cold cabinet. The cooled semen was packaged into 0.25 mL straws(IMV, L’Aigle, France), arranged horizontally, by using a cooled rack over liquid nitrogen vapor[19] after which the straws were dipped into liquid nitrogen for at least one week.
2.4. Evaluation of frozen-thawed spermatozoa
Frozen semen samples were thawed in water bath at 37 ℃ for 30 s. Sperm motility was subjectively assessed immediately after thawing (0 h) and at 1, 2 and 3 h post-thawing. The rate of abnormal acrosome was recorded after thawing in smears stained by a dual staining procedure[20]. The procedure described by Akhteret al[21]was used to determine the percentage of hypo-osmotic swelling positive sperm cells in each semen sample.
2.5. Determination of antioxidant status of frozen-thawed spermatozoa
Malondialdehyde (MDA) concentration, an index of lipid peroxidation, was determined according to Kumaret al[22] using Thiobarbituric acid reactive substances assay kit (Cayman Chemical Company).
2.6. Single cell gel electrophoresis (Comet assay)
The alkaline comet assay for frozen-thawed spermatozoa was carried out according to Codringtonet al[23]. 1×105sperm cells in 50 µL phosphate buffer saline (pH 7.2) mixed with 50 µL of 1.2%low melting point agarose were laid on frosted glass slides covered with 100 µL of 0.5% normal melting point agarose. The slides were lysed for 1 h in lysis buffer (2.5 M NaCl, 100 mM ethylenediaminetetraacetic acid, 10 mM Tris, 1% Triton X at a pH of 10), then incubated at 37 ℃ in 100 µL/mL of proteinase-K in lysis buffer overnight.To denature DNA, slides were placed in freshly prepared alkaline electrophoresis solution containing 300 mM NaOH and 1 mM ethylenediaminetetraacetic acid for 20 min. Electrophoresis was performed at room temperature, at 25 V (0.714 V/cm) and 300 mA for 10 min. After neutralization in 0.4 M Tris solution (pH 7), the slides were stained with 50 µL of 20 µg/mL ethidium bromide and mounted with a coverslip. A total of 200 sperm cells were examined under fluorescent microscope (×400). The intensity of the stain in the comet tail region was presumed to be related to DNA content. DNA damage was estimated from measurements of the DNA percentage in tail, tail length and Olive tail moment by using image analysis software (Comet-Score program). Spermatozoa with fragmented DNA (damaged) displayed an increased migration of the DNA from the nucleus towards the anode, while spermatozoa with non-fragmented DNA (undamaged) did not form a “comet”[24].
2.7. Statistical analysis
By using SPSS Verion16[25], data were analyzed and presented as mean±standard deviation (mean±SD) with two-way analysis of variance. Multiple comparisons of the means were done with Duncan test. The difference among breeds was obtained by independent-samplettest.α= 0.05 was considered as statistically significant level.
2.8. Ethical approval
All the procedures were accomplished according to the Ethics for Humane Treatment of Animal Use in Research Guidelines and complied with the relevant legislation of Animal Reproduction Research Institute and Faculty of Veterinary Medicine, Benha University, Egypt (Ref. No. 000R0105-2015).
3. Results
3.1. Impact of BHT supplementation in buck semen extender on post-thawing spermatozoa motility
The evaluation of frozen-thawed semen of Boer and Zaraibi buck breeds in terms of spermatozoa motility at different postthawing times (0, 1, 2 and 3 h) and different BHT concentrations(0.0-4.0 mM) in Tris-eggy yolk and Tris-soya lecithin extenders was presented in Table 1. Data verified significant differences in the post-thawing buck spermatozoa motility between breeds and BHT concentrations. More specifically, with the different times of examination of post-thawing, Boer buck spermatozoa showed higher motility rate than its contemporary of Zaraibi buck in the presence of BHT at 0.5 mM and 1.0 mM concentrations either with egg yolk or soya lecithin. In the meantime, there was a significant difference in spermatozoa motility of both breeds in relation to BHT concentrations either with egg yolk or soya lecithin at all times of examination where the post-thawing motility rate of both breeds significantly (P<0.001) improved at 0.5-2.0 mM of BHT compared to the control (0.0 mM BHT). However, high BHT (4.0 mM) negatively impacted buck semen activity along the examined time in both breeds.
3.2. Impact of BHT supplementation in buck semen extender on post-thawing spermatozoa membranes integrities and lipid peroxidation
Evaluation of frozen-thawed buck semen verified a highly significant impact of BHT level on plasma membrane and acrosome integrities, in addition to lipid peroxidation (Table 2).
In Tris-egg yolk extender, the inclusion of BHT at levels 0.5-1.0 mM improved plasma membrane integrity of Boer more obviously than that of Zaraibi semen (P<0.05). At the same time, Zaraibi bucks showed higher plasma membrane integrity than Boer bucks at 2.0 mM (P<0.05). In contrast, there were no differences between the two breeds in plasma membrane integrity in Tris-soya lecithin extender except for BHT at 0.4 mM regarding the effect of BHT supplementation level on plasma membrane integrity of buck semen.It was noticed that the highest rate of plasma membrane integrity in Boer buck was at BHT 1.0 mM (with egg yolk) and 0.5 mM (with soya lecithin), and in Zaraibi bucks at BHT 2.0 mM (in egg yolk) and 0.5 mM (in soya lecithin). The lowest plasma membrane integrity was noticed in both breeds in the two extenders at BHT 4.0 mM.
Regarding the acrosomal intactness, in Tris-egg yolk extender,Zaraibi bucks showed higher (P<0.001) acrosomal intactness than Boer bucks at BHT 2.0 mM. In the meantime, in Tris-soya lecithin extender, BHT 0.5 mM enhanced (P<0.05) Zaraibi bucks acrosomal intactness compared to Boer bucks. Meanwhile, BHT at 1.0 and 2.0 mM markedly increased acrosomal intactness of Boer bucks than Zaraibi bucks (P<0.001 for Tris-egg yolk extender andP=0.001 for Tris-soya lecithin extender, respectively). Regarding BHT levels influence on buck semen acrosomal intactness, significant differences were found between the two breeds compared to the control group (0.0 mM) in both extenders (P<0.001) except for 4.0 mM concentration. Boer buck semen showed the highest rate of acrosomal intactness at BHT 1.0 mM in both extenders, while Zaraibi buck semen expressed the highest acrosomal intactness at BHT 2.0 mM (with egg yolk) and 0.5 mM (with soya lecithin).
The level of lipid peroxidation (MDA) in frozen-thawed semen was markedly lower in Boer buck than Zaraibi buck in Tris-egg yolk extender at 2.0 mM BHT (P<0.01), and in Tris-soya lecithin extender at 0.5 mM (P<0.05), 1.0 mM (P<0.001) and 2.0 mM(P<0.05). Lipid peroxidation (MDA) was markedly decreased with BHT at concentrations of 1.0 mM in Boer and 2.0 mM in Zaraibi bucks in the presence of egg yolk (P<0.001). In the meantime, the lowest MDA levels were noticed in the semen of Boer (P<0.001)and Zaraibi (P<0.05) bucks at 2.0 mM BHT in the presence of soya lecithin.
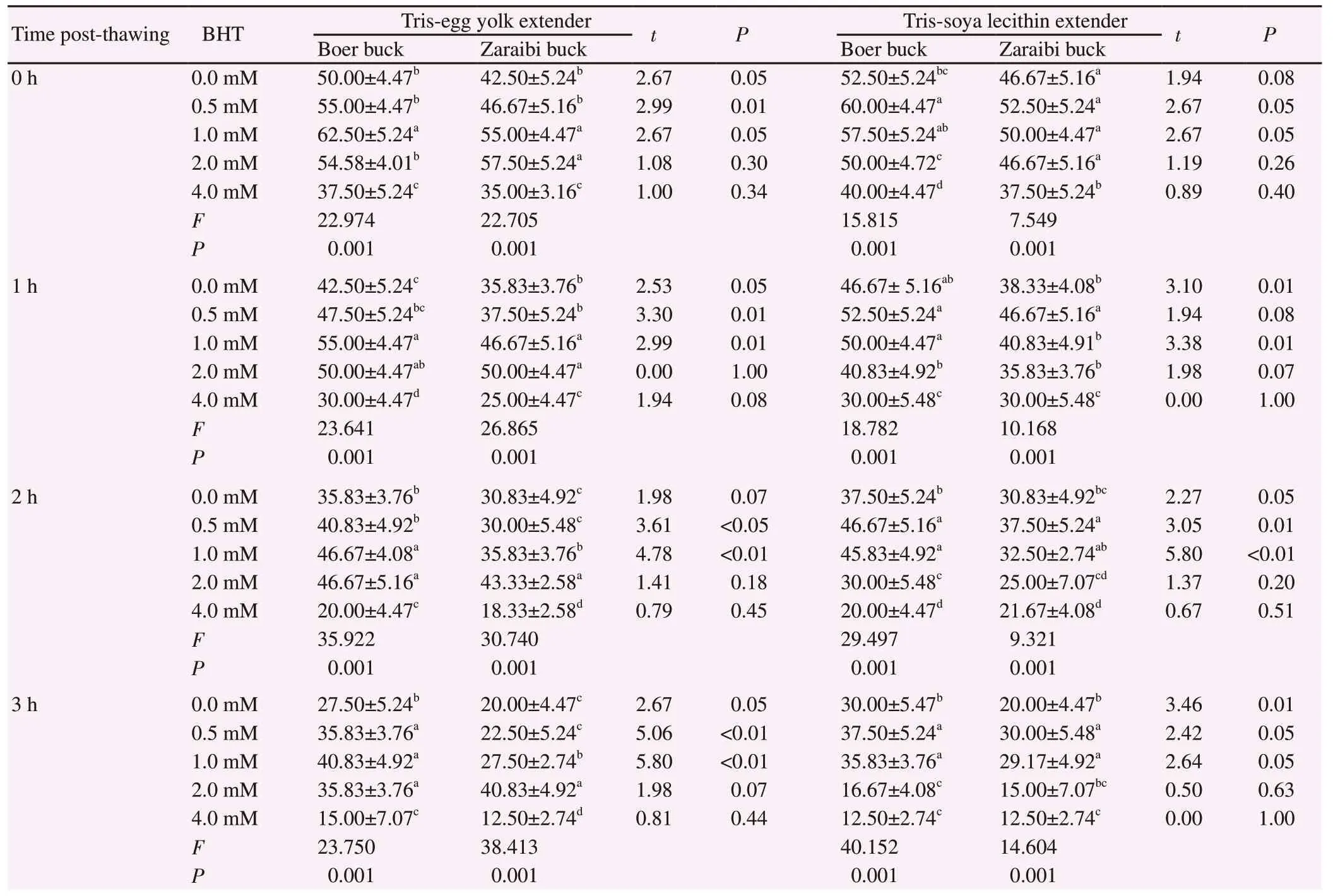
Table 1. Effect of butylated hydroxytoluene (BHT) on post-thawing motility (%) of frozen-thawed Boer and Zaraibi buck semen.

Table 2. Effect of butylated hydroxytoluene (BHT) on plasma membrane and acrosome integrities, and lipid peroxidation of frozen-thawed Boer and Zaraibi buck semen.
3.3. Impact of BHT supplementation in buck semen extender on post-thawing spermatozoa DNA integrity
Figure 1 represented photomicrographs of Boer and Zaraibi bucks’ spermatozoa examined for DNA integrity using comet assay.Table 3 presented the data analysis of comet assay (percentage of non-fragmented DNA, head DNA, tail DNA, tail length, and tail moment). Zaraibi buck semen showed higher non-fragmented DNA than Boer bucks at BHT 0.5 mM (P<0.05) in Tris-egg yolk extender, while no difference was observed between the two breeds in Tris-soya lecithin extender. Nevertheless, BHT level affected buck semen DNA integrity in both extenders. In Tris-egg yolk extender,the highest rate of spermatozoa DNA intactness was recorded in Boer buck at 2.0 mM BHT, and in Zaraibi buck at BHT 0.5 mM. In Tris-soya lecithin extender, the highest rate of spermatozoa DNA intactness was recorded in both breeds buck at 0.5 mM BHT. In Tris-egg yolk extender, Boer and Zaraibi buck semen showed highest head DNA and lowest tail DNA at BHT 2.0 mM. In the meantime, the shortest tail length and tail moment were in Boer buck at BHT 1.0 mM and in Zaraibi buck at 2.0 mM. In Tris-soya lecithin extender, the highest head DNA, lowest tail DNA and shortest tail length and tail moment were found at BHT 0.5 mM in Boer and 1.0 mM in Zaraibi buck semen.
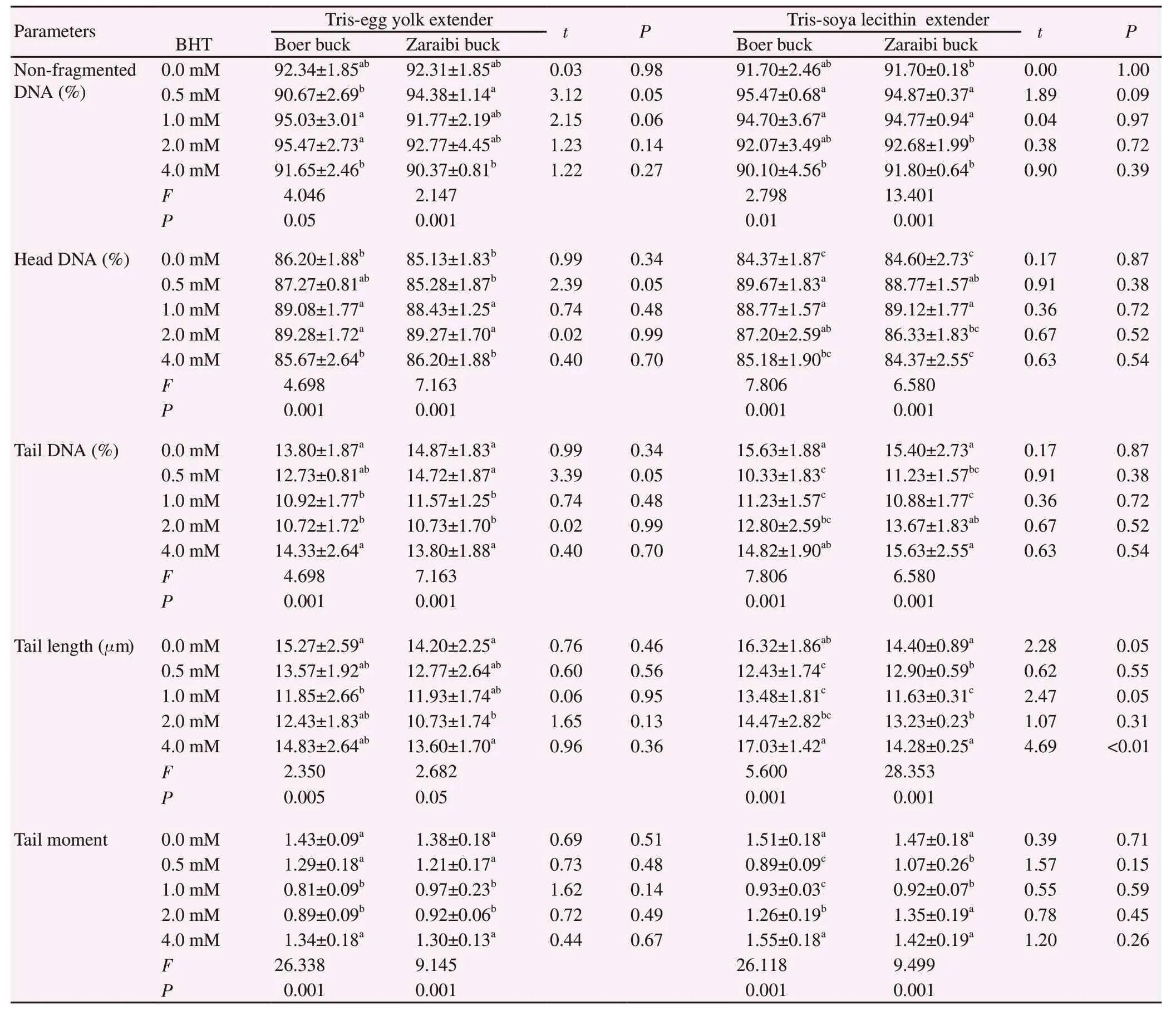
Table 3. Effect of butylated hydroxytoluene (BHT) on DNA integrity of frozen-thawed Boer and Zaraibi buck semen.

Figure 1. Representative photomicrographs demonstrating the influence of butylated hydroxytoluene addition to cryodiluents containing egg yolk (A)or soya lecithin (B) on DNA integrity of frozen-thawed Boer and Zaraibi buck semen evaluated by comet assay (single-cell gel electrophoresis). BHT:butylated hydroxytoluene; EY: egg yolk; SL: soya lecithin.
4. Discussion
Buck sperm is sensitive to the temperature fluctuations due to its high contents of polyunsaturated fatty acids[4]. Egg yolk as well as soybean lecithin are essential components of semen extenders to preserve spermatozoa against cold shock due to their high contents of phospholipids and several fatty acids[10]. From the aims of antioxidants, implementation in semen extenders is their role in minimizing ROS. However, the appropriate dose of antioxidant controls their effect in regulation of the amount of ROS. In the current study, we verified that either egg yolk or soya lecithin-based extenders when combined with proper dose of BHT sustain high semen quality post-thawing in Boer and Zaraibi buck breeds.
In the present study, the evaluation of buck frozen-thawed semen verified a highly significant impact of BHT level on spermatozoa motility. In Tris-based soya lecithin extender, the highest motility rates were recorded with BHT at concentration 0.5 mM in both buck breeds, while in Tris-based egg yolk extender, the best motility rates were accompanied to BHT concentrations at 1.0 mM in Boar and 2.0 mM in Zaraibi breeds. High concentration (4.0 mM)adversely affected spermatozoa motility in both extenders and both buck breeds. BHT is a synthetic analogue of vitamin E, as a lipid soluble antioxidant has the property of protecting the membrane lipids from chemical denaturation by ROS as well as increasing the fluidity of sperm membranes[26], thereby improving the viability of sperm by protecting the cell membrane. The positive impact of BHT on semen quality has been formerly recorded at concentration 5.0 mM in buck[13], 2.0 and 3.0 mM[15] in rams, 1.0 and 2.0 mM in buffalo[27], and at 0.5 and 1.0 mM in cattle[26]. These differences between studies and among species about the optimal BHT could be attributed to multiplex factors affecting the counteracting action of antioxidant against oxidative damage such as extender composition,dilution rate, cooling and freezing/thawing method[13], and cholesterol/phospholipid ratio in the plasma membrane[28]. In the present study, higher concentration of BHT (4.0 mM) had deleterious effect on spermatozoa motility. Similar results were also verified the toxicity of BHT for spermatozoa at higher concentrations in bull[16],human[29] and buffalo bull[17]. An inclusion of antioxidants at higher concentrations may counteract the ROS induced oxidative stress and thus hamper the ROS associated functions of spermatozoa[30].Besides, higher BHT, the more fluidity of plasma membrane beyond the desired point, making spermatozoa more prone to acrosomal damage[16].
In the present study, BHT has shown to maintain plasma and acrosomal membrane integrities after freezing and thawing at level of 1.0-2.0 mM in Tris-based egg yolk extender and at 0.5-1.0 mM in Tris-based soya lecithin extender in Boer and Zaraibi buck breeds.This activity was associated with the reduction of lipid peroxidation(malondialdehyde) at 1.0-2.0 mM of BHT (irrespective to the breed or extender type). Similar studies in rams showed that acrosomal damage was reduced in BHT spermatozoa compared to the control when exposed to cold shock[15]. It seems that freezing and thawing gamete results in inducing oxidative stress that attacks sperm plasma membrane fluidity in addition to DNA integrity[31]. The role of BHT in sustaining membrane intactness is through its integration in sperm membranes and protection of membrane damage against cold shock[13]. BHT has lipophilic properties that could control the change of peroxy radicals to hydro peroxides and reduce autooxidation. Patelet al[32] hypothesized that BHT serves as a scavenger of oxygen free radicals and minimizes damage to the sperm motility apparatus and membranes.
The foremost goal of fertilization is the successful delivery of an undamaged genetic material through the zona pellucida to the oocyte cytoplasm. Therefore, the quality of fertilization and the subsequent embryos are affected by the DNA integrity[33]. Cryopreservation causes oxygen free radical production in spermatozoa and leads to lipid peroxidation and protein alternations, causing sperm membrane and DNA damages or cell death[16]. The DNA integrity was highly improved in the current study with the addition of 1.0 and 2.0 mM of BHT in Tris-based egg yolk extender in both breeds, and at 0.5 and 1.0 mM in Tris-based soya lecithin extender in Boer and Zaraibi bucks, respectively. Former studies assessed the effect of BHT on buck sperm DNA integrity are limited. Results in bulls showed that supplementation of BHT in Bioxcell (at 0.5 mM/mL) or Tris- (at 1.0 mM/mL) and citrate- (at 1.5 mM) based extenders improved DNA integrity[34].
In conclusion, the effect of BHT on buck frozen-thawed spermatozoa characteristics varies with the levels of supplementation, buck breed and the source of phospholipid in the extender (egg yolkversussoya lecithin). In general, the response of Boer buck semen is better than that of Zaraibi bucks. The level of BHT 1.0-2.0 mM represents the best concentrations and cost-effectively upgrades the semen quality of buck semen. In egg yolk-based extender, 1.0 mM and 2.0 mM BHT are likely associated with an improved frozen semen quality in Boer and Zaraibi bucks, respectively. On the other hand, BHT at 0.5 mM in Tris-based soya lecithin extender is the optimal level in both buck breeds.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Authors’ contributions
Ahmed R.M.El-Khawagh and Mohamed M.M.Kandiel designed the work and helped in evaluating frozen-thawed semen, determining DNA damages and preparing the final manuscript. Zaher M.Rawash and Diya A.El-Badry made semen collection, processing and post thawing evaluation. All authors read and approved the final manuscript.
 Asian Pacific Journal of Reproduction2020年2期
Asian Pacific Journal of Reproduction2020年2期
- Asian Pacific Journal of Reproduction的其它文章
- Erectile dysfunction and statins: The assorted view of preponderance
- Effect of routine iron supplementation on copper level and oxidative stress status in pregnant women
- Effect of short-term gavage of ethanolic extract of cogon grass (Imperata cylindrica L)root on the ovarian activity and estrus behavior of female mice
- Effect of aqueous seed extract of Mucuna pruriens on arsenic-induced testicular toxicity in mice
- Effects of ciprofloxacin on testicular tissue and sperm quality in rabbits
- Influence of N-acetylcysteine on pituitary-gonadal axis hormones and protamine expression level in streptozotocin-induced diabetic male rats
