Dengue virus infections and anti-dengue virus activities of Andrographis paniculata
Mohamed Ali-Seyed, Kavitha Vijayaraghavan
1Department of Clinical Biochemistry, Faculty of Medicine, University of Tabuk, Tabuk, Saudi Arabia, Tabuk 71491, Saudi Arabia
2Department of Chemical Engineering, Agni College of Technology, Chennai, Tamil Nadu, India
ABSTRACT Dengue is a debilitating disease that poses a perpetual threat to human health and increases the global economic burden every year.Despite advances in medical sciences, dengue virus (DENV)infects approximately 200 million people every year.To date, no effective antiviral is valiable to treat DENV in individuals despite great efforts in accomplishing these goals.Numerous approaches have been used in the search for dengue antiviral like screening of combinatorial compounds against DENV enzymes and structurebased computational discovery.In recent years, investigators have turned their focus into medicinal plants, trying to identify compounds that can be used as dengue antiviral.Nature represents a great reservoir of potential substances that can be explored with the aim of discovering new drugs that can be either used directly as pharmaceuticals or can provide drug leads, which can be scrutinized further for the development of new anti-dengue natural product.Many previous investigations have dealt with numerous plant extracts or bioactive principles for their antiviral property as they normally considered being safer when compared to synthetic drugs.Andrographis paniculata belongs to family Acanthaceae and is generally known as ‘king of bitters’.Diverse bioactive compounds from this plant such as diterpenes, flavonoids,xanthones, noriridoides and other miscellaneous compounds have exhibited their potential as therapeutics for various chronic as well as infectious diseases.This review is based on literature review on scientific journals, books and electronic sources, which highlights the pathogenesis of DENV and describe an assortment of bioactive principles that have been possessing antiviral potential, which include dengue and discuss the therapeutic efficacy and mechanism of action of Andrographis paniculata.However, a detailed and more comprehensive clinical trial on mammalian tissues and organs is needed in future studies.
KEYWORDS: Andrographis paniculata; Dengue; Phytochemicals;Natural products; Andrographolide
1.Introduction
The emergence of microbial infectious diseases constantly threatens the humankind[1].Viruses, with few base pairs of genetic material have the ability to enter inside the cell and nuclear level of the human cells owning a few million base pairs of the genome and can influence them at the molecular level[2] and responsible for host cell lysis.Viral outbreaks in endemic areas trigger panic among the world population because of the upsetting surge in infection spread due to the reduced traveling speed and many other multifactorial facts exploiting the host defenselessness[3].Previous investigations have well demonstrated that infectious disease transmission patterns always continued to change because of various human activities including travel mostly by humans’ contribution to changing patterns of risks[4].Other than the above, it is important to identify other factors, which may contribute to disease spread among species, and viral duplication mechanisms in new variants at basic science level to precisely evaluate the risk of a newly identified viral strain spread to the human subjects[4].
In the last two decades, investigators have shown tremendous interests towards virus causing unexpected illness and epidemics among humans, wildlife and livestock and their changing patterns of infectious diseases[5].These outbreaks often have seriously stretched both local and national resources at a time when healthcare spending in the economically developed world has been constrained.On the other hand, methods to control the viral spread is inadequate or undeveloped and remain poor in some regionswhere many of these diseases have their origin, e.g.Ebola outbreak in a few African countries including Democratic Republic of Gongo[6].
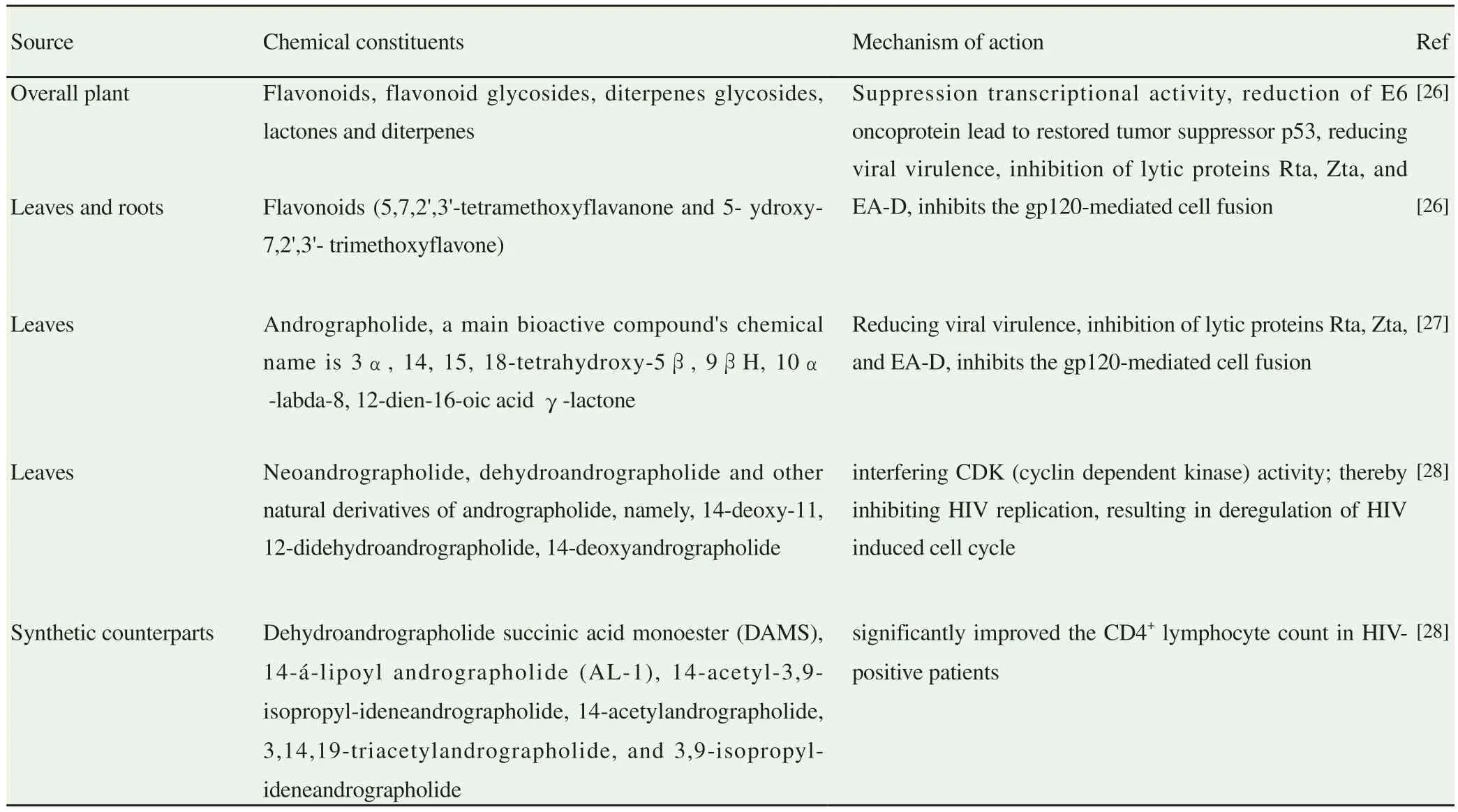
Table 1.Source and antiviral potentials of important chemical constituents of Andrographis paniculata.
Despite the progress made in immunization and drug development,many viruses lack preventive vaccines and efficient antiviral therapies, which resulted in the generation of viral escape mutants[7].Therefore, identification of new potential antiviral drugs is crucial.In this regard, plants and phytochemicals are an excellent source for such discoveries[8].In recent years, medicinal plants were chosen as imminent origin for the development of new drugs to treat various diseases caused by viruses.Phytochemicals provide a rare practice for the discovery of antiviral drugs with significant pharmacological outcomes[9].Till date, limited numbers of anti-viral drugs are available for effective treatment, yet many of them pose viral resistance and produce side effects when the drug is consumed for longer period of time[10].
Herbals used by traditional medicine practitioners for various human ailments play a vital role and their need continues to increase in the coming years[11].According to World Health Organization’s(WHO) recent estimation, approximately 2 500 medicinal plant species[12] are available globally to treat an innumerable number of ailments and diseases[13].Native plants spread throughout tropical Asian countries including India, China, Indonesia, Malaysia, and Sri Lanka, as they are a rich depository of medicinal plants[14].Having close life existence with the nature and herbal plants, a vast majority of world-wide population still depend on these herbs and learn to utilize them for their immediate health care needs[15].These remedial plants yield a wide variety of secondary natural products(nearly around 100 000) and are classified based on their structural compositions and their synthesis pathways[16].
Natural product research is always viable to explore, extract and establish their therapeutic properties[7].With the advent of many new as well as improved advance, technologies including genetically engineering approaches help to elucidate the complexity of plantderived natural products in terms of structural and functional features[17].Currently, 25% of herbal-derived prescribed drugs are being used, and among those, many are anticancer and anti-infective in nature[18].In recent years, these findings place herbal plants in greater position, for they are the source of bioactive compounds which will be utilized for the development of new drugs against various viruses.In fact, phytochemicals provide a unique and innovative way to find various new antiviral drugs with substantial outcomes[9].However, these new natural products and their possible drug leads with well-deciphered mechanisms of action are important for the development of medicine to avoid any existing therapeutic difficulties.
Andrographis (A.) paniculata (Burm.f.) Nees is a common medicinal plant (Table 1), which is predominantly found as isolated patches in most of the tropical Asian countries[19-23].Numerous beneficial effects of A.paniculata described in various reports, are involved in the treatment of hypertension, cardioprotective, anti-cancer,anti-inflammatory and antioxidant action[24].It also used to treat gastrointestinal tract, upper respiratory and herpes infections, sore throat, hepatitis and a variety of other chronic metabolic diseases like obesity, diabetes[25].
Many potential bioactives from A.paniculata have been reported,including flavonoids, glycosides, lactones and diterpenes[26].Among these compounds, flavonoids were prominent in most parts of plants including leaves.The following two flavonoids such as 5,7,2’,3’-tetramethoxyflavanone and 5-hydroxy-7,2’,3’- trimethoxyflavone were principal ones.Gorter et al.[27] were the first to isolate a bitter colorless bioactive principle known as andrographolide from A.paniculata.The chemical name of andrographolide is 3α, 14, 15,18-tetrahydroxy-5β, 9βH, 10α-labda-8, 12-dien-16-oic acid γ-lactone.
In recent years, many studies have investigated substantial anti-viral potentials of A.paniculata[28,29].These studies have identified various anti-viral bioactive compounds such as polyphenols, alkaloids and flavonoids, saponins, quinones, terpenes,proanthocyanidins, lignins, tannins, polysaccharides, steroids,thiosulfonates and coumarinsfor nearly 23 viral induced ailments[30].These compounds have inhibited viral entry into host cells by various mechanisms[31].These reports highlighted the therapeutic efficacy and action mechanism of A.paniculata against various viral infections including dengue.Hence the present review discusses the antiviral potency of A.paniculata derived natural products and their roles in inhibiting the viral replication with special focus on dengue virus and proposes A.paniculata derived bioactive agents as an important antiviral herbal medicine.
2.Dengue virus (DENV)
2.1.Description of DENV
DENV, a positive single strand RNA containing enveloped virus,belongs to flaviviridae family and genus of Flavivirus.The DENV virion typically presents as 40-50 nm spherical particle with a genomic core containing single-strand positive-sense RNA (@11kb in length) covered by a glycoprotein envelope.Dengue is a fast and widespread mosquito-borne viral disease, typically, by mosquitoes(Aedes aegypti and Aedes albopictus)[32] and the viral infection has been reported as the second commonest cause for hospitalization of tropical travelers in Europe next to malaria[33].
DENV has the following structures such as capsid, membrane, and envelope as well as seven non-structural proteins which are identified as NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5[34].Simmonds et al.[35] have briefly explained the details of the current International Centre for Taxonomy of Viruses (ICTV 2017) classification of DENV and the viral proteins replication.Normally, the viral replication occurs in the hosts cytoplasm with the involvement of both viral and host cellular proteases cleavage.The viral protein is formed initially as a polyprotein after the translation of viral genomic RNA[36].
The replication process follows a complicated topology by placing the different proteins at a different level around the endoplasmic reticulum (ER)[34].During the replication and assembly, the proteins of capsid (C), NS3 and NS5 remain in cytoplasm, the NS1,membrane and envelope proteins move towards the tubules of the ER; whereas NS2A and B, NS4A and B stay inside the plasma membrane of the ER[37].The various spatial presentation effectively guides the successful assembly of the virus by strongly influencing the host.The virions are transported as cytoplasmic vesicles and exported via exocytosis[35].
52. Then Hansel sprang like a bird out of a cage when the door is opened: Here we have more bird imagery with this simile123 describing Hansel s release from the stable.
According to WHO, four different kinds of dengue viruses are listed: they are DENV1-4.Among those, DENV-2 serotype is the most prevalent and continues to be a threat to society[38].All four serotypes were reported to be emerging from the sylvatic strains from Southeast Asian forests[39].The environmental condition in tropical countries like Southeast Asian region is an important factor in the increased prevalence and incidence of outbreaks.The emergence of new fifth type threatens the future[40].DENV-2 is one of the highly virulent serotypes[41].
2.2.Classical dengue fever and its outbreak
DENV infects 200 million people per year and disables 3.6 billion people approximately.The recounted threat to the public never declines in spite of advances in medical therapeutic science[42].The endemic area for DENV has stretched over 128 countries.DENV-2 appearance was noticed in many countries like Malaysia, Indonesia,and Thailand in 1960, and ten years later (1970) in China, Srilanka,India, and Singapore[43].Philippines and Thailand reported the first cases of DENV-3 and DENV-4; and thereafter, it is reported every year in the Asian countries including Thailand, Malaysia, Indonesia,China, Cambodia and Singapore[43].It is noteworthy.DENV is known as arbovirus in Southeast Asian countries.The co-circulation of all the DENV types in endemic areas were increasingly reported even though the reports were inconsistent due to restricted supply of specific diagnostic aids.
Currently, co-infections with different serotypes of DENV has become an alarming threat in hyper-endemic countries including Southeast Asia, India, and China[41].The virus infects the host cell by adsorption mechanism by binding to receptors and penetrating plasma membrane to uncoat the viral genetic material.Integrated episomic genetic element then interferes various processes including transcription, replication, translation, assembly respectively and followed by the release of material upon host cell lysis.
Although there are four distinct types of verified dengue serotypes imposing enormous research works to achieve the control, but emergences of new fifth type threaten the future[40].However,currently, DENV-2 is one of the highly virulent and predominant serotypes compared to all the other four-dengue types[41].Clinical signs of DENV infection consist of apparent/mild feverish presentation, as well as other symptoms like skin rash, myalgias,headache, vomiting, nausea and joint pains and deadly hemorrhagic condition.Additionally, severe dengue cases may have hemorrhagic dengue shock syndrome (DHF/DSS)[44].
DENV largely targets and causes maximum damages to blood vessels mainly on the vascular endothelial cells and its supporting structures[45].The crucial issues of severe DENV infection are due to the dysregulated immune response leading to extensive bleeding resulting with platelet reduction and hypovolemia, which was not responding to the fluid and platelet replacement rather worsening the situation[45].Treatment outcomes with corticosteroids to modulate the immune system were inconclusive and not widely accepted[46].Intravenous immunoglobulin intervention suggested for a clinical trial with limited evidences[47].A tetravalent vaccine against all the serotypes of DENV is still under elaboration and need worldwide approval for its clinical use[48].In line with this, a recent study by Biswas et al.[49] have demonstrated that the early results of an ongoing phase 3 randomized trial of a tetravalent dengue vaccine candidate (TAK-003) in regions of Asia and Latin America in which the disease is endemic have shown promise achieving an overall vaccine efficacy of 80.9% (95% CI, 75.2% to 85.3%; 78 cases per 13 380 (0.5 per 100 person-years) in the safety.
2.3.DENV pathophysiology
Despite the urgency regarding the control and treatment of DENV infections, the pathogenesis remains insubstantial[50].Currently there are no effective antiviral therapeutic as well as vaccines available or under clinical trials to halt or eliminate the viral spread.DENV infected host does develop lifetime immunity but it is restricted to the particular serotype.Consecutive DENV infection with another serotype might end with the worst situation due to original antigenic sin, which was due to the cross-reaction of the antibodies developed for the first infection binding ineffectively with the antigens of new infections and leaving them uninhibited[51].
Emerging clinical reports alarm the troubles of post-infection complications and co-infections, and co-morbidities accumulating the fatalities[55].There are many fatal infections leading to postinfection complications including DENV and lack of enough renewal cells during repairment due to the extensive involvement[56].Furthermore, other fatal complications such as dengue hemorrhagic fever and dengue shock syndrome are also enumerated[57].Currently suggested DENV treatment options are immunomodulatory and immunosuppressive medicines, however, these suggested drugs do possess strong contraindication during viral infections[58].
2.4.Dengue fever control measures
In recent years, dengue is a fast emerging pandemic viral disease worldwide and its incidence is alarmingly increasing both urban and semi-urban residential areas.There is an alarming 30-fold increase of dengue cases which results not only financial burden but leads to huge health care fund allocation every year[59].The affected country and regional public health administration face huge challenges to curb dengue disease and find out some effective control methods.In general, there are three solutions to prevent and control dengue disease and its epidemic: (a) to inhibit the action of DENV inside the human body by inducing specific immunization by the administration of the vaccine (b) by eliminating the infectious chains by vector control and (c) by epidemiological surveillance at the early stages in both probable affected and new areas.
Although mosquito control measures are paramount protective practice, yet, successful implementation and control measures maintaining face many practical difficulties[60].Since all these approaches have their own independent merits, suggesting their implementation is highly required to find their effectiveness.Until now, there is no accepted treatment with synthetic or natural medicines, however, they are widely accepted because of costeffective for prophylaxis and further treatments.Thus, there remains a clear need for novel treatment strategies aiming at developing safe anti-dengue drugs for future treatment.
However, on the positive note, the continued search for new natural compounds with appropriate validation process is required to reveal new structure-activity relationships.These approaches help to develop many new drug leads like glycyrrhetinic acid for human papilloma virus (HBV), acetoxime as an inhibitor of herpes simplex virus (HSV-1) and caffeic acid as an influenza NA antagonist[61].The finding of drug leads like punicalagin and chebulagic acid has the capacity of blocking the entry of many viruses because of their GAG-competing ability, which could aid to develop multi-spectrum antivirals for the control and prevention of many viruses[7].
3.Beneficial attributes of A.paniculata
3.1.Antiviral potentials of A.paniculata
In recent years, many investigations have reported substantial anti-viral potentials of A.paniculata besides its spectrum of other pharmacological activities.However, most of these studies have reported A.paniculata’s antiviral potential only against few selected viruses like influenza A,HIV antigen-positive H9 cells[62,63], herpes simplex type 1, flaviviruses,pestiviruses[64] and DENV-1[65].Besides the above, A.paniculata reduced viral virulence and protected from the life-threatening ability of human papilloma virus type 16 (HPV16) by suppressing its transcriptional activity and reduction of E6 oncoprotein expression in the host[63].Moreover, A.paniculata’s potential bioactive compound, andrographolide effectively inhibited the expression of various Epstein-Barr viral lytic proteins such as Rta, Zta, and EA-D in P3HR1 cells.To support further, Uttekar et al.[66] demonstrated that this compound and its by-products inhibited the gp120-mediated cell fusion of HL2/3 cells with TZM-bl cells signifying that andrographolide might be ideal candidates for better prevention and treatment of HIV infection.
3.2.Anti-platelet activity
It is well established that in DENV infected patients, platelets are one of the most vulnerable cell population by direct and/or indirect mechanisms of infection; however, the exact mechanism triggering platelet reduction is yet to be elucidatedin detail[67].It is common to observe both thrombocytopenia and platelet dysfunction in dengue,both strongly related to the clinical outcome[67].Thus, platelets are frequently affected in dengue, either for alteration of their own functionality, for “silent transport” of virus, or as an anti-viral immune cell[68].
Phytoconstituents extracted from A.paniculata have exhibited various anti-platelet activities by modulating platelet activating factor through various signaling pathways like eNOS-NO/cyclic-GMP PLCγ2-PKC and PI3 kinase/Akt-MAPKs[69].One of the active phytocompounds of A.paniculata diterpenoid, especifically 14-deoxy-11,12-didehydroandrographolide exhibits dose and time-dependent inhibitory action on thrombin-induced platelet aggregation[70], whereas, neoandrographolide exhibits none.The observed results suggest that the other new compounds like two flavanoids such as andropaniculosin A and ropaniculoside A present in the extracts may be responsible and attributed for high antiplatelet activity[71].To support further, a study by Amroyan et al.[72]has shown that andrographolide inhibits PAF-induced human blood platelet aggregation in a dose-dependent manner.However, study results suggest that the mechanism of action of andrographolide is entirely different from a known NSAIDs action.The andrograpahlide actions are most probably related to cardiovascular and antithrombotic activity described elsewhere for A.paniculata
3.3.Anti-dengue activity
Methanol extracts of A.paniculata[65] exhibit anti-dengue activity on DENV-1.The study has shown that the extracts have the ability to inhibit the viral activity of DENV-1.It is very interesting to note that the treatment of Nilavembu Kudineer and Adathodai Manapagu combination to twenty DENV affected patients for the scheduled period of 7 d showed substantial symptomatic relief and improvement in the management of dengue fever.It is very important to note that the crude extract of A.paniculata has shown anti-larval activity for many insects including mosquito species like Culex quinquefasciatus[73,74] and Aedes aegypti[75].The following main phytochemical compound bicyclic diterpenoid lactone and some constituents of diterpenoids like andrographolide,neoandrographolide (NAND), 14-deoxy andrographolide (DAND),and 14-deoxy-11, 12-didehydroandro-grapholide (DDAND)[46] have been identified and they are responsible for larvicidal, anti-microbial and anti-inflammatory activities[76].Since andrographolides are not soluble in water, they have some limitations and cannot be directly used as a healing agent[77].
Many in vitro and in vivo investigations are conducted with plantderived bioactive principles against dengue viruses[76,78,79].In this regard, various flavonoid compounds from ethanol/methanol derived extract of A.paniculata have been described that they are possessing antiviral properties including dengue with 75% of inhibition[78].The antiviral activity might be due to the presence of the following natural compounds such as terpenes, polyphenols,and flavonoid of ethanol extracts.Although flavonoid compounds are the main constituent of interest in search of an anti-dengue agent, the other potential compounds like diterpenes which include andrographolide, 14-deoxy andrographolide, and 14-deoxy-11, 12-didehydroandrographolide should not be neglected in future clinical trials to decipher their anti-dengue mechanism of action as these two compounds have exhibited their anti-HIV activity in various investigations[79].
In summary, based on the available evidence, the exact antiviral mechanisms of action of andrographolide and other bioactive compounds are yet to be explored in detail.However, existing data suggest host cell proteins such as actin and NF-κB are the direct targets of andrographolide and may thus exert its effect through these proteins as proposed[80].In addition, andrographolide modulates many molecular and cellular mechanisms including oxidative stress[81], autophagy[82], mitochondrial function[83] and the unfolded protein response pathway[84].Thus, the compound andrographolide and other derivatives from A.paniculata exert their action on multiple pathways within the cell causing various antiviral effects.To explore further, the anti-DENV efficacy, especially the detailed mechanism of action of neoandrographolide, dehydroandrographolide and other derivatives of andrographolide, namely, 14-deoxy-11,12-didehydroandrographolide, 14-deoxy andrographolide and their synthetic counterparts such as dehydroandrographolide succinic acid monoester (DAMS), 14-á-lipoyl andrographolide (AL-1), 14-acetyl-3,9-isopropyl-ideneandrographolide, 14-acetylandrographolide,3,14,19-triacetylandrographolide, and 3,9-isopropylideneandrographolide need to be investigated by employing multiple advanced technical approaches.
DENV, one of the tribulations of the world, has no specific treatment with antivirals and the clinical trials with the tetravalent vaccines have been reported with limited results.With the available encouraging reports, this review highlights and recommends A.paniculata as an antiviral therapy for DENV infection, which could potentially open a novel scientific area to embark its antiviral potentials.Yet, some limitations still exist like post-infection challenges, which need to be rigorously subjected to various preclinical and clinical trials by investigating at the different period of its lifetime to verify the permanent damages induced by DENV.A.paniculata potency assays need to be standardized and experimented to predict the therapeutic efficient dosage as mentioned in earlier studies.
 Asian Pacific Journal of Tropical Medicine2020年2期
Asian Pacific Journal of Tropical Medicine2020年2期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Human leukocyte antigen class-Ⅱ DRB1 alleles and Giardia lamblia infection in children: A case-control study
- A PCR and RFLP-based molecular diagnostic algorithm for visceral leishmaniasis
- In vitro biological activities of aqueous extracts of Tetrapleura tetraptera (Schumach.& Thonn.) taub.and Aframomum citratum (C.Pereira) K.Schum from three Agroecologic Zones in Cameroon
- Predicting the number of visceral leishmaniasis cases in Kashgar, Xinjiang, China using the ARIMA-EGARCH model
- Co-existence of renal hydatid cyst and renal cell carcinoma in one kidney: A case report
- First evidence of Bartonella phoceensis and Candidatus Mycoplasma haemomuris subsp.ratti in synanthropic rodents in Malaysia
