Novel genetic variants of transferrin receptor 2 exon 4 and cytokines profile of anemic and nonanemic pregnant women in Central Java, Indonesia
Dono Indarto, Budiyanti Wiboworini, Amelya A. Ayusari, Arisanty N. Restuti, Isnar N. Alfiyah,Aniki Puspita, Yohanes C. Wibowo, Yoga M. Pratama
1Department of Physiology, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia
2Postgraduate Program of Nutrition Sciences, Universitas Sebelas Maret, Surakarta, Indonesia
3Department of Nutrition Sciences, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia
4Biomedical Laboratory, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia
5Health Program, State Polytechnics of Jember, East Java, Indonesia
6Department of Internal Medicine, Faculty of Medicine, Jenderal Soedirman University, Purwokerto, Indonesia
ABSTRACT Objective: To assess the association between genetic variants of transferrin receptor 2 (TFR2) exon 4 and anemia status and to describe the expression levels of several cytokines, hepcidin, soluble transferrin receptor and erythropoietin.Methods: Institutional based comparative study was done randomly to recruit 106 pregnant women who attended antenatal care in three different health centers in Boyolali Regency, Central Java from May 2015 to September 2015. DNA was extracted from peripheral blood samples of selected pregnant women and sequencing was done for TFR2 exon 4. Furthermore, enzyme-linked immunosorbent assay was conducted to measure the expression levels of interleukin 6,interleukin 4, transforming growth factor β and iron-metabolism related proteins such as hepcidin, soluble transferrin receptor,and erythropoietin. Gene alignment was performed by using a CLUSTAL W program. Collected data were analyzed statistically by using parametric and nonparametric tests with Statistical Product and Service Solutions (SPSS) 20.0 for Windows.Results: Three novel genetic variants from TFR2 exon 4 (position 603, 605 and 606) were associated with anemia status. Moreover,the expression levels of interleukin 6, interleukin 4, transforming growth factor β and erythropoietin were higher in anemic pregnant women than those of nonanemic pregnant women but only erythropoietin level reached statistical significance. These results were followed by decreases of hepcidin and soluble transferrin receptor levels.Conclusions: Various factors contribute to anemia prevalence among pregnant women in Boyoali Regency, Central Java,Indonesia. Our novel findings showed that TFR2 exon 4 has 3 mutational sites in position 603, 605 and 606. These novel genetic variants may provide a new insight into the role of TFR2 in anemia.
KEYWORDS: Cytokines; Iron deficiency anemia; Pregnancy;Transferrin receptor 2 mutation
1. Introduction
Nutritional status during pregnancy plays a vital role in maternal and fetal outcomes[1]. In developing countries, undernutrition often results in adverse conditions during pregnancy[2]. It has publicly been reported that this condition can increase the risk of some causative factors of higher maternal mortality rate such as anemia,postpartum hemorrhage and hypertension[1].
Nutritional iron deficiency anemia is a major disorder that occurs in 56% of pregnant women in developing countries including Indonesia[3]. Even though iron supplementation has widely been implemented in anemic pregnant women, it showed that the iron supplementation program was less effective due to different individual responses. For example, data from the Indonesian Ministry of Health show that the national coverage for iron supplementation has recently reached 73.2%, lower than the national coverage for iron supplementation in 2013. Furthermore,this decreased achievement is followed by the increased anemia prevalence among pregnant women[4].
Some possible factors that can influence the effectiveness of iron supplementation have been reported. Firstly, the adverse effects of iron supplementation such as nausea and vomitus may cause decreased iron absorption[5]. Secondly, genetic variations may also influence iron metabolism such as transmembrane serine protease 6 gene polymorphism[6].
A previous study suggested that lack of matriptase-2 activity might cause the accumulation of hemojuvelin in the cell membrane of hepatocytes, which modulated the bone morphogenetic protein(BMP)- Son of Mothers Against Decapentaplegic (SMAD) signaling pathway[7]. Furthermore, it can upregulate the hepcidin transcription,which is the main regulator of iron homeostasis, and decline iron efflux via ferroportin[8]. Besides the BMP-SMAD signaling pathway,hepcidin expression is also regulated by transferrin receptor 2(TFR2) and interleukin (IL)6[8]. The TFR2 receptor has an important role in sensing plasma iron by interaction with different transferrin,hemojuvelin and hemochromatosis-associated molecule, leading to the induction of hepcidin expression[9]. Moreover, co-expression of TFR2 and erythropoietin (EPO) receptor enhances EPO sensitivity and maturation of erythroid cells[10]. Several studies have revealed that mutated exon 4 of TFR2 gene can cause hereditary iron overload(hemochromatosis)[11-13] but genetic variants of this gene in anemic pregnant women have not been documented yet. Therefore,this study aimed to investigate the genetic variation of exon 4 in TFR2 gene and its association with anemia in Indonesian pregnant women. The study also investigated other parameters such as several cytokines, hepcidin, soluble transferrin receptor (sTFR), and EPO serum levels.
2. Materials and methods
2.1. Study design, area, and period
The institutional-based comparative study was conducted in three different health centers (Ampel, Klego and Nogosari Primary Health Centers), Boyolali Regency, Central Java Province, Indonesia from May to September 2015. Boyolali Regency was located in the south eastern part of Central Java Province and approximately 82.2 and 29.4 km distances from to the nearest two major cities in Central Java, Semarang and Surakarta respectively. The population was dominated by Java ethnics and the majority of Boyolali people worked in agriculture, textile industry, and cattle farming for milk production. Many areas in Boyolali regency were rural and undeveloped, including three health centers used in this present study.
2.2. Eligibility criteria
2.2.1. Inclusion criteria
Study participants were eligible to participate in this study if they aged 18-36 years old and had 10-32 gestational weeks, and hemoglobin (Hb) 7<Hb<11 g/dL for anemia or Hb ≥11 g/dL for normal.
2.2.2. Exclusion criteria
Study participants who had a history of chronic and hematological diseases and severe anemia (Hb ≤ 7 g/dL) and those who refused to give consent were excluded.
2.2.3. Sample size and data collection methods
Based on Yamane[14] formula for sample size, 109 study participants were needed as minimum samples. A total of 113 pregnant women who attended antenatal care at three health centers were recruited as study participants at the end of September 2015.
Sample size formula:
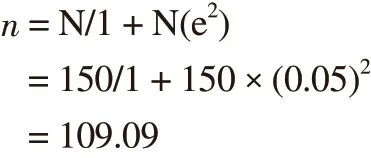
n = sample size; N = study population (150, in three health centers according to the preliminary study); e = allowed margin of error(0.05).
But one day the Princess, wandering sadly by the river, spied Prince Featherhead fast asleep in the shade of a tree, and stole nearer to enjoy the delight of gazing at his dear face unobserved
A purposive sampling method was used to select study participants,according to our inclusion and exclusion criteria during the data collection period.
2.3. Ethical clearance
The protocol of this study was approved by the Ethical Committee of the Faculty of Medicine, Universitas Sebelas Maret (UNS)/Dr.Moewardi Hospital, Surakarta (No: 278/IV/HREC/2016). Further additional permissions were also obtained from the administrative office of three respective health centers. Moreover, before commencing this study, informed consent was taken from all study participants and collected data were kept confidentially.
2.4. Data collection
Pregnant women who attended antenatal care in three health centers and fulfilled inclusion and exclusion criteria during five months,from May to September 2015 were included in this study. Personal and clinical data of study participants were carefully recorded and stored. All study participants were treated by clinicians according to the national standard protocol. Blood samples were then drawn for a routine laboratory test of antenatal care procedure in Indonesia.A total of 5 mL venous blood was taken and was collected in a vacutainer containing ethylenediaminetetraacetic acid and kept in a cool box. Hemoglobin level was measured directly by using a routine method for hematological analysis (cyanmethemoglobin method). Each 20 μL blood sample from study participants was added to tubes containing 50 mL Drabkin’s reagents. Afterward,it spectrophotometrically read the absorbance at 540 nm. The remaining blood samples were stored in the fridge for further analysis of cytokines, proteins-related to iron metabolism and molecular study.
2.5. Measurement of cytokines and iron metabolism indicators
The protein expression of hepcidin, sTFR, IL6 and EPO was determined by using enzyme-linked immunosorbent assay.Measurements of hepcidin and sTFR (Elabscience, Wuhan, China),IL6 (Sigma-Aldrich, St. Louis, MO, USA), IL4, transforming growth factor β (TGFβ) and EPO serum levels (Biolegend; San Diego, CA,USA), were performed by using manufacturer’s protocols.
2.6. Molecular studies
We prepared 34 blood samples from 11 anemic and 23 nonanemic pregnant women. Total DNA was extracted from 200 μL blood samples by using TIANamp Genomic DNA Kit(Tiangen Biotech, Beijing, China). For amplification of exon 4 in TFR2 gene, the primer design was based on a published study(forward - 5’ TCTGGCATCCTTCCCTCTTC 3’ and reverse -5’ ACGGATCCGGGAATTGCAG 3’)[15]. The gene target was amplified by using polymerase chain reaction which was initiated by denaturation at 95 ℃ for 5 min and followed by 35 polymerase chain reaction cycles: denaturation at 95 ℃ for 1 min, annealing at 57 ℃ for 1 min and elongation at 57 ℃ for 1 min. Therefore,amplified DNA products were confirmed by 2.5% gel agarose. DNA sequencing was then performed by using a Big Dye Terminator Cycle Sequencing (Applied Biosystems, CA, USA). CLUSTAL W (version 1.4) was performed for multiple alignments with the reference sequence (accession number: NG_007989).
2.7. Statistical analysis
All data were presented as mean±standard deviation (mean±SD)and were analyzed by using SPSS 20.0 for Windows (SPSS,Inc., Chicago, IL, USA). Normality and homogeneity tests were performed to determine whether the data were analyzed using parametric or non parametric test. The Student’s t-test was used to analyze parametric data and the Mann Whitney test was used for the analysis of nonparametric data. The Spearman correlation test was also conducted to determine the association between two variables.The significant value was set up at P less than 0.05.
3. Results
3.1. Socio-demographic characteristics
A total of 113 study participants joined to this study but 7 study participants were excluded due to incomplete data (Table 1). Anemia was found in 27(25.47%) pregnant women. The range of age in both anemia and non anemia groups was similar from 25 to 30 years old and they were mostly within the second trimester period of pregnancy. Among anemic and nonanemic pregnant women,most cases included in this study were in the secundigravida and primigravida, respectively. The highest proportion of education in both groups was in senior high school (data not shown).
3.2. Hb levels, erythrocyte indices and white blood cell count
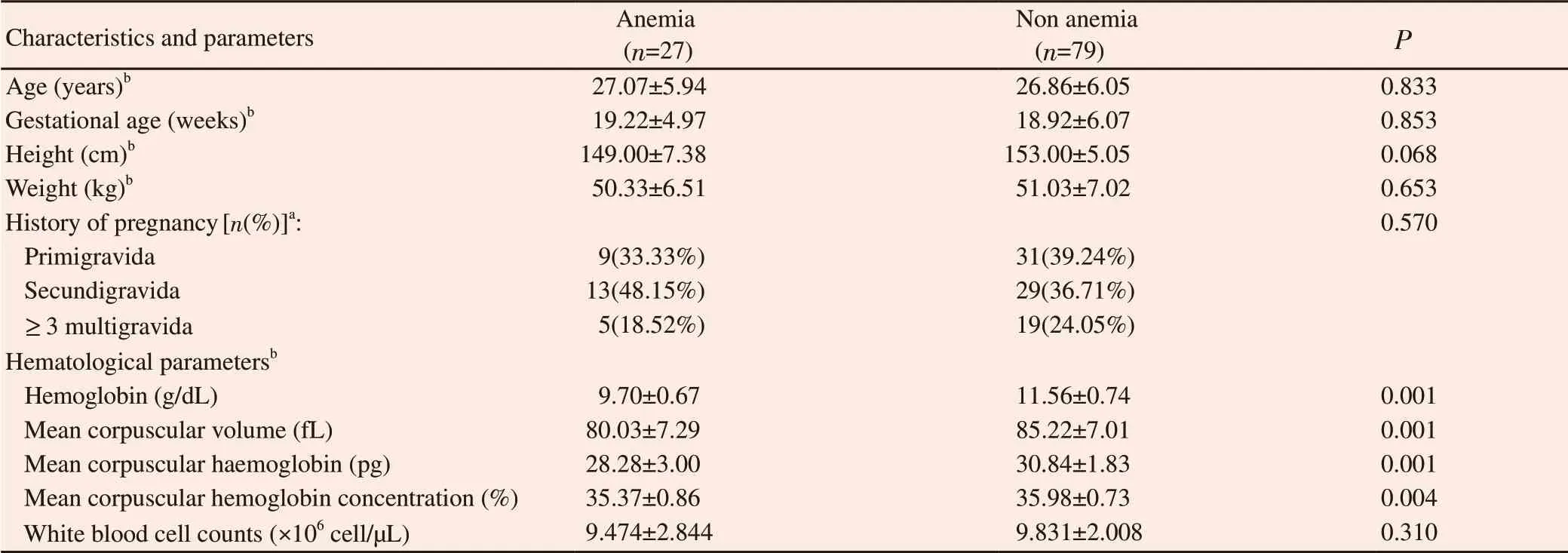
Table 1. Sociodemographic characteristics and hematological parameters among anemic and nonanemic pregnant women.
3.3. Cytokines levels increased among anemic pregnant women
The protein levels of several cytokines such as IL6, IL4 and TGFβ which may influnce iron homeostasis were shown in Figure 1. Slightly increases but not significant in those of IL6, IL4 and TGFβ were observed in the anemic group when compared to the non anemia group (Figure 1A-C). However, hepcidin and sTFR levels were lower among anemic pregnant women than that of nonanemic pregnant women but the differences were not significant.EPO level was significantly (P<0.01) higher in the anemia group,compared to the non anemia group (Figure 1D-F). Elevated EPO protein stimulated erythropoiesis process, which resulted in inhibition of hepcidin level.
3.4. Novel genetic variants of TFR2 exon 4
Growing evidence suggested that exon 4 of TFR2 gene was frequently mutated in some iron-related diseases such as hemochromatosis. Even we recognized that hemochromatosis had totally different symptoms from anemia, but both diseases were underlined by iron metabolism disorder. Therefore, we assessed whether or not mutation of TFR2 gene in exon 4 had an impact on anemic condition (Table 2).
A 605T>C synonymous mutation was the most frequent mutation in both groups. A 603A>T mutation which may led to the substitution of isoleucine to phenylalanine were also found in both groups.Furthermore, 4 different variants in nucleotide 606 were observed in two groups but less frequent.
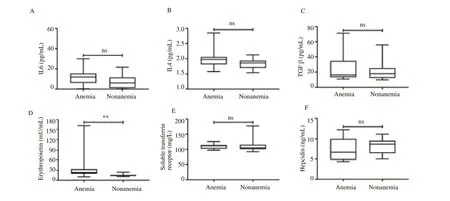
Figure 1. Protein levels related to iron homeostasis in pregnant women. (A, B) Expression levels of interleukin 6 (IL6) and interleukin 4 (IL4) were slightly higher in anemic pregnant women but not significant; (C) Increase of transforming growth factor β (TGF β) in anemic condition; (D) Erythropoietin levels increased significantly in anemic pregnant women; (E) Expression level of soluble transferrin receptor decreased in anemic pregnant women but not significant; (F) Decreased hepcidin level but not statistically significant was shown in the anemia group. ns= non significant; **P<0.01.
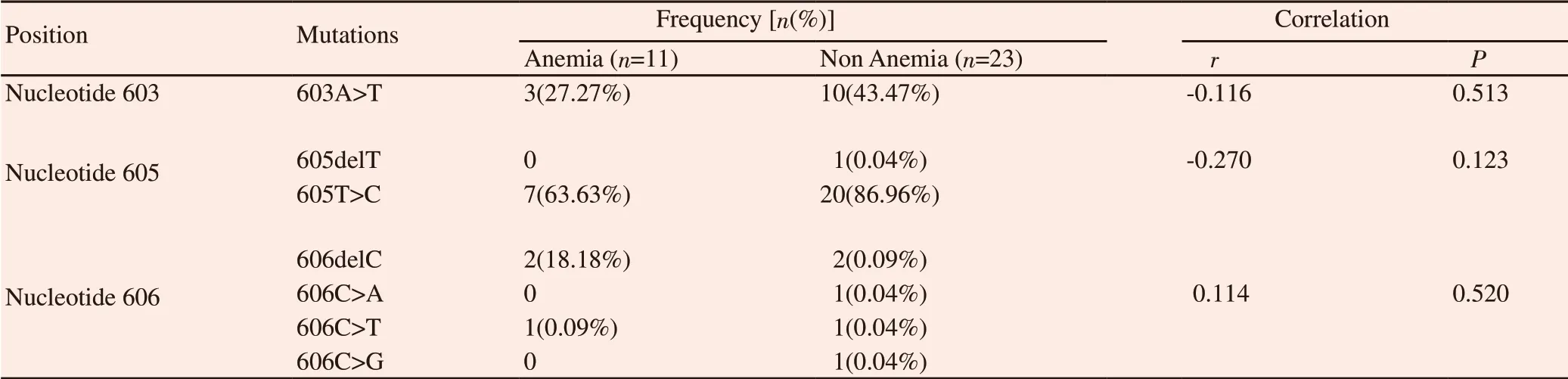
Table 2. Genetic variants identified in exon 4 of TFR2 gene.
4. Discussion
The overall prevalence of anemia in pregnant women was 25.47%in this study and was lower than the national prevalence, according to National Health Services data (56%)[4]. This low prevalence of anemia was also similar to another study within a different city in Java Island[16]. However, other studies in Bali and Sumatra Islands reported that anemia among pregnant women was 46.2% and 40.7%,respectively[17,18]. In the present study, our data were based on small numbers of samples and only localized in some small areas, Boyolali Regency. Boyolali is located in Central Java where has more educated individuals with good nutritional intake than other areas in Indonesia. Based on our data, we can exclude such factors that affect anemia incidences from the sample population.
The TFR2 protein forms a complex with Fe-loaded transferrin and hemochromatosis-associated molecule to stimulate hepcidin expression[8]. Consistent to the hepcidin level, the expression of TFR2 was also decreased as shown by decrease of sTFR level.It was also supplemented by the significant increase of EPO expression, the inhibitor of hepcidin expression and the marker of erythropoiesis activity[19]. Therefore, it suggested that TFR2 might be more physiologically relevant to the signaling process of hepcidin expression than other regulators such as IL6. Thus, we determined the molecular profile of TFR2 in exon 4, which had previously reported to be involved in iron homeostasis[12,13]. Our results showed that TFR2 exon 4 exhibited novel 3 mutational sites in position 603,605 and 606.
The TFR2 protein has two isoforms, TFR2α and TFR2β respectively. All exons are responsible for transcriptional process of TFR2α whilst TFR2β transcription site is within exon 4. The TFR2α protein can be found in hepatocytes and erythroid cell line and the TFR2β is primarily expressed in reticuloendothelial cells on various tissues[20]. A previous animal study[21] found that TFR2β KO mice exhibited anemia at young age even with normal transferrin saturation, liver iron concentration, and serum levels of hepcidin,and BMP6. Our result showed that anemia occurred in mutated exon 4 TFR2 gene among pregnant women, suggesting the important role of exon 4 variants in TFR2 gene.
Previous study suggested that anti-inflammatory cytokines related to T-helper 2 are essentials in maintaining pregnancy processes.Maternal immune tolerance towards fetal growth is needed during pregnancy because fetus is a semiallograft, which may cause an immune reaction, similar to the rejection of allograft in transplantation. IL4 and IL10 are major anti-inflammatory cytokines that are produced and activated by particular immune cells such as T cells, mast cells, basophils, eosinophils, and natural killer T cells. IL4 supports T-helper 2 response and induces the activation of signal transducer and activator of transcription signaling, which later inhibit T-helper 1 activity and its cytokines such as IL6 through down-regulation of interferon-gamma. In our study, we found different results where IL4 levels were higher in anemic pregnant women than the controls but not significant. It may suggest that IL4 did not play an important role in regulating immune processes among pregnant women in Central Java[22].
In theory, the increased expression of IL4 was followed by decreased IL6 expression, but our data indicated a slightly elevation of IL4 and IL6 but not statistically significant in anemic women.This is a paradoxical finding because IL6 regulates hepcidin transcription and causes hypoferremia[8]. We speculated that bacterial infections may also have an impact on anemia condition among pregnant women since a previous study[23] reported that IL6 increased among Japanese adults with iron deficiency and history ofHelicobacter pyloriinfection.
Current understanding indicates that hepcidin controls iron homeostasis to reduce iron efflux via its binding to ferroportin.However, some authors discovered that hepcidin level declined[24].In vivo approach has proved that the lower level of hepcidin is required to maintain fetal viability because excessive level of hepcidin caused severe iron deficiency anemia in fetus, which was lethal during its birth[25]. Reduced hepcidin level might also be an indicator of iron deficiency anemia during pregnancy[24].
In addition, other authors investigated that increased IL4 expression was correlated to hepcidin levels in different spectrum diseases such as malaria-related anemia and Kawasaki disease[26,27].Meanwhile, TGFβ which regulates hepcidin level through SMAD signaling was also elevated[28,29].
With regards to EPO and hepcidin levels in our study, these parameters can vary between iron deficiency anemia and control groups, which were also similar to the previous study[30]. These results might raise a preliminary insight if the hematology parameters and cytokines only would not be the ideal indicators of anemia status and progression in anemic pregnant women.
In conclusion, our findings suggest that there are various factors contributing to anemia incidence among pregnant women in Central Java, Indonesia. Our novel findings showed that exon 4 of TFR2 gene has 3 mutational sites in position 603, 605 and 606. These novel genetic variants may give a new insight towards the TFR2 role in anemia. However, the number of our study population is limited and does not represent ethnic heterogeneity in Indonesia. Hence,further investigation from different regions in Indonesia is required to understand the specific role of TFR2 variation among anemic pregnant women.
Conflict of interest statement
All authors declare that there is no conflict of interest.
Acknowledgments
We gave our appreciation to the Head of District Health Office and Community Health Centers in Boyolali Regency, Central Java for providing access to pregnant women who underwent their antenatal care. We would also kindly acknowledge Dr. Lionel Larribère for his valuable suggestions.
Authors’ contributions
Dono Indarto and Budiyanti Wiboworini made the concept and study design as well as supervised directly the clinical and experimental studies. Amelya A. Ayusari, Arisanty N. Restuti, Isnar N. Alfiyah and Aniki Puspita performed clinical and experimental studies. All authors acquired and analysed the data. Dono Indarto, Budiyanti Wiboworini, Yohanes C. Wibowo and Yoga M. Pratama prepared and edited the manuscript. At the end of this study, all authors reviewed and agreed with the final manuscript. Dono Indarto and Budiyanti Wiboworini are the guarantors of this manuscript.
 Asian Pacific Journal of Reproduction2020年1期
Asian Pacific Journal of Reproduction2020年1期
- Asian Pacific Journal of Reproduction的其它文章
- Testosterone is a surrogate and proxy biomarker for severity of late-onset preeclampsia: A cross-sectional study
- Assessment of antioxidant status of women with polycystic ovarian syndrome
- Comparative proteomic analysis of mature and immature oocytes in domestic cats
- Estrogenic activity of hydroalcoholic extract of Clitoria ternatea Linn. leaves on rats
- Residual impact of 17α-methyltestosterone and histopathological changes in sexreversed Nile tilapia (Oreochromis niloticus)
- Identification of pathogenic microorganisms of repeat breeder dairy cows and a hyperimmune treatment approach
