Identification of pathogenic microorganisms of repeat breeder dairy cows and a hyperimmune treatment approach
Seyed Morteza Aghamiri, Mohammad Rahim Ahmadi, Masoud Haghkhah, Abdollah Derakhshandeh
1Department of Clinical Sciences, School of Veterinary Medicine, Shahid Bahonar University of Kerman, Kerman, Iran
2Department of Clinical Sciences, School of Veterinary Medicine, Shiraz University, Shiraz, Iran
3Department of Pathobiology, School of Veterinary Medicine, Shiraz University, Shiraz, Iran
ABSTRACT Objective: To investigate the bacterial infections in repeat breeder dairy cows and to evaluate the treatment effects of hyperimmune serum against two main endometritis bacteria, Escherichia (E.) coli and Trueperella pyogenes.Methods: A total of 29 Holstein multiparous cows with three or more unsuccessful artificial inseminations were carried out and examined to confirm the absence of reproductive tract abnormality and vaginal discharges. Uterine lavage was performed to collect uterine samples for bacterial and fungal cultures. In addition,polymerase chain reaction (PCR) was done to detect Trueperella pyogenes, E. coli, Fusobacterium necrophorum, and Prevotella melaninogenicus. The cytological study was done on cervical mucus.The hyperimmune serums produced against Trueperella pyogenes and E. coli were infused into the uterus of repeat breeder cows and two doses of prostaglandin F2α were administrated intramuscularly within 14 days' interval.Results: There were 10 positive samples in the bacterial culture with 19 isolations and no growth of the main causative bacteria of endometritis. In the PCR method, no Trueperella pyogenes,Fusobacterium necrophorum, and Prevotella melaninogenicus were found. However, 11 positive samples of E. coli were identified by PCR. Cladosporium was detected in one case of repeat breeder cows. The median (interquartile range) of neutrophils in vaginal discharge was 12 (22.5). Eighteen from twenty-nine (62.1%) repeat breeder and eight from eleven (72%) cows with E. coli infections in PCR were made pregnant in the first artificial insemination after treatment by intrauterine infusion of hyperimmune serum and prostaglandin F2α .Conclusions: Intrauterine administration of hyperimmune serum could be an alternative to antibiotics for the treatment of repeat breeder cows.
KEYWORDS: Cow; Repeat breeder; Bacterial and fungal culture;Polymerase chain reaction; Hyperimmune serum
1. Introduction
The cows with healthy clinical and functional genital tracts that do not get pregnant after three or more artificial inseminations are called repeat breeder cows[1]. About 9%-24% of dairy cows are shown to have this problem[1-3] and cause wastage cost via increasing calving interval, insemination cost, and culling rate[4,5].The causes of repeat breeder syndrome are categorized into three main reasons including ovulation delay, uterine environment and luteal deficiency[6]. Many studies have reported that various factors lead to repeat breeder syndrome such as infectious agents by affecting the sperm or embryo, hormones deficiency and luteal problems, immunologic problem and anti-sperm antibodies, oocytes quality, nutrition, feeding, weather and season[1,3,4,7,8]. There are various proposed protocols for treatment of repeat breeder cows such as injection of gonadotropin-releasing hormone or its analogue around artificial insemination[4,9,10], post-insemination supplementation with progesterone[4,11,12], treatment of human chorionic gonadotropin 5 days after artificial insemination[11,13],intrauterine antibiotics[14-16] and antiseptics[16], uterine lavage by saline[14] or even acupuncture and moxibustion[16]. Also, the effect of dimethyl sulfoxide was suggested on clearing the uterus from bacterial biofilm as well as improving the fertility of repeat breeder cows after antibiotic treatment[15].
This study was to detect the presence of the main bacterial causes of endometritis [Escherichia coli (E. coli), Trueperella pyogenes(T. pyogenes), Fusobacterium necrophorum (F. necrophorum), and Prevotella melaninogenicus (P. melaninogenicus)] by polymerase
chain reaction (PCR) method in repeat breeder cows and to evaluate of treatment effects of hyperimmune serum against two major pathogen bacteria of endometritis, E. coli and T. pyogenes, on repeat breeder dairy cows.
2. Materials and methods
2.1. Animals
A total of 29 repeat breeder multiparous dairy cows were chosen from a dairy farm in the north of Shiraz, Fars Province,Iran (29°58′34″N, 52°40′45″E). Cyclic cows with three or more unsuccessful artificial inseminations were classified as repeat breeders[5]. The animals had no detectable abnormality in the reproductive tract and their vaginal discharges were clear during estrus. The average of days in milk of these cows was (346.78 ± 67.18) days. The mean of artificial insemination number was 6.19 ± 2.06. The annual average milk yields of the cows were 10 000 L. The body condition score of all cows were 2.75-3.50(scale 1-5). The cows were housed in free-stall barns and fed by alfalfa hay, corn silage, and concentrates (a balanced mixture of barley and corn grain, soybean meal, wheat bran, vitamins, and minerals).
2.2. Clinical examination
To confirm the absence of clinical abnormalities, the uterine horns and ovaries of the cows were examined by rectal palpation and 5-MHz linear transducer ultrasonography (Easi-Scan®, BCF, UK).The vaginal discharges of the cows were evaluated by a gloved hand and only the animals with clear mucus[17] were chosen for this study.
2.3. Uterine samples collection
To collect uterine samples for bacterial culture and PCR, uterine lavage was performed on 29 clinically healthy repeat breeder cows[18] by infusion of 60 mL saline solution into the uterus and retraction about 10 mL of it. The collected samples were transferred to the laboratory on ice. All bacterial and fungal cultures were done in less than 6 h from sampling.
2.4. Cervical cytology of samples
The discharges of cervical mucus were collected for cytological study. Gentle collection of the discharge was done by a 50 mL syringe connected to a plastic uterine pipette. By putting a drop of cervical mucus between two slides, thin smears were prepared[19].The smears were stained with Giemsa and neutrophils were counted in 20 microscopic fields (×1 000).
2.5. Bacteriological and fungal culture
Aerobic culture on sheep blood agar and MacConkey agar(Merck, Germany) was done at 37 ℃ for 48 h. Anaerobic culture on sheep blood agar was performed up to seven days. The isolates were identified by standard biochemical tests[20]. Spot inoculation technique was used on Sabouraud Dextrose agar to diagnose fungal infection[21]. The inoculated uterine discharges on Sabouraud Dextrose agar were incubated at 25 ℃ for 2 weeks. Based on colony characteristics and staining reaction, the identification of fungal agents was performed.
2.6. DNA extraction from uterine fluid
The samples were centrifuged at 5 000 × g for 10 min. After transferring the sediment into 2 mL micro tubes, 200 μL of the suspension was used to extract DNA by AccuPrep®Genomic DNA Extraction Kit (Bioneer, South Korea).
2.7. Detection of E. coli, T. pyogenes, F. necrophorum and P.melaninogenicus by PCR
Standard PCR method was performed to detect E. coli, T.pyogenes, F. necrophorum, and P. melaninogenicus by predesigned primers (Table 1)[18]. The positive controls were E. coli ATCC 35218, P. melaninogenicus ATCC 25845, native T. pyogenes and F. necrophorum. Amplification products were visualized by electrophoresis through 1% (wt/vol) agarose gel with 0.5 μg/mL ethidium bromide. If the amplicons had expected molecular size,they were considered as positive (Figure 1).
2.8. Treatment of repeat breeder cows using hyperimmune serum
Yavari et al showed T. pyogenes and E. coli were the most prevalent bacteria in the Iranian dairy cows with clinical endometritis[22],In this study we used the hyperimmune serums that had been produced before examination of cows based their inoculation. For the preparation of the hyperimmune serums, soy broth cultured bacteria pellets had been sonicated. Then by two-week intervals,each bacterial antigen had been injected subcutaneously to two healthy heifers. Antibodies had been reached peak value after eight injections. The hyperimmune serums had been prepared for uterine infusion by mixing 25 mL of hyperimmune serums separated fromheifers’ blood with 25 mL sterile 1 × phosphate buffer saline[23].Following sampling, hyperimmune serum was infused into uterus and two doses of prostaglandin F2α, 500 mg of cloprostenol sodium(EstroPlan®, IM, UK) were administrated intramuscularly within 14 days’ interval. Subsequently, the cows were inseminated about 12 h after the standing heat by the same person. Pregnancies were diagnosed by ultrasound examination (Easi-Scan®, BCF, UK) about 35-40 days after artificial insemination.
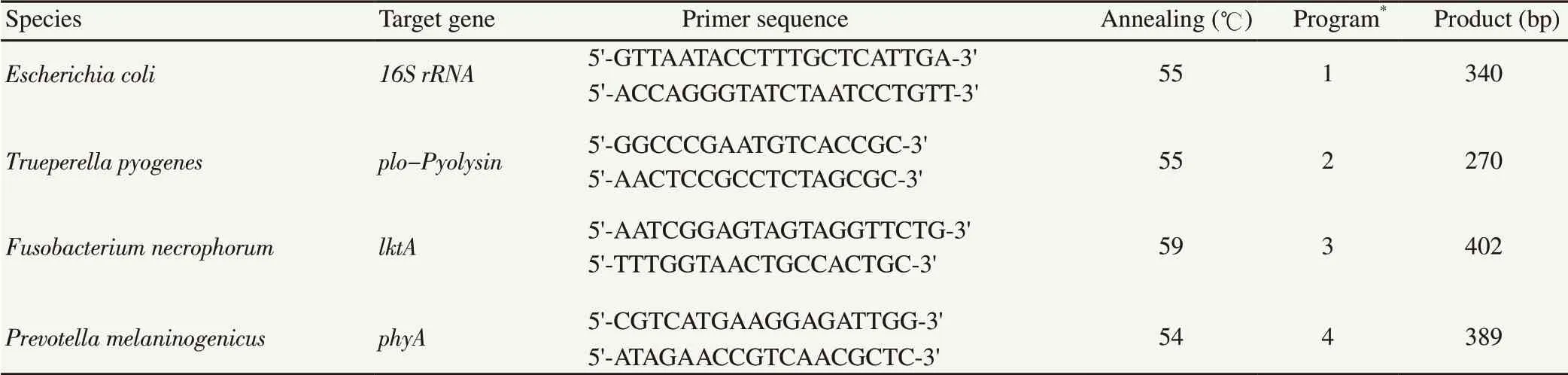
Table 1. Primer pairs used to amplify each target genes[18].
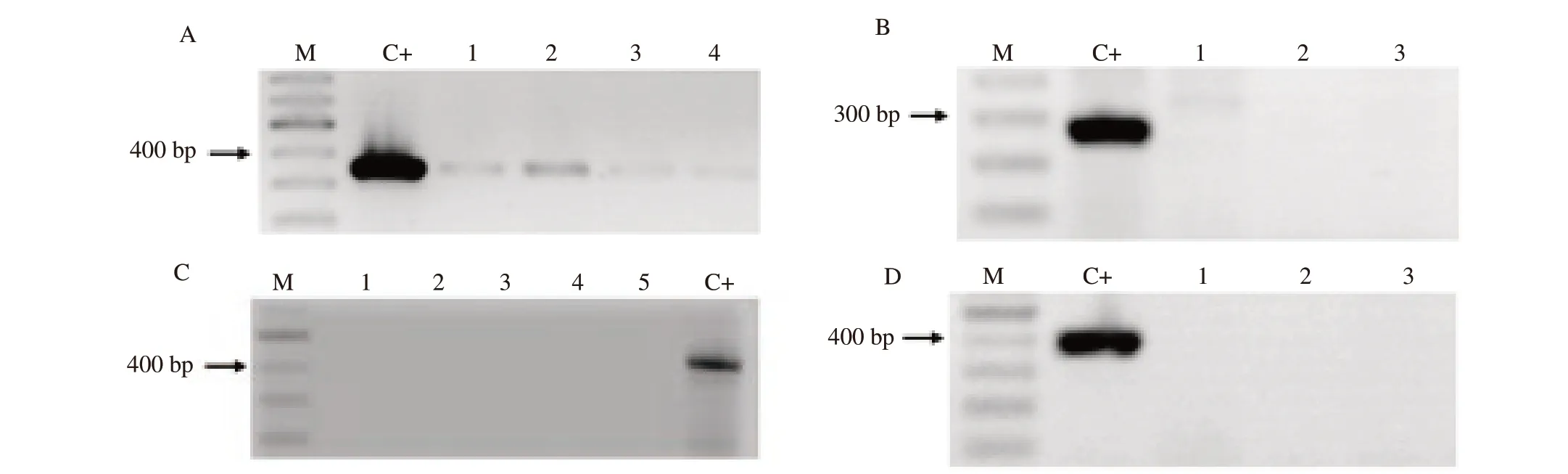
Figure 1. PCR amplification products of genomic DNA of uterine discharges. A: Escherichia coli (340 bp); B: Trueperella pyogenes (270 bp); C: Fusobacterium necrophorum (402 bp); D: Prevotella melaninogenicus (389 bp). M: molecular weight marker; C+: positive control; Lane 1-5: samples from uterine discharges.
2.9. Statistical analysis
Data were analyzed by SPSS software (SPSS for Windows,version 11.5, SPSS Inc, Chicago, Illinois). Pregnancy among different groups of culture and PCR results were compared by the Chi-square test. Significant difference was considered when P<0.05.
2.10. Ethics approval
All experiments were approved by the State Committee on Animal Ethics, Shiraz University, Shiraz, Iran (ethical approval number:90GCU1M1599). The recommendations of the European Council Directive (2010/63/EU) of September 22, 2010, were followed by the Ethics Committee.
3. Results
3.1. Bacteriological and fungal culture
There were 10 (34.5%) positive samples in the bacterial culture(19 isolations) and the remaining 19 (65.5%) showed no bacterial growth. The isolated bacteria were Actinobacter spp. (21.05%),
Micrococcus spp. (10.52%), Pasteurella caballi (10.52%), Staphylococcus saprophyticus (5.26%), Staphylococcus lentus (5.26%), Streptococcus dysgalactiae (5.26%), Bacillus coagulans (5.26%), Bacillus lentus(5.26%), Kurthia spp. (5.26%), Corynebacterium renale (5.26%),Rhodococcus equi (5.26%), Actinomyces spp. (5.26%), Burkholderia spp.(5.26%), Nocardia spp. (5.26%). By fungal culture, Cladosporium was detected in the one case of repeat breeder cows.
3.2. PCR detection
Following the PCR method to diagnose the major pathogens of chronic subclinical endometritis, no T. pyogenes, F. necrophorum,and P. melaninogenicus were found. However, 11 positive samples of E. coli were identified by PCR.
3.3. Cervical cytology
The median of polymorphonuclear neutrophils (PMNs) in the vaginal discharge was 12 (min: 0, max: 50, interquartile range: 23).The median of PMNs in the positive and negative bacterial culture and also, in the positive and negative PCR were shown in Table 2.In addition, there were grouping repeat breeder cows by the cut-off point at 8% PMNs. The percentage of PMNs was ≤ 8 in 10 and > 8 in 19 cows. Positive results of bacterial culture and PCR were not caused more cows positioning on > 8% PMNs category (Table 2).
3.4. Pregnancy results
Eighteen cows from twenty-nine (62.1%) were pregnant in the first artificial insemination after treatment by intrauterine infusion of hyperimmune serum and PGF2α. Pregnancy of positive bacterial culture was higher than negative cows after treatment insignificantly (90% versus 47.4%). Also, more positive PCR cows were pregnant after treatment than negative that it was not significant (Table 3). The median (interquartile range) of PMNs in pregnant and non-pregnant cows after treatment was 15 (23.5) and 11 (19), respectively. On the PMNs grouping, 50% (5/10) of ≤ 8%category and 68% (13/19) of > 8% category were pregnant after treatment that their difference was not significant.
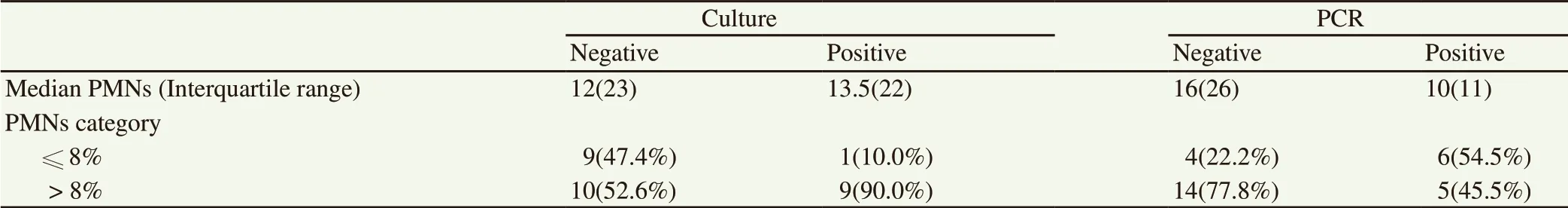
Table 2. Comparison of PMNs (median and interquartile range) and PMNs categories with culture and PCR detection of bacteria in repeat breeder cows.

Table 3. Comparison of pregnancy with culture and PCR detection of bacteria in repeat breeder cows.
4. Discussion
Repeat breeder syndrome is one of the main problems in the reproduction of cattle, especially in high producing dairy cows.Infectious agents are one of the multiple causes that were mentioned for repeat breeder syndrome[5,6,16,24]. E. coli is the most common bacteria isolated from the uterus in the first week after parturition but in weeks 2 and 3 T. pyogenes and its related bacteria, F. necrophorum and P. melaninogenicus are common causes of uterine diseases such as endometritis[25,26]. These four bacteria are the major pathogens of endometritis[26]. It is suggested that non-specific genital infections are most prevalent in repeat breeder cows and many different bacteria were isolated from their uterus[16]. Bacterial cultures of this study have shown many different infections. But any of the pathogen bacteria were not grown. However, in the PCR method 11 cows were positive for E. coli. Some studies have not found relations between infectious agents and repeat breeder syndrome[27] but maybe using better methods to detect microorganisms could change it. Just like there are some reports on many cases of the negative bacterial culture in the clinical endometritis of cows[14,28] and also, bacterial biofilms detected in 60% of the uterus of the repeat breeder cows[15]showing probable inefficiency of the traditional methods to detect bacteria and infectious agents in the uterus. Nowadays, PCR is an accurate and fast method of the culture-independent identification of microorganisms[29] and there are numbers of PCR studies on uterine discharges[18,29-31]. These observations could probably show the necessity of more bacterial investigation of the repeat breeder cows with accurate methods.
In this study, one case of Cladosporium was grown from uterine lavage fluids. There are some reports of fungi and yeast in uterine samples of cows with repeat breeder syndrome[32] and endometritis[21]. Some studies have mentioned the subclinical endometritis as a cause of repeat breeder syndrome[27,33,34]. The median of PMNs of cervical discharges was 12 (22.5). Because of the different techniques for acquiring samples, various PMNs number threshold was used such as 1%[33], 3%[35] and 5%[27] in uterine discharges. In this study, cut-off points at 8% PMNs have set for cervical discharges and there was no difference in post-treatment pregnancy. In this study, lesser cows with positive PCR (45.5%)compared to negative (77.8%) were positioned on the > 8% PMNs category (Table 2). Inactive forms of bacteria such as biofilms may not stimulate increasing uterine PMNs but could be detected by PCR methods. Also, some other bacteria may increase PMNs that not found in the present study.
In our study, 18 from 29 cows (62.1%) with repeat breeder syndrome were conceived after treatment with hyperimmune serum.Recently, researches were focused on hormonal and functional causes of repeat breeder syndrome mostly[4,9,11-13] because of physiological changes of high producing cows and their problems with ovulation time and corpus luteum function[36]. Also, in many studies, infectious agents were not a serious problem in repeat breeder cows[27] but it has been advised that reproductive indexes could be improved by antibiotic treatment[16]. There was no significant difference in treatments of repeat breeder cows between uterine lavage with normal saline with or without cephapirin[14].
It appears that causes of repeat breeder syndrome in dairy cows vary in different countries, even from farm to farm, which is related to the management of transition period, parturition and the postpartum period, genital tract infections, nutrition, genetics, estrus detection,and artificial insemination protocols[9,37,38].
Ahmadi et al treated clinical endometritis in dairy cows by the administration of the hyperimmune serum and reported acceptable clinical improvement compared to oxytetracycline and prostaglandin F2α[23]. The limited effects of most of the antibiotics on major pathogenic bacteria in the uterus, inhibition of cellular immune response and withdrawal times for milk consumption[6,22,26]have reduced the tendency for the intrauterine administration of antibiotics. In this study, the hyperimmune serum against two major uterine pathogens, T. pyogenes, and E. coli were produced. Eighteen out of 29 (62%) repeat breeder cows were conceived by first posttreatment insemination. Also, eight from 11 (72%) cows with E.coli infections in PCR were pregnant after hyperimmune treatment.So, hyperimmune serum could be a specific, effective and harmless replacement of antibiotics in the treatment of repeat breeder cows.Besides the bacterial result, the improvement effects of hyperimmune serum may show bacterial presence and their importance in the occurrence of repeat breeder syndrome. The presence of these bacteria in the uterus could interfere conception by exerting toxic effects on the embryo or endometrium[37,39,40]. Although the effects of other components of serum such as complement proteins,anti-inflammatory, growth factors should not be ignored. Lange-Consiglio et al reported 70% of repeat breeder cows were conceived by intrauterine administration of platelet concentration 48 h after artificial insemination and it is because of improvement of the uterine environment and counteract subclinical endometritis[34].
In conclusion, this study proved that PCR is an accurate method of detecting uterine bacterial infections including repeat breeder cow syndrome. The intrauterine administration of hyperimmune serum could be a harmless alternative to antibiotics, reduce the loss brought by repeat breeder syndrome and prevent the culling of the valuable cows.
Conflict of interest statement
All authors declare that there is no conflict of interest.
Authors’ contributions
Seyed Morteza Aghamiri participated in the sampling and laboratory tests such as PCR, bacterial culture and cervical cytology. Mohammad Rahim Ahmadi designed the project, prepared hyperimmune serum and performed sampling and treatments. Masoud Haghkhah and Abdollah Derakhshandeh participated in the PCR test and bacterial and fungal culture.
 Asian Pacific Journal of Reproduction2020年1期
Asian Pacific Journal of Reproduction2020年1期
- Asian Pacific Journal of Reproduction的其它文章
- Testosterone is a surrogate and proxy biomarker for severity of late-onset preeclampsia: A cross-sectional study
- Assessment of antioxidant status of women with polycystic ovarian syndrome
- Novel genetic variants of transferrin receptor 2 exon 4 and cytokines profile of anemic and nonanemic pregnant women in Central Java, Indonesia
- Comparative proteomic analysis of mature and immature oocytes in domestic cats
- Estrogenic activity of hydroalcoholic extract of Clitoria ternatea Linn. leaves on rats
- Residual impact of 17α-methyltestosterone and histopathological changes in sexreversed Nile tilapia (Oreochromis niloticus)
