Synthesis, Structure and Properties of a Novel Copolymer with Photochromic and Photoluminescence Functions
YANGLong(杨龙),ZHAOTianxiang,SHUDengkun,XIPeng(西鹏),2,3
1 School of Materials Science and Engineering, Tiangong University, Tianjin 300387, China 2 State Key Laboratory of Separation Membranes and Membrane Processes, Tianjin 300387, China 3 Tianjin Key Laboratory of Advanced Fibers and Energy Storage, Tianjin 300387, China
Abstract: Photochromic and photoluminescence materials show bright colors under different excitation conditions, and thus, these functional materials have been applied in many fields. Based on the photochromic and photoluminescence theories, a block copolymer, which could be directly processed into nanofibers by electrospinning, was successfully prepared through atom transfer radical polymerization (ATRP). To synthesize the block copolymer, a vinyl monomer containing a spiropyran unit was employed to prepare the photochromic chain segment, and a polymethyl methacrylate (PMMA) chain segment was introduced to improve the processability of the block copolymer. Acting as the photoluminescence unit, the rare earth complex was linked to the side chain through coordination bonding between the rare earth ions and the ester groups of PMMA. When the photochromic and photoluminescence block copolymer was exposed to different wavelengths of ultraviolet (UV) light and visible (Vis) light, it could show white, red, green, yellow, and blue-purple. These results provide the potential of the as-prepared photochromic and photoluminescence block copolymer for application in fibers and fabrics.
Key words: photochromic material; photoluminescence material; spiropyran; atom transfer radical polymerization (ATRP); block copolymer
Introduction
In recent decade years, photochromic materials have attracted much attention because of their superior properties and wide applications in various fields such as sensors and authentication systems[1], smart inkjet printing[2], data storage devices[3], smart fibers and textiles[4], and biomedical research[5]. Typical photochromic materials mainly include spiropyran[6], diarylethene[7], azobenzene[8], and schiffbase[9]. Among these, spiropyran and its derivatives display unique photochromic properties and good recyclability. Under ultraviolet (UV) light irradiation, the ring-closed and colorless spiropyran form can completely transform into the ring-opened and colored merocyanine form. In dark or in a heated environment, the transformation of merocyanine to spiropyran occurs (Fig. 1). Based on the photochromic mechanism of spiropyran, many photochromic materials were prepared by dispersing spiropyran in polymer matrices[10-12]. However, physical dispersion decreases the photostability, photochromic properties, coloration efficiency, and reversibility due to the precipitation of photochromic units from the polymer matrix[13]. In addition, the low thermal stability of spiropyran limits their direct application[14].

Fig. 1 Reversible transformation of molecular structures of (a) spiropyran and (b) merocyanine units
Recently, many efforts have been devoted to preparing novel photochromic materials with good thermal stability and photochromic properties. Tianetal.[15]prepared cellulose-based dynamic fluorescent materials with phototunable full-color emission by covalently attaching spiropyran and other photochromic units onto cellulose chains. Wangetal.[16]synthesized poly(ethylene glycol)-co-poly(spiropyran methacrylate) copolymers by modifying the linkage of the benzospiropyrane side chain functionality to the copolymer backbone. Sharifianetal.[17]synthesized a photochromic copolymer with acrylic-spiropyran, butyl acrylate, and methyl methacrylate (MMA) comonomers by emulsion polymerization. Through UV-Vis spectroscopy and the corresponding kinetic studies, they demonstrated that the flexibility and polarity of the polymer chains significantly affect the photoisomerization of spiropyran and merocyanine, and these nanoparticles were highly photostable. These results indicate that the functional modification of polymers is a successful strategy to immobilize spiropyran units onto other substrates through chemical bonding. Chemical bonding between the photochromic unit and polymeric chain can increase the photostability, fatigue resistance, lifetime, and photochromic efficiency of photochromic materials[18-19].

In this work, we prepared a novel photochromic and photoluminescence copolymer. In the design of the molecular structure of the copolymer, we selected 2-(3′,3′-dimethyl-6-nitrospiro[chromene-2,2′-indolin]-1′-yl)ethyl methacrylate (SPMA) as the first monomer to synthesize a spiropyran-containing poly(2-(3′,3′-dimethyl-6-nitrospiro[chromene-2,2′-indolin]-1′-yl) ethyl methacrylate) (PSPMA) and MMA as the second monomer to prepare a photoluminescence unit by the coordination of PMMA chain segments with an organic rare earth complex. The atom transfer radical polymerization (ATRP) technology was used to control the molecular chain structure of the as-prepared photochromic and photoluminescence copolymer. The intrinsic relationship between the structure and the properties of the as-prepared samples was determined by1H nuclear magnetic resonance (1H-NMR) spectroscopy, Fourier transform infrared (FTIR) spectroscopy, scanning electron microscopy (SEM), and thermo gravimetric analysis (TGA). These results open a path for the design and synthesis of multi-primary color functional polymer materials.
1 Experiments
1.1 Materials
Ethyl 2-bromoisobutyrate (EBIB, analytical grade), dichloromethane (DCM, analytical grade), tetrahydrofuran (THF, analytical grade),N,N-dimethylformamide (DMF, analytical grade) and copper (I) bromide (CuBr) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China.N,N′,N″,N″,N″-pentamethyldiet-hy-lenetriamine (PMDETA, analytical grade) was obtained from Tianjin Heowns Biochemical Technology Co., Ltd., Tianjin, China. 3,3′-Dimethyl-1′-(beta-hydroxyethyl)-6-nitrospiro [2H-1-benzopyran-2, 2′-indoline] (SP-OH, 93%) was purchased from Sigma-Aldrich. The organic rare earth complex [BCMT-Tb(III) complex] was prepared as reported in Refs.[28-29].Other chemicals were purchased from Tianjin Kemiou Chemical Reagent Co., Ltd., Tianjin, China. All reagents were dried and purified according to the standard process before use.
1.2 Synthesis of photochromic and photoluminescence copolymer
SPMA was synthesized according to the procedure reported in Ref.[30]. The photochromic copolymer (PSPMA-b-PMMA) was synthesized by the following procedures (Fig. 2). In a Schlenk bottle, SPMA (2.38 mmol), initiator EBIB (0.12 mmol), PMDETA (0.24 mmol), and THF (61.50 mmol) were added. The reaction system was degassed by three freeze-pump-thaw cycles. Then, CuBr (0.12 mmol) was added quickly at the last frozen state. The sealed Schlenk bottle was placed in an oil bath at 40 ℃ under a strong flow of nitrogen gas. After 6 h, MMA was added into the above reaction solution, and the reaction continued until the monomer completely disappeared. The as-prepared sample was precipitated by adding an excess of methanol and then vacuum filtered. The obtained sample was washed with methanol 10 times and dried in a vacuum oven at 60 ℃.
The photochromic and photoluminescence copolymer was prepared as follows. Firstly, the photochromic copolymer (1.670 g) and [BCMT-Tb(III) complex] (0.018 g) were dissolved in DMF (50 mL) and the solution was stirred at 50 ℃. Then after 30 min, the pH of the mixed solution was adjusted to 8.5 with NH3·H2O. After the mixed solution was stirred at 50 ℃ for 2 h, it was cooled to 25 ℃ and maintained at this temperature for 24 h. Finally, the precipitate was quickly filtered, and the as-prepared product was washed five times with ethanol (total volume: 50 mL) and deionized water (total volume: 50 mL). The sample was dried in a vacuum oven at 60 ℃ for 24 h to obtain the final product of white powder with 86.52% yield. Samples with different mass ratios were prepared by the same process.

Fig. 2 Synthesis route of photochromic and photoluminescence copolymer by ATRP
1.3 Measurements and characterization of the samples
1H-NMR spectra were recorded on the Avance 400 MHz instrument (Bruker, Germany) in CDCl3. FTIR spectra of the samples formed into KBr disks were collected by using the Nicollet NEXUS-670 FTIR spectrometer in the wavenumber range of 4 000-400 cm-1. Gel permeation chromatography (GPC) was performed by using the Viscotek 270 Gel chromatograph (Malvern, America). The test samples were prepared by chromatography using pure THF of 5-10 mg/mL samples. The molecular weights were expressed relative to polystyrene (PS) standards. The SEM images of the samples were recorded by using the Hitachi S4800 (Hitachi Co., Ltd., Japan) field-emission SEM operated at an acceleration voltage of 10 kV.
The thermal stabilities of the samples were investigated by TGA. The TGA curves were obtained on the Netzsch STA 449F3 TGA at a heating rate of 10 ℃/min in the temperature range of 25-800 ℃. Steady-state luminescence spectra were collected by using the Gangdong F-380 spectrofluorometer at an excitation wavelength range of 200-400 nm and an emission wavelength range of 400-750 nm with a slit width of 2 nm. The UV-Vis spectra of the samples were collected by using the TU-1901 UV-Vis spectrophotometer (Puxi Co., Ltd., China). The sample was added into a sample pool. The spectral bandwidth was 2 nm, response time was 0.2 s, and the scanning range was 200-800 nm. All the above-mentioned measurements were carried out at room temperature.
2 Results and Discussion
2.1 Structure of photochromic copolymer
The structure of PSPMA-b-PMMA was determined by1H-NMR and FTIR spectroscopy. Figure 3 shows the1H-NMR spectra of PSPMA-b-PMMA and SPMA. The characteristic proton peaks of SP appeared at 8.01, 6.72-7.28, 4.38, 3.4-3.58, and 1.25-1.31 [Fig. 3(b)][31], and the proton peaks at 5.58 and 6.15 corresponded to vinyl protons. The characteristic proton peaks of spiropyran shown in Fig. 3(a) are broader than those of SPMA, which indicates that the spiropyran moieties are attached on the polymer chains instead of being physically mixed. The disappearance of proton peaks at 5.58 and 6.15 further proves that the polymerization of SPMA occurred. The proton peak at 1.31 increases significantly, and two new proton peaks appear at 1.61 and 1.84. These results indicate that MMA repeat units are introduced into the polymer chains[32-33]. The1H-NMR analysis confirms the successful synthesis of the photochromic copolymer.
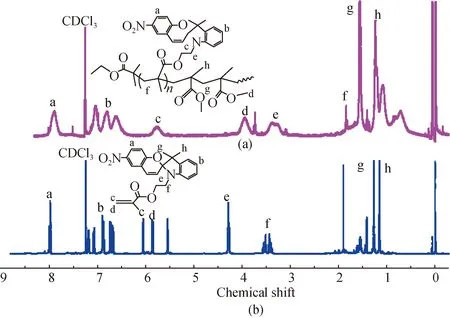
Fig. 3 1H-NMR spectra of (a) PSPMA-b-PMMA; (b) SPMA


Fig. 4 FTIR spectra of (a) PMMA; (b) SPMA; (c) PSPMA-b-PMMA
Moreover, the intensities of the absorption peaks of —CH2— and —CH3at 2 860-2 960, 865, and 748 cm-1were increased. These results indicate the formation of the photochromic copolymer, and the structure of the as-prepared photochromic copolymer determined by FTIR spectroscopy are consistent with those data shown in Fig. 3(a).
2.2 Structure optimization of photochromic and photoluminescence copolymer
The photochromic and photoluminescence copolymer is composed of PSPMA, PMMA chain segments, and a photoluminescence unit [BCMT-Tb(III) complex]. Electrospinning is an efficient technique for the fabrication of polymer nanofibers under mild conditions. To optimize the structure of the photochromic and photoluminescence copolymer, the electrospinning technique is employed[36-38]. Figure 5(a) shows the GPC curve of PSPMA. The number average molecular weight of PSPMA is 5 669 g/mol. Table 1 presents the solubility and thermal properties of PSPMA. As can be seen from Fig. 5(b), the process of PSPMA into fibers using different solvent systems by electrospinning is difficult. Moreover, since the initial decomposition temperature of PSPMA is only 136 ℃, the process of PSPMA directly by melting is also difficult.
PMMA has good processability. With the introduction of PMMA segments to the PSPMA chains, the processability of the as-prepared PSPMA-b-PMMA copolymer was improved. Figure 6 presents the GPC and TGA curves of PSPMA-b-PMMA.

Table 1 Solubility and thermal properties of PSPMA and PSPMA-b-PMMA

Fig. 5 Characterization of PSPMA: (a) GPC curve; (b) SEM images of electrospun PSPMA with different solvent systems
Through the coordination of rare earth ions with the ester groups of PMMA, polymeric rare earth photoluminescence materials can be prepared[39]. As rare earth elements are strongly paramagnetic[40], the1H-NMR and13C nuclear magnetic resonance (13C-NMR) spectroscopy of the photochromic and photoluminescence copolymer are difficult to obtain. In the FTIR spectra of the photochromic and photoluminescence copolymer containing the rare earth complex (Fig. 7), the stretching vibration peak of the carbonyl groups in the ester group of PMMA shifts to 1 728 cm-1, which indicates the coordination of Tb3+ions with the carbonyl groups of PSPMA-b-PMMA[41-43]. Figure 8 shows the fluorescence spectra of the photochromic and photoluminescence copolymer with different contents of the rare earth complex. And the fluorescence intensity of the photochromic and photoluminescence copolymer gradually increased with an increase in the content of rare earth complex[44]. When the content of rare earth complex is more than 6% (mass percent of the PSPMA-b-PMMA copolymer), the increase in the fluorescence intensity of the photochromic and photoluminescence copolymer is no longer obvious.
Based on the above analysis, the optimized composition of the photochromic and photoluminescence copolymer is presented in Table 2. A schematic diagram of the molecular structure of the as-prepared photochromic and photoluminescence copolymer is shown in Fig. 9. The photochromic unit is located at one end of the polymer chain, which is wound around and protected by the flexible PMMA chain. Therefore, PSPMA-b-PMMA shows good thermal stability [Fig. 6(b)]. The photoluminescence units are attached to the branched polymer chain through the coordination of the ester group of PMMA with the Tb3+ion. The mole number ratio of the PMMA segments and [BCMT-Tb(III) complex] units is 365.65∶2.49. In the aggregated structure of the photochromic and photoluminescence polymer, the photoluminescence units are distributed in many MMA units. Because of the coordination of the PMMA unit with the Tb3+ion of the rare earth complex, the photochromic and photoluminescence units are formed in a unitary manner and uniformly distributed in the copolymer, thereby realizing a multi-primary color photoluminescence function.

Fig. 8 Fluorescence spectra of the photochromic and photoluminescence copolymer with different contents of rare earth complex

Table 2 Optimized composition of photochromic and photoluminescence copolymer

Fig. 9 Schematic diagram of the molecular structure of photochromic and photoluminescence copolymer
2.3 Photochromic properties of photochromic and photoluminescence copolymer
The photochromic properties of the as-prepared samples were determined by UV absorption spectroscopy, and the absorption curves were recorded in every minute under UV light irradiation at 365 nm wavelength. Figures 10 (a) and (b) show the process of color change. As can be seen, new absorption peaks appeared at 450-700 nm, which are attributed to the ring-opening reaction of spiropyran under UV light irradiation. The intensity of the absorption peak increased with the duration of UV irradiation. After 2 min, the intensity of the absorption peak was 50% of the total intensity, and the as-prepared sample began to turn pale blue. After 10 min, the intensity of the absorption peak reached the maximum value, and the as-prepared sample turned deep blue with complete spiropyran to merocyanine transformation of the photochromic units.
Under dark conditions, merocyanine to spiropyran transformation of the photochromic units will occur. Figures 10(c) and (d) show the retained intensity of the UV absorption peak of the photochromic and photoluminescence fibers after different time. The result indicates that the transformation of merocyanine to spiropyran is slow. After 4 h, the sample retains a conspicuous blue color. The sample did not turn white until after 30 h. However, upon heating, merocyanine rapidly transformed into spiropyran. For example, merocyanine to spiropyran transformation was completed within 2 min at 60 ℃.
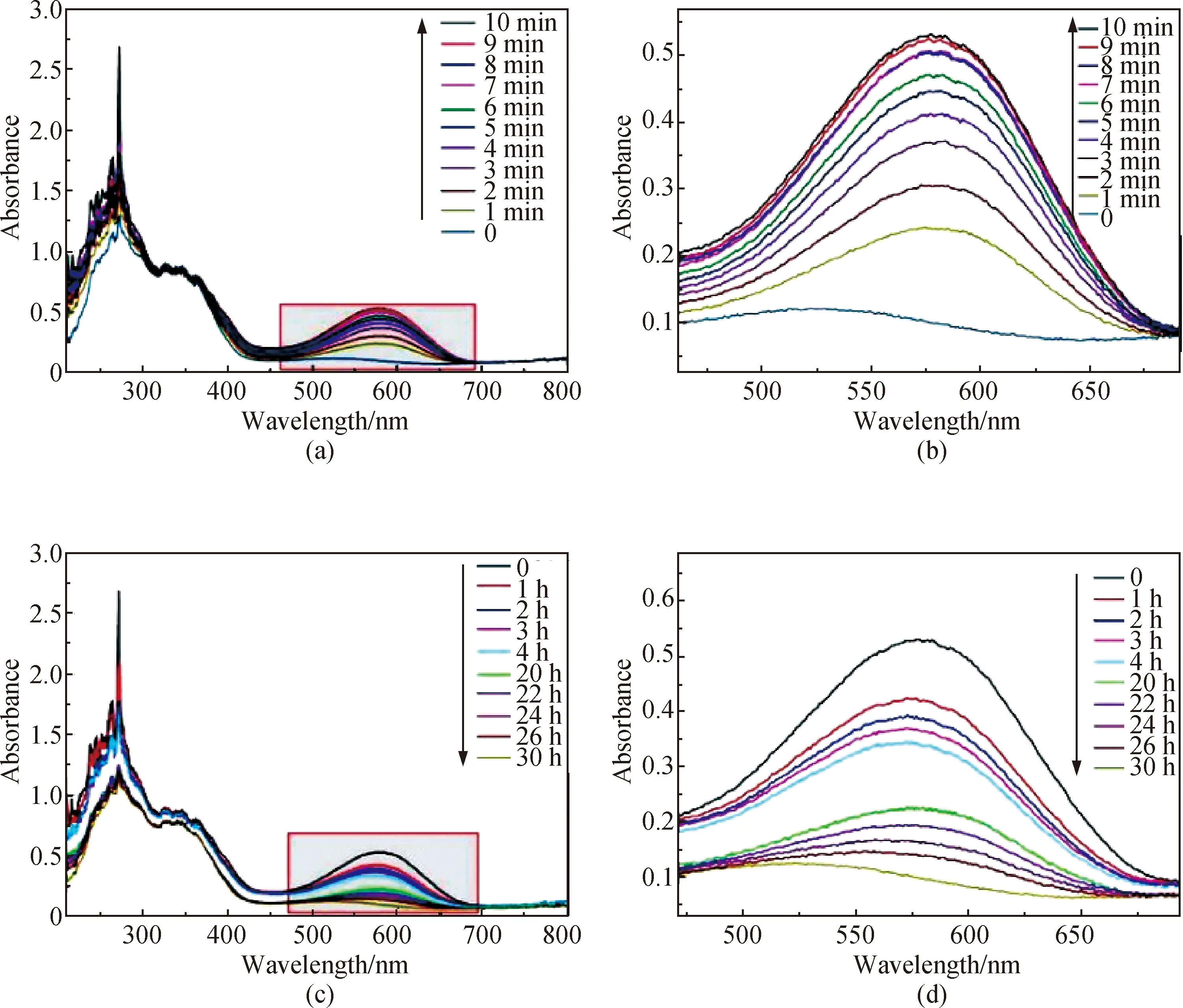
Fig. 10 Variation in absorption intensity of photochromic and photoluminescence copolymer at different time intervals: (a) and (b) under UV light irradiation at 365 nm; (c) and (d) under dark conditions
2.4 Multi-primary color photochromic functions of photochromic and photoluminescence nonwoven fabrics
The as-prepared photochromic and photoluminescence copolymer was electrospun to nonwoven fabrics with photochromic and photoluminescence functions. The multi-primary color photochromic functions of the as-prepared nonwoven fabrics are shown in Fig. 11.
The nonwoven fabrics exhibited different colors, and turned white, red, green, yellow, and blue-purple under excitations of 295 and 365 nm UV light and Vis light, thus exhibiting multi-primary color photochromic and photoluminescence properties.

Fig. 11 Photographs of multi-primary color of the photochromic and photoluminescence nonwoven fabrics excited by different UV wavelengths
The stability of the photochromic and photoluminescence properties of the as-prepared nonwoven fabrics is essential for practical applications. The optical switching of UV-Vis absorption was repeated 500 times to study the reversibility and reproducibility of the photochromic and photoluminescence properties of the as-prepared nonwoven fabrics. The reversible conversion between spiropyran and merocyanine could be realized by alternating UV light/Vis light irradiations. As shown in Fig. 12, after 500 cycles, the photochromic and photoluminescence properties of the nonwoven fabrics did not decrease significantly, which was significantly improved compared with the reversibility of conventional organic photochromic luminescent materials.

Fig. 12 Reversibility and reproducibility of the photochromic and photoluminescence properties of photochromic and photoluminescence copolymer
3 Conclusions
A multi-primary color photochromic and photoluminescence copolymer was prepared, which could be directly processed into nanofibers by electrospinning. The as-prepared photochromic and photoluminescence copolymer exhibited good thermostability and prolonged photochromic and photoluminescence functions. Furthermore, a nonwoven fabrics made of the photochromic and photoluminescence copolymer turned white, red, green, yellow, and blue-purple under the excitations of 295 nm and 367 nm UV light and Vis light. Moreover, after 500 cycles, the photochromic and photoluminescence properties of the nonwoven fabrics did not decrease significantly. These results provide a foundation for the wide application of the as-prepared photochromic and photoluminescence copolymer.
 Journal of Donghua University(English Edition)2020年6期
Journal of Donghua University(English Edition)2020年6期
- Journal of Donghua University(English Edition)的其它文章
- Physicochemical Properties of Poly (Vinyl Alcohol)/Chitosan/Soluble Starch Composite Hydrogel
- Dynamic Modeling of Variable Stiffness and Damping for Spatial Linkage Weft Insertion Mechanism with Clearance
- Design and Lamination Process of Composite Fabric for Automobile
- Energy-Delay Tradeoff for Online Offloading Based on Deep Reinforcement Learning in Wireless Powered Mobile-Edge Computing Networks
- Optimization Method of Bearing Support Positions in a High-Speed Flexible Rotor System
- Design and Application of Coordinator App Based on Needs Survey
