Preparation of CeO2/Flake-like CdS Composites as High-performance Photoanodes for Photoelectrochemical Cathodic Protection
WEI Ke-Nian,LIU Zhan,ZUO Shi-Xiang,YAN Xiang-Yu,WU Feng-Qin,LI Xia-Zhang,YAO Chao,LIU Xiao-Heng
(1.Key Laboratory for Soft Chemistry and Functional Materials,Ministry of Education,Nanjing University of Science and Technology,Nanjing 210094,China;2.Advanced Catalysis and Green Manufacturing Collaborative Innovation Center,School of Petrochemical Engineering,Changzhou University,Changzhou 213164,China)
Abstract:CeO2/CdS nanocomposites with the function of storing electrons and physical barrier were fabricated for photocathodic protection through a stepwise synthetic route.The obtained smples were characterized by XRD,TEM,UV-Vis and PL.The photoelectrochemical properties of CeO2/CdS with different mass ratios were investigated under white light irradiation.The maximum photocurrent density of CeO2/CdS (mass ratio 0.2 :1.0) is 700 μA·cm-2 and the potential of the 304 stainless steel coated with CeO2/CdS (mass ratio 0.2 :1.0) shifts to -650 mV (vs.SCE),which is significantly lower than the corrosion potential (-200 mV vs.SCE).The above results indicate that the CeO2/CdS composites exhibit remarkable photo cathodic properties which are attributed to the heterostructure of CeO2 nanoparticles and CdS nanosheets,and the structure facilitates the separation of photo-induced electron and hole.Furthermore,the CeO2/CdS composites can also provide cathodic protection for 12 h in the dark.
Key words:CeO2/CdS composite;heterostructure;physical barrier;photoelectrochemical cathodic protection
Steels have been widely used in marine engineering such as ship hulls,boilers and subsea equipment.However,steel corrosion has been getting more and more attention.Photoelectrochemical (PEC) cathodic protection is considered as an eco-friendly method for metals anticorrosion.The n-type semiconductor photoanode provides the photo-generated electrons for metals to achieve cathodic protection.At present,the semiconductors used for photocathode protection are mainly TiO2,SrTiO3and ZnO[1].However,the semiconductors with a wide band gap (Eg) only respond to the ultraviolet light which accounts for ~5% of sunlight,however,visible light that accounts for ~50% of sunlight cannot be fully utilized[2].In addition,these semiconductors have a high probability of recombination of photo-generated electrons and holes,resulting in the low utilization of photo-generated electrons.Moreover,the metals cannot be cathodically protected in dark condition.Therefore,it is necessary to develop a photocathode composite with low cost,visible light response,high electron utilization and electronstorage.
CdS with a direct band gap of 2.42 eV is a kind of important semiconductor which is currently considered as a significant material for scientific and technological applications[3-4].At present,CdS is widely used in photocatalytic reduction of CO2,photocatalytic hydrogen production and photocatalytic degradation[5-7].Unfortunately,the ultrafast recombination of photo-generated charge carriers and the strong self-oxidation of CdS significantly restrict the practical applications in the photoelectrochemical field.The combination of CdS with another semiconductor is an efficient approach to improve the photocatalytic activity and stability[8].Feng,et al[9].reported that the formation of the CdS/Bi2MoO6heterojunction contributed to the separation of photoinduced charge carriers.However,CdS has not been reported for photocathodic protection.Here,the combination of CeO2and CdS is examined for photoelectrochemical cathodic protection due to the suitable band gap(3.2 eV) of CeO2.CeO2has been recently used as a photoactive material in solar cells and hydrogen evolution[10].In addition,as reported,CeO2is used as an electron storage in photocathode protection because of the following conditions:(1) redox activity;(2) more posi-tive redox potential than the conduction band (CB) potential of the employed semiconductor;(3) more negative redox potential than the self-corrosion potential of the metal;(4) stability during repeated redox cycles[2,11].
Herein,CeO2/CdS nanocomposites were prepared by a stepwise synthetic method to achieve a high photocathodic protection efficiency.CeO2nanoparticles were distributed on the surface of CdS sheets.The photocathodic protection properties of CeO2/CdS including Tafel plots,the photo-induced current density,electrochemical impedance spectroscopy (EIS) and the photogenerated potential were investigated.
1 Experimental
1.1 Preparation of CdS nanosheets
Sulfur powder (0.374 g) and CdCl2·2.5H2O (0.526 g)were dissolved in 70 mL diethylenetriamine (DETA) and stirred for 1 h.The mixed liquid was transferred to a Teflon liner and kept at a constant temperature of 80 ℃for 24 h.Then,it was cooled to room temperature,centrifuged and washed with deionized water and ethanol.The product was dried at 60 ℃ for 12 h to obtain CdS nanosheets.
1.2 Preparation of CeO2/CdS composites
Firstly,Ce(NO3)3·6H2O (0.76 g) was dissolved in 30 mL mixed solution consisted of water and ethylene glycol(mass ratio:1/1),and stirred for 10 min.CdS (1.0 g) was added and stirred at 60 ℃.25wt% ammonia solution(4.8 mL) was added in the mixture until the color of the mixed solution changed from dark green to yellow.Finally,the above mixture solution was centrifuged and washed with deionized water,then dried at 60 ℃ for 12 h to obtain 30wt% CeO2/CdS whose ratio was 0.3 :1.0 For comparison,10wt% CeO2/CdS (0.1 :1.0) and 20wt%CeO2/CdS (0.2 :1.0) composites were prepared by the similar method.
1.3 Characterization
The morphology was determined by JEOL-2100 transmission electron microscopy (TEM).X-ray diffraction (XRD) was recorded on a D/MAX 2500/PC powder diffractometer (Rigaku) applying Cu Kα radiation source from 5° to 80° (2θ) at a scanning rate of 3(°)/min.The UV-Vis diffuse reflectance spectra (DRS) were recorded on a UV-Vis spectrometer (Thermo Nicolet Evolution 500) equipped with an integrating sphere.The Photoluminescence (PL) spectra were carried on a fluorescence photometer (LS45) with the excitation wavelength of 300 nm.
1.4 Photoelectrochemical measurements
All electrochemical measurements were tested in a three-electrode experimental system at room temperature using CHI660D electrochemical workstation (Chenhua Instrument Co.Ltd,Shanghai,China).The experimental setup for photoelectrochemical protection measurements was shown in Fig.1.
The electrolyte was 3.5wt% NaCl aqueous solution.The prepared film photoanode served as the working electrode.Pt foil and a saturated calomel electrode (SCE)were used as the counter electrode and the reference electrode,respectively.The light source was a 300 W Xenon lamp in front of a quartz window incorporated into a conventional electrolytic cell.The working electrode was prepared as follows:the as-prepared samples were well dispersed in distilled water with proper content.The dispersion was uniformly dipped using a pipette gun(50 μL) on the surface of circle 304SS electrode.Simultaneously,Nafion solution was also coated on them until the dried composite film formed.
2 Results and discussion
Fig.2 shows XRD patterns of CeO2/CdS,pure CdS and CeO2.The diffraction peaks at 2θ=26.5°,28.2°,43.7°,47.8° and 51.8° are attributed to the (002),(101),(110),(103) and (112) planes of pure CdS (JCPDS 41-1049)respectively[6,12-13](Fig.2(a)).The peaks at 2θ=28.5°,33.1°,47.5° and 56.3° correspond to the (111),(200),(220) and(311) planes of pure CeO2(JCPDS 43-1002),respectively[12,14](Fig.2(e)).In Fig.2(b-e),the peaks of CeO2/CdS composites correspond to both CeO2and CdS with varying intensities,confirming the successful loading of CeO2nanoparticles on CdS nanosheets.

Fig.1 Schematic sketch of the experimental setup for photoelectrochemical cathodic protection investigation
Fig.3 shows TEM images of different samples.CdS exhibits a sheet-like structure with a size of about 300 nm (Fig.3(a)).A nanoparticle with a size of 3-5 nm can be observed (Fig.3(b)).CeO2NPs are uniformly dispersed on CdS NSs with intimate interfacial contact which is beneficial to the efficient transfer of photogenerated charge (Fig.3(c,d)).The HRTEM image confirms the formation of crystalline CeO2and CdS (Fig.3(e)).The lattice spacing of 0.33 and 0.31 nm are assigned to(002) plane of CdS (JCPDS 41-1049) and (111) plane of CeO2(JCPDS 43-1002),respectively.
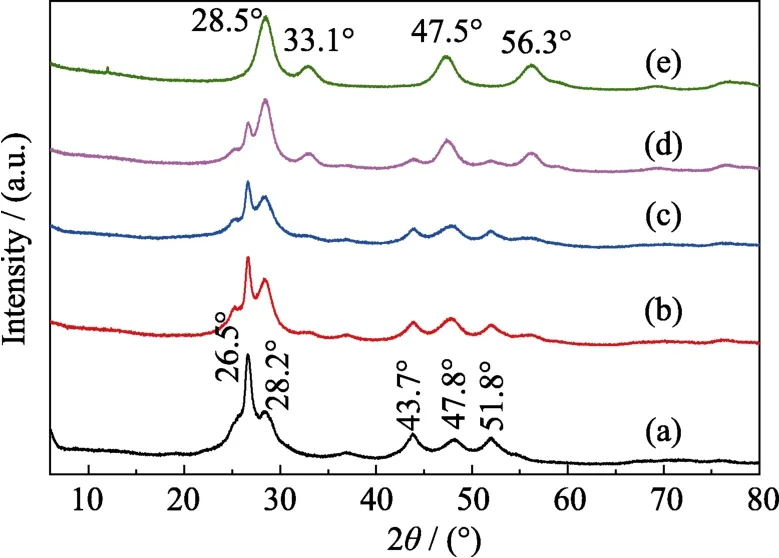
Fig.2 XRD patterns of (a) CdS,(b) CeO2/CdS (0.1 :1.0),(c) CeO2/CdS (0.2 :1.0),(d) CeO2/CdS (0.3 :1.0) and (e) CeO2
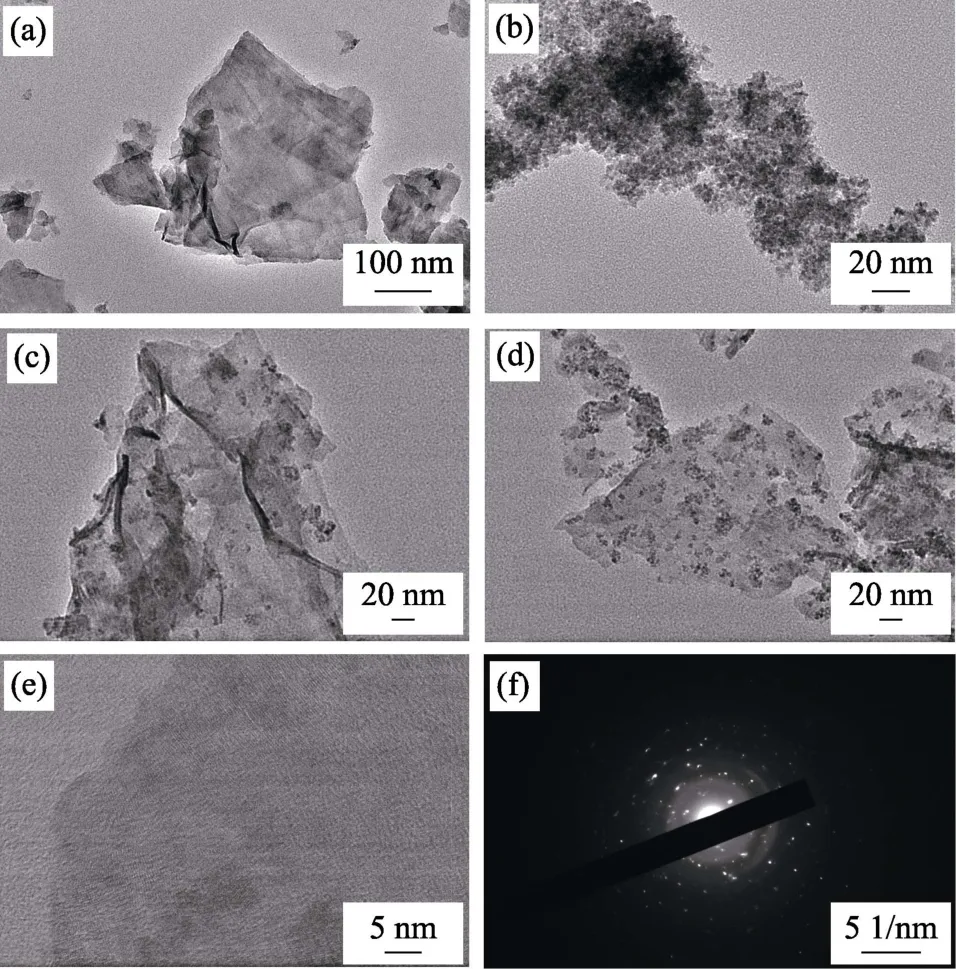
Fig.3 TEM images of (a) CdS,(b) CeO2,(c) CeO2/CdS (0.1 :1.0),(d) CeO2/CdS (0.2 :1.0),and (e) HRTEM image and EDX pattern of CeO2/CdS (0.2 :1.0)

Fig.4 UV-Vis diffuse reflection spectra of (a) CdS,(b) CeO2/CdS(0.1 :1.0),(c) CeO2/CdS (0.2 :1.0),(d) CeO2/CdS (0.3 :1.0)and (e) CeO2

Fig.5 PL spectra of CdS and CeO2/CdS(0.1 :1.0),CeO2/CdS(0.2 :1.0) and CeO2/CdS(0.3 :1.0)
The light absorption of the samples are investigated by UV-Vis DRS (Fig.4).Pure CeO2can absorb the visible light shorter than 460 nm while pure CdS can absorb the visible light shorter than 540 nm[6,12].Compared with CdS and CeO2,CeO2/CdS composites exhibit an obvious red-shift in absorption edge while the absorption intensity in the range of 350-540 nm increases.This phenomenon is attributed to the formation of heterojunction between CdS and CeO2which reduces the recombination of electron-hole and enhances light absorption.Fig.4(c)shows that CeO2/CdS (0.2 :1) has the highest absorption intensity (Fig.4(c)).
Fig.5 shows PL spectra of CdS,CeO2/CdS with the different contents of CeO2(0.1wt%,0.2wt%,and 0.3wt%).The lower the intensity,the lower the recombination rate is[15].CdS presents the highest peak intensity,revealing the highest recombination rate.PL intensity of CdS is higher than that of CeO2/CdS,implying the charge carrier recombination.CeO2nanoparticles are loaded on CdS nanosheets,which can efficiently separate the photogenerated charge carriers and inhibit the recombination of photogenerated charge carriers,leading to enhance photoelectrochemical cathodic protection.In particular,CeO2/CdS (0.2 :1.0) shows the lowest peak intensity,resulting in high photocatalytic activity.
Fig.6 displays potentiodynamic polarization curves of 304SS coated with different materials in 3.5% NaCl under on-off response of white light irradiation.The corrosion potential of the uncoated 304SS is -192 mV (vs.SCE) corresponding to current density of about 1.15×10-5mA·cm-2.After coating,the corrosion potential of the coated 304SS in the dark becomes more positive,and the current density decreases by one order of magnitude,which can be ascribed to the barrier effect of the sheet-like CdS[16].The 304SS coated with the CeO2/CdS (0.3 :1.0) shows the highest potential (-129 mV) and the lowest current density (1.58×10-6mA·cm-2),which is related to the denser structure of CeO2nanoparticle and CdS nanosheets.Compared with bare 304SS,the corrosion potentials of 304SS coated with different composites are more negative and the current densities are higher under illumination,indicating that the composites generate photo-electrons and transfer to 304SS substrate[17].Therefore,the 304SS substrate is under cathodic protection.The corrosion potential of the 304SS with CeO2/CdS (0.2 :1.0) coating is more negative(~-640 mV) and the photocurrent density is higher(~1.99×10-3mA·cm-2) under the light compared with the 304SS with CeO2/CdS (0.1 :1.0) coating and CeO2/CdS(0.3 :1.0) coating.It may be due to the formation of heterojunction between CeO2and CdS which reduces the recombination rate of electrons and holes,and transfer more electrons to the metal.For comparison,the corrosion potential of CeO2/CdS (0.2 :1.0) coating is more positive than that of CeO2/CdS (0.2 :1.0) nanoparticles,suggesting that flake-like CdS possesses excellent physical barrier.
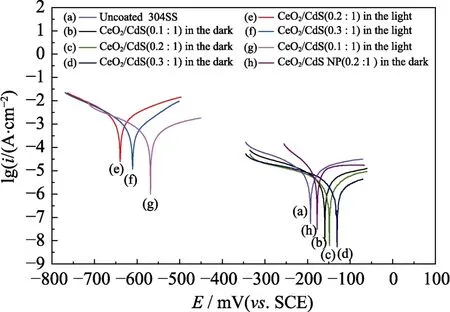
Fig.6 (a-g) Polarization curves for uncoated 304SS,304SS coated with CeO2/CdS (0.1 :1.0),CeO2/CdS (0.2 :1.0) and CeO2/CdS(0.3 :1.0) in the white-light illumination and in the dark;(h)polarization curves for CeO2/CdS (0.2 :1.0) nanoparticles in the dark
The transient photo-current responses of the coatings were observed upon on/off cycles of intermittent visible light illumination in Fig.7.All the samples exhibit fast reproducible photoresponse under visible light illumination.Obviously,the current density rises sharply when the light is turned on.The current density rapidly decays to almost zero when the light is turned off.The photocurrent density of the composites is higher than that of pure CdS,which is attributed to the formation of heterojunction between CeO2and CdS.Under light illumination,the electrons in the CB of CdS can be more easily transferred to more positive CB of CeO2,which leads to the higher photocurrent of the composites[18].The photocurrent density of CeO2/CdS (0.2 :1.0) is the highest (~700 μA/cm2) which is consistent with the potentiodynamic polarization curves in Fig.5.Moreover,no noticeable photocurrent degradation is detected after several on/off cycles of light illumination,indicating that the prepared films possess good photostability.
In order to evaluate the photoelectron separation,transport efficiency and corrosion resistance of the composites,EIS was tested (Fig.8).In this model,CfandRfat high frequencies represent the capacitance and resistance of the coating whileCdl,RctandRsat low frequencies represent the double-layer capacitance,charge transfer resistance and electrolyte resistance,respectively[18].Table 1 shows the fitting results of EIS spectra with different coated electrodes in 0.5 mol/L H2SO4solution with and without visible light irradiation.
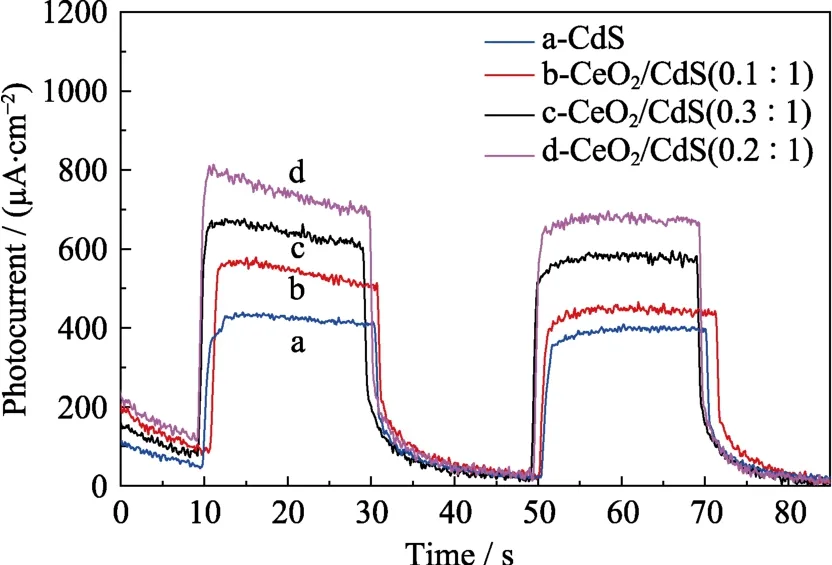
Fig.7 Photocurrent-time curves of (a) CdS,(b) CeO2/CdS(0.1 :1.0),(c) CeO2/CdS (0.3 :1.0) and (d) CeO2/CdS (0.2 :1.0)
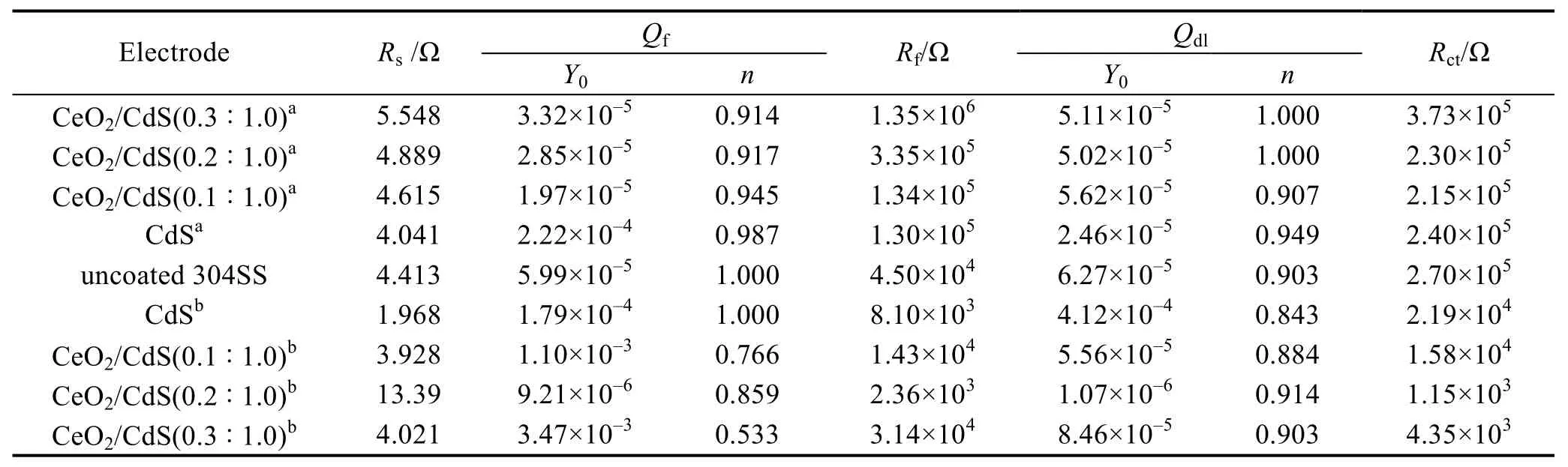
Table 1 Fitting results of the Nyquist plots using the equivalent circuits of Fig.8
In Fig.8(a),CeO2/CdS presents an excellent corrosion resistance in the dark because the resistance of CeO2/CdS coating is much larger than that of bare 304SS electrode[16].From the fitting results in Table 1,theRfvalue of CdS nanosheets (1.30×105Ω) is one order of magnitude higher than that of bare 304SS electrodes (4.50×104Ω).The sheet material shields the penetration of corrosion factors such as water,oxygen and ions,and the overlapping flakes that are parallel to each other can prolong the path of corrosion factor penetration,thus improving the corrosion resistance of the 304SS.With the increasing content of CeO2,theRfof the composite coatings becomes larger.TheRfof CeO2/CdS (0.3 :1.0) coating(1.35×106Ω) is one order of magnitude higher than that of CdS nanosheet (1.30×105Ω),which is the result of the dense structure of CeO2nanoparticles and overlapping CdS nanosheets.
The arc diameter of the 304SS electrode with composite coatings is significantly smaller than that of the bare 304SS electrode under light irradiation (Fig.8(a)).The charge transfer resistance (Rct) decreases significantly(Table 1),which is consistent with the trend of the Tafel polarization curve.These photoelectrons can reach 304SS and lead to the negative shift of the corrosion potential.In this process,the heterojunction between CeO2and CdS promotes the separation and transport of electrons and holes[19].The charge-transfer resistance(1.15×103Ω) of CeO2/CdS (0.2 :1.0) is the smallest in the composites,which is two orders of magnitude lower than that of bare 304SS electrode (2.70×105Ω).Therefore,CeO2/CdS (0.2 :1.0) composite under light irradiation has the highest charge separation and transfer efficiency.
Fig.9 shows the open circuit potential (OCP) curves for 304SS in 3.5wt% NaCl solution under the visible light irradiation.The uncoated 304SS electrode potential is -189 mV (Fig.9(a)).In the dark,304SS electrode with CdS nanosheet coating and CeO2/CdS (0.2 :1.0) coating are -160 mV (SCE) and -130 mV (SCE),respectively,which are consistent with the results of Fig.6.The sheet-like structure of CdS plays a good barrier,indicating that the composites can be used as a protective layer on the substrate[17,19].
When the light is turned on,the potential of the 304SS electrode drops sharply and stays at a more negative potential due to the generation of photo-generated electrons.The potential of the 304SS electrode rises when the light is turned off[19-20].Consistent with the results of Fig.6 and Fig.7,potential of the 304SS with CeO2/CdS (0.2 :1.0)coating electrode (-632 mV (SCE)) is more negative than that of CdS coating (-510 mV (SCE)),due to the formation of a heterojunction between CdS and CeO2.The potential of 304 SS electrode with CdS coating rapidly rises back to the initial potential value while the potential 304 SS electrode with CeO2/CdS (0.2 :1.0) coating slowly rises and remains at a lower potential when the light is turned off,which can be attributed to the storage electron function of ceria[19-20].The coupled semiconductor CeO2is capable of storing the excess electrons which can be released during the dark.The cathodic protection can still continue before illumination is restored.Fig.9(b) shows that CeO2/CdS (0.2 :1.0) composites can provide electrons for 304SS in the dark for up to 12 h.
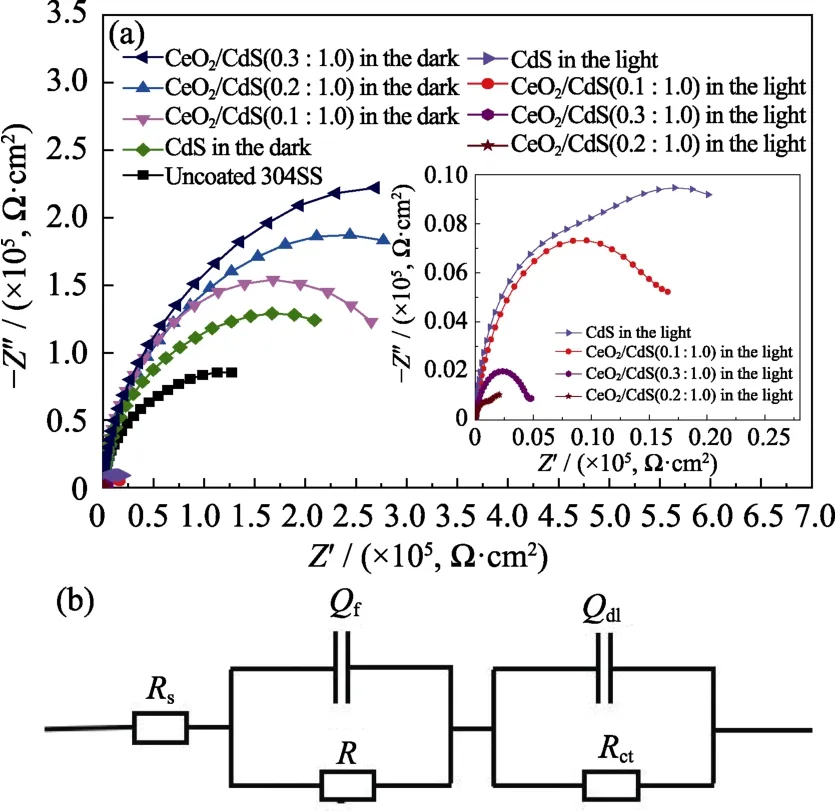
Fig.8 (a) Nyquist plots of pure 304SS,304SS coupled with CdS and CeO2/CdS(0.1 :1),CeO2/CdS(0.2 :1) and CeO2/CdS(0.3 :1) in the dark and white-light illumination;(b) Schematics of the equivalent circuit obtained by EIS result fitting
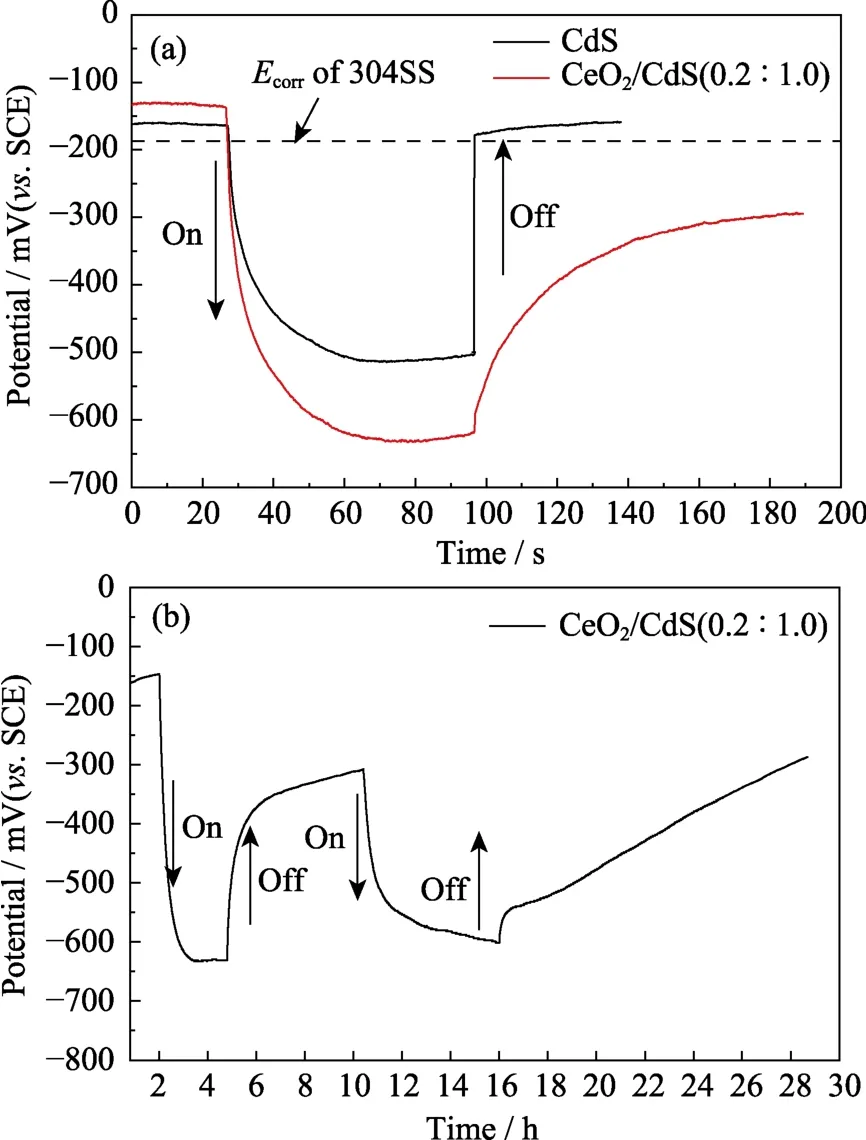
Fig.9 OCP variations of 304SS electrodes coupled with the as-obtained samples
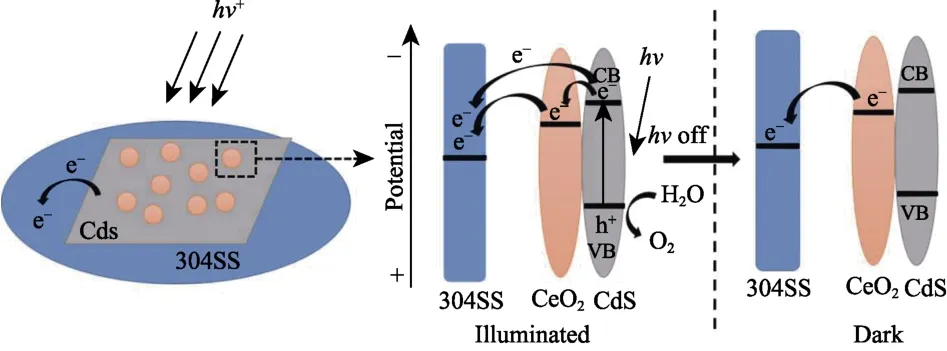
Fig.10 Mechanism of CeO2/CdS composite for 304SS photocathodic protection
Based on the above results and discussion,the possible photocathodic protection mechanism of CeO2/CdS composite for 304SS is proposed in Fig.10.Photogenerated electrons on the CB of CdS can easily be transferred to 304SS under light irradiation,because the conductive potential of CdS is more negative than the self-corrosion potential of 304SS[21].Some photogenerated electrons of CdS is conducted to CeO2attributed to the conductive potential of CdS which is more negative than the redox potential of CeO2[11].CeO2achieves the storage and release of electrons through the following redox reactions:
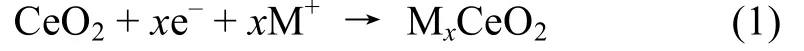
where M=H andx=1[11,21].When the light is turned off,the electrons stored in CeO2are released and transferred to the 304SS to maintain the cathodic protection effect.Therefore,304SS can be protected even in the dark.In summary,CeO2/CdS composite can provide an enduring protection for 304SS.
3 Conclusion
CeO2/CdS composites were fabricated by a stepwise synthetic approach.Under the light illumination,CeO2/CdS exhibits an enhanced photo-induced current density and a more negative photo-potential shift as compared with pure CeO2and pure CdS due to the formation of a heterojunction between them,which reduces the recombination of electron-hole pairs and improves the efficiency of photo-electric conversion.Furthermore,CeO2/CdS can continue to provide cathodic protection in the dark because CeO2has the function of storing electrons.At last,flake-like CdS can slow down the penetration of corrosion factors and act as a physical barrier.A variety of mechanism work synergistically to make CeO2/CdS nanocomposites have a more effective photo cathodic protection on 304SS.

