Breed ing w heat for resistance to Fusarium head blight in the Global North: China,USA,and Canad a
Zhnw ng Zhu,Yunfng Ho*, Mohm Mrgoum, Guihu Bi,Gvin Humphrys, Sylvi Cloutir, Xinhun XiZhonghu Hf,*
a Institute of Crop Sciences,Chinese Academy of Agricultural Sciences,Beijing 100081,China
b Food Crops Institute,Hubei Academy of Agricultural Sciences,Wuhan 430064,Hubei,China
c Department of Crop and Soil Sciences,University of Georgia,Griffin,GA 30223,USA
d US Department of Agriculture-Agricultural Research Service,Hard Winter Wheat Genetics Research Unit, Manhattan,KS 66506,USA
e Agriculture and Agri-Food Canada,Ottaw a Research and Development Centre,Ottawa,Ontario K1A 0C6,Canada
f CIMMYT-China Office,c/o CAAS,Beijing 100081,China
Keywords:Fhb1 Fusarium head blight resistance Fusarium graminearum Triticum aestivum Wheat breeding
ABSTRACT The objective of this paper is to review progress m ade in w heat breeding for Fusarium head blight(FHB)resistance in China,the United States of Am erica(USA),and Canada.In China,numerous Chinese landraces possessing high levels of FHB resistance were grown before the 1950s. Later, pyram iding m ultiple sources of FHB resistance from introduced germ plasm such as Mentana and Funo and locally adapted cultivars played a key role in com bining satisfactory FHB resistance and high yield potential in com m ercial cultivars.Sum ai 3, a Chinese spring w heat cultivar, becam e a m ajor source of FHB resistance in the USA and Canada,and contributed to the release of m ore than 20 m odern cultivars used for w heat production, including the leading hard spring w heat cultivars Alsen, Glenn, Barlow and SY Ingm ar from North Dakota, Faller and Prosper from Minnesota, and AAC Brandon from Canada. Brazilian wheat cultivar Frontana, T. dicoccoides and other local germ plasm provided additional sources of resistance. The FHB resistant cultivars m ostly relied on stepw ise accum ulation of favorable alleles of both genes for FHB resistance and high yield,w ith m arker-assisted selection being a valuable com plem ent to phenotypic selection.With the Chinese Spring reference genome decoded and resistance gene Fhb1 now cloned, new genom ic tools such as genom ic selection and gene editing w ill be available to breeders,thus opening new possibilities for developm ent of FHB resistant cultivars.
Contents
1. Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 731
2. Progress in breeding FHB resistant cultivars . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 732
2.1. China . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 732
2.1.1. Wheat landraces . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 732
2.1.2. Contributions of Italian germplasm. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 732
2.1.3. Cultivars with accumulated resistance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 732
2.1.4. Deployment of Fhb1 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 732
2.1.5. Breeding in the Yellow and Huai River Valleys. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 733
2.2. The USA. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 733
2.2.1. Exotic FHB resistance sources . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 733
2.2.2. Local sources of FHB resistance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 734
2.2.3. Triticum dicoccoides . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 735
2.2.4. Regional genotyping laboratories and marker-assisted selection . . . . . . . . . . . . . . . . . . . . . 735
2.3. Canada . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 735
3. Lessons learnt from the past . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 735
4. Future perspectives . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 736
Declaration of Competing Interest . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 736
Acknowledgments. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 736
References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 736
1. Introduction
Fusarium head blight (FHB), mainly caused by Fusarium graminearum Schwabe,is an economically devastating disease of wheat (Triticum aestivum L.) worldwide. FHB epidemics significantly reduce grain yield and quality[1,2].Infected grain contains toxic fungal secondary metabolites known as mycotoxins, particularly deoxynivalenol (DON), that make it unsuitable for food and feed. Outbreaks of FHB could cause severe social and economic turmoil[3].
In China,FHB was historically prevalent in the Middle and Lower Yangtze Valleys Autumn-Sown Spring Wheat Zone and the Northeastern Spring Wheat Zone but was not officially recorded until 1936 when an epidemic occurred in southern China [4]. During the second half of the last century seven severe epidemics and 14 medium outbreaks occurred in either one or both zones [5]. The frequency and severity of FHB epidemics have significantly increased in recent years, and currently the average infected area is more than 5.3 Mha annually. The 2012 epidemic was the most widespread with FHB occurring in about 9.9 Mha,including the Yellow and Huai River Valleys,the major wheat producing area in China where FHB was not previously endemic [6]. Apart from the impacts of climate change, FHB expansion to the Yellow and Huai River Valleys was mainly due to the long-term wheat-maize rotation combined with the increasing practice of reduced tillage and highly susceptible wheat cultivars grown throughout the region[7].Public concerns regarding DON contamination from FHB infection have been growing,particularly in the flour processing industry.
The earliest record of FHB infection in the USA, was in the 1890s when Chester [8], Arthur [9], and Detmers [10]independently reported the spread of FHB on wheat in Delaware,Indiana and Ohio.Later,the disease was reported throughout the cereal growing areas of the USA[11].In the 1980s and 1990s,frequent outbreaks occurred in the soft red winter (SRW) wheat areas characterized by high moisture and widespread maize cultivation.When rainfall is above average,the hard red winter(HRW)and hard red spring(HRS)wheat production areas of the eastern Great Plains can also experience severe outbreaks[3].The most recent severe epidemic in 1993 in the northern HRS wheat growing areas of Minnesota, North Dakota and South Dakota caused yield losses of 4.8 million tonnes amounting to loss of approximately 704 million US dollars, and led to a supply shortage and significant increases in wheat grain prices [12].Since 2000, FHB has been more frequent and severe in most of the hard winter wheat region in the Great Plains and has spread to other regions such as Oklahoma and Montana,where FHB had not been seen previously. Severe FHB epidemics in 2014-2016 resulted in more than 80%of infected spikes in some production fields in Georgia where wheat was planted on summer maize debris (http://swvt.uga.edu/). Increased maize production areas in these regions and climate change that favors FHB development are likely causes of the new FHB epidemics.
The first report of FHB in Canada was in 1919 [13]. From 1927 to 1980,epidemics were sporadic and largely confined to Ontario, Quebec and Manitoba (https://phytopath.ca/publication/cpds/). In the early 1980s, major outbreaks were reported in eastern Canada and part of Manitoba. The outbreak in 1980 was the first widespread FHB epidemic recorded in Canada[14]. Since then,the disease has occurred frequently in eastern Canada and become a serious problem in the west, particularly in Manitoba from 1993 [3]. The FHB epidemic in 2014 caused significant yield losses in Saskatchewan,the largest wheat producing province in Canada,where FHB had never been problem previously [15]. Ward et al. [16]reported that the more toxigenic 3-acetyl-deoxynivalenol chemotype of Fusarium graminearum, which produces more DON, was rapidly displacing the 15-acetyl-deoxynivalenol chemotype and moving westwards from the Red River Valley in Manitoba. This chemotype shift may be signaling an escalation of the FHB threat to wheat production on the Canadian Prairies[17].
No single control measure has provided complete FHB control. Some agronomic practices can reduce FHB losses,whereas wheat-maize rotations and no-till farming facilitate FHB epidemics.Some fungicides can reduce FHB severity,but significantly increase costs and are more effective for cultivars with some FHB resistance [1]. The use of host resistance remains the most effective, economical and environmentally friendly way to minimize disease losses. Significant progress has been made in developing FHB resistant cultivars in China,the USA, and Canada during the past several decades. This article reviews progress from long-term efforts in breeding for FHB resistance in the Global North.
2. Progress in breeding FHB resistant cultivars
2.1. China
2.1.1. Wheat landraces
Due to favorable environmental conditions, FHB was historically prevalent in southern wheat growing areas including Fujian, Hubei, Jiangsu, and Zhejiang. Through long-term natural and artificial selection under conditions with high FHB pressure, more than 60 FHB resistant or moderately resistant (MR) landraces were selected by local farmers,including Wangshuibai (Jiangsu), Chongyanghongmai(Hubei), Pinghujianzimai (Zhejiang), Fanshanxiaomai (Fujian)and Taiwanxiaomai (Taiwan) [18-20]. These landraces had been predominant in the region until the 1950s.They are now invaluable genetic resources in breeding for FHB resistance.
2.1.2. Contributions of Italian germplasm
In 1932, the Italian cultivar Mentana (Rieti/Wilhelmina//Akakomugi) was introduced into China and was later renamed as Nanda 2419 [21]. Its higher grain yield, early maturity, good stripe rust resistance, and moderate susceptibility(MS)to FHB ensured a quick adoption in the Middle and Lower Yangtze River Valleys and neighboring regions after 1949. The peak area of Nanda 2419 reached 4.7 Mha in 1958.Later,Wannian 2,Wangmai 15 and Emai 6 with improved FHB resistance were reselected from Nanda 2419 and became leading cultivars in the Middle and Lower Yangtze River Valleys[19,22,23].
Funo was another Italian cultivar introduced to China in 1956 with an MS response to FHB.It was selected from a cross between Duecentodieci and Damiano (a sister line of Mentana) and became a predominant cultivar during the 1960s and 1970s with a peak area of 1.2 Mha in 1977 [22]. At least five cultivars (Yangmai 1, Yangmai 2, Yangmai 3, and Wumai 1 in Jiangsu, and Ewusan 3 in Hubei) with high yield and FHB resistance were reselected from Funo. These cultivars were widely grown in the Middle and Lower Yangtze River Valleys in the 1970s and 1980s [19,24]. Funo was not only an outstanding cultivar for direct wheat production but also a milestone parent for more than 98 cultivars released prior to 1983, including the highly FHB resistant cultivar Sumai 3[22].
2.1.3.Cultivars with accumulated resistance
Sumai 3, released in 1970, is a highly resistant spring wheat cultivar developed from the cross Funo/Taiwanxiaomai by Suzhou Institute of Agricultural Sciences in Jiangsu province.Funo was MS to FHB and Taiwanxiaomai was MR to highly resistant, indicating that the resistance of Sumai 3 was contributed by both parents. Sumai 3 is recognized as the best source of FHB resistance in the world and has been widely used as an FHB resistant parent in many Chinese and international wheat breeding programs[25].
Yangmai 4 was a leading cultivar in the Middle and Lower Yangtze River Valleys released in 1984 from a cross between Line 1-3-2 (Nanda 2419/Triumph) and Axuan 2 (a reselection of Funo).Both parents were MS to FHB,but Yangmai 4 was MR,suggesting that the resistance in Yangmai 4 came from transgressive segregation.Yang 9-16,a sister line of Yangmai 4, was crossed with Italian line St1472/506, and derived MR cultivars Yangmai 5 and Yangmai 158 [24]became leading cultivars in the Middle and Lower Yangtze River Valleys from the late 1980s to 1990s [26]. Yangmai 158 with a combination of high yield potential and moderate FHB resistance was the most popular cultivar in the 1990s with a peak planting area of 1.7 Mha in 1997 [27]. The success of Yangmai 158 indicates that pyramiding of genes for both FHB resistance and high yield potential is critical to successful breeding for FHB resistance. Three additional cultivars derived from Nanda 2419 or Funo were subsequently released including Jingzhou 1 and a sister cultivar Jingzhou 47 (Nanda 2419/Jingzhou rye),and Jingzhou 66 (Funo/durum wheat//Nanda 2419/Jingzhou rye) [28]. They all had much better FHB resistance than their parents and became predominant cultivars in Hubei province.
Since 2000, several new Yangmai cultivars with moderate resistance to FHB have been released and become predominant cultivars in the Middle and Lower Yangtze River Valleys.These included Yangmai 11 with a peak planting area of 0.29 Mha in 2006, Yangmai 12 with a peak planting area of 0.14 Mha in 2006, Yangmai 16 with a peak planting area of 0.39 Mha in 2013,and Yangmai 20 with a peak planting area of 0.16 Mha in 2015(http://202.127.42.47:6006/home/bigdataindex).
2.1.4.Deployment of Fhb1
Sumai 3 continues to give high levels of resistance [25,29],which is largely conferred by gene Fhb1 and several minor QTL[30]. Sumai 3 has been used as a resistant parent in many crosses in China, but only very few cultivars actually carry Fhb1 [7]. This is probably due to linkage drag associated with Fhb1 in this donor.
Ningmai 9, a soft red wheat cultivar with Fhb1 that was released by the Jiangsu Academy of Agricultural Sciences in 1997, is an important Fhb1 donor in Chinese breeding programs; it combines high yield potential, broad adaptation and FHB resistance. Zhu et al.[7]found that various cultivars from Jiangsu province carried Fhb1, including Ningmai 13,Ningmai 14, Ningmai 16, Ningmai 18, Ningmai 26, Yangmai 18,Yangmai 21,and Zhenmai 5,and all had Ningmai 9 in their pedigrees. Among these cultivars, Ningmai 13 was widely grown with a peak planting area of 0.35 Mha in 2016 (http://202.127.42.47:6006/Home/BigDataIndex). Molecular markers and pedigree analysis traced Fhb1 to Japanese cultivar Norin 129, in which Fhb1 is likely from Shinchunaga, a well-known Japanese FHB resistant cultivar released in the 1930s [31].Norin 129 was originally used as a parent in Jiangsu Academy of Agricultural Sciences for its earliness rather than FHB resistance, but serendipitously served as an Fhb1 donor and played an important role in deployment of Fhb1 in that province. Jiangsu was the only province in China where Fhb1 was successfully deployed in commercial cultivars [7]. After the severe FHB epidemic in the Yellow and Huai River Valleys in 2012, breeders started to employ Fhb1 in their breeding programs as a means of improving the level of FHB resistance.
2.1.5. Breeding in the Yellow and Huai River Valleys
FHB epidemics have been more frequent in the Central Shaanxi Plain than other locations in the Yellow and Huai River Valleys [27]. Using Sumai 3 as a resistance donor,breeders from Northwest Agricultural and Forestry University in Shaanxi province developed various resistant lines including 84(14)43 that was later used as a major source of FHB resistance in the region [32]. The leading cultivars Zhengmai 9023(with a peak planting area of 1.8 Mha in 2005)and Xinong 979 (with a peak area of 0.82 Mha in 2013) with some level of FHB resistance were developed from 84(14)43 [33]. Neither of them contains Fhb1, indicating that the resistance was probably contributed by QTL other than Fhb1 from Sumai 3[7]. Thinopyrum ponticum as an alternative source of FHB resistance could contribute to the release of Xinong 511 and Xinong 529 with improved FHB resistance, but further confirmation is needed. Guo et al. [34]identified resistance gene Fhb7 from Th. ponticum derivatives developed by US breeding program at Purdue University, and Fu et al. [35]reported that a 7E chromosome from Th. elongatum harbored FHB resistance. These reports support the possible contribution of Thinopyrum spp.to FHB resistance in wheat.Due to the utilization of Sumai 3 and Th. ponticum as the sources of FHB resistance in breeding, cultivars from Shaanxi province generally have better FHB resistance than those from neighboring provinces [7]. Due to lack of consistent disease development in breeding nurseries, phenotypic selection might not be sufficiently effective for reliable selection of resistant genotypes.Thus,marker-assisted selection(MAS)of resistance genes is of great importance. Recently, Fhb1 was introduced into elite cultivars such as Zhoumai 16 [36]and Zhoumai 22 (Guihong Yin, personal communication, 2018)using MAS and the derivatives showed improved resistance.However, the level of FHB resistance in cultivars released from the Yellow and Huai River Valleys needs to be further increased to minimize FHB damage. In many ways, breeding for resistance to FHB in that region is still in its infancy and development of high yielding cultivars with moderate to high FHB resistance remains a huge challenge. Speed breeding could accelerate development of resistant cultivars by transplanting winter wheat seedlings at Sanya in Hainan province(18°N,109°E)after natural vernalization in Beijing in late November.Harvested seed from Sanya can be sown again in Beijing in late January or early February the following year.Using this strategy, breeders in several breeding programs in northern China are growing two generations in a traditional single crop season. To develop MS to MR cultivars more quickly for this region, we propose to: (1) firstly deploy Fhb1 using newly-released cultivars such as Yangmai 30, Ningmai 26 or Shengxuan 6 as Fhb1 donors rather than Sumai 3,(2)use locally adapted high yielding cultivars as recipients, and (3)use the diagnostic marker to select Fhb1 in large backcross populations (about 1000 plants) and background markers to recover the desirable features of the recurrent parents.For the longer term, other important genes/QTL such as Fhb2 [37],Fhb4[38],Fhb5[39],Fhb7[34],and the QTL on chromosome 2DL[40]will be gradually introduced into new cultivars after Fhb1 is transferred. Continuing investigations of FHB resistance in indigenous accessions could also lead to the discovery of new sources of FHB resistance.
2.2. The USA
After experiencing severe losses from frequent FHB outbreaks in the soft winter and hard spring wheat regions across the country, scientists from federal, state and private sectors established a unique program called the“U.S.Wheat&Barley Scab Initiative” (USWBSI, http://scabusa.org/) in 1997. The objective was to develop effective control measures to reduce the threat of FHB [41]. Over the past two decades, this national, multi-disciplinary, and multi-institutional research system has been fully supported by US government appropriated special funding to ensure long-term continuation of research and cultivar development.After more than 20 years,the levels of FHB resistance in US cultivars have been improved by combining major QTL from exotic resistant sources of eastern Asian and Brazil, with minor variations in well-adapted local cultivars. Alsen that was released as a MR cultivar in the northern HRS wheat regions in 2000 is one example. Subsequent release of new cultivars led to a great increase in planting area of MR cultivars in the region[42,43].
2.2.1.Exotic FHB resistance sources
With successful application of MAS for Fhb1 and speed breeding using shuttle breeding or off-season greenhouse generation advancement strategies [44], significant progress was made in breeding for FHB resistance in HRS wheat breeding programs. From 1999, at least 18 HRS cultivars were released in North Dakota, Minnesota and South Dakota, all with Sumai 3 in their pedigrees (http://www.wheatpedigree.net) (Fig. 1). These three states account for most of the HRS wheat production in the USA with 2,650,691, 651,544, and 424,920 ha planted in 2018, respectively (https://www.nass.usda.gov). Several Sumai 3-derived MR cultivars have been predominant in North Dakota (http://www.wheatpedigree.net), including Alsen (2002-2006), Glenn (2007-2011), Barlow(2012-2015), SY Soren (2016), and SY Ingmar (2017-2018).Taking 2017 as an example, cultivars SY Ingmar, SY Soren,Barlow, Glenn, Prosper and Faller were ranked 1st, 2nd, 4th,7th, 8th and 9th, respectively, in planted area and occupied 48.6% of the area of spring wheat in North Dakota (https://www.nass.usda.gov/Statistics_by_State/North_Dakota/Publications/Annual_Statistical_Bulletin/index.php). In South Dakota, Sumai 3-derived cultivars Prevail, Focus and Brick ranked 1st, 5th and 6th, respectively, and occupied 36.1% of the HRS wheat area in that state in 2016 (https://www.nass.usda.gov/Statistics_by_State/South_Dakota/Publications/Annual_Statistical_Bulletin/index.php). In Minnesota, Sumai 3-derived Ning 8331 was used as a source of resistance for McVey (released in 1999) and Sabin (released in 2009) [45].Norden with FHB resistance contributed by Alsen was released for its FHB resistance in 2012. Two sister cultivars,Faller and Prosper,were jointly released by North Dakota and Minnesota and were leading cultivars in Minnesota in 2009-2012 and 2013-2015,respectively[46].
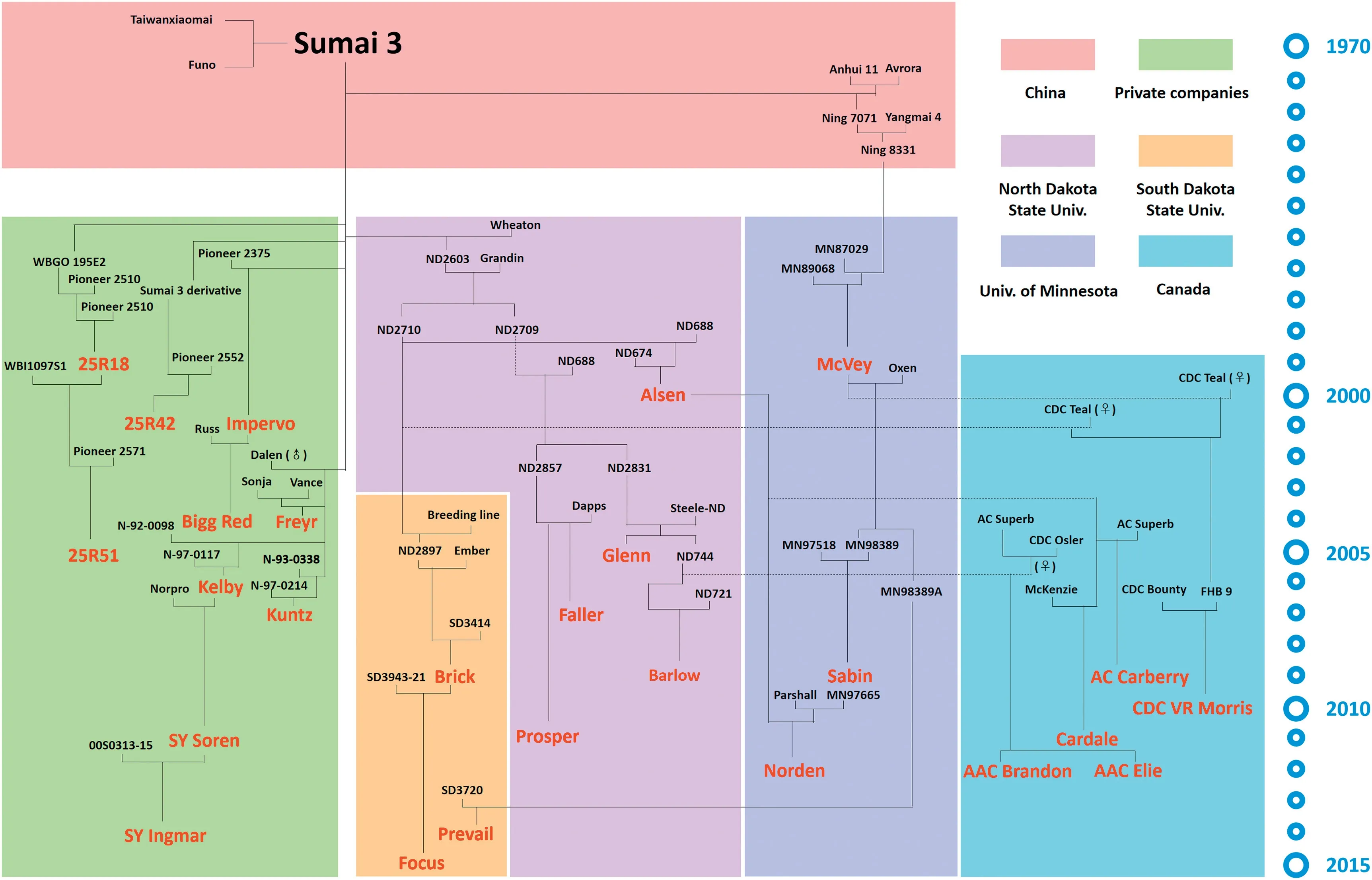
Fig. 1-Sumai 3-derived wheat cultivars(marked in red)released in North America by public and private breeding programs.
Sumai 3 was also used to develop FHB resistant winter wheat cultivars.The SRW wheat cultivars Pioneer 25R18,Pioneer 25R42 and Pioneer 25R51 were developed by the Pioneer breeding company (Fig. 1). In the US HRW region, Sumai 3 and its derivatives were used as a source of resistance for decades, but resistance was never incorporated into HRW cultivars apparently due to poor adaptation traits in Asian germplasm.
Prior to utilization of Sumai 3, the FHB-resistant Brazilian cultivar Frontana(Fronteira/Mentana)was extensively used in US wheat breeding programs primarily as a source of resistance to leaf rust. Willet (Frontana/Thatcher), the first released cultivar with Frontana as a parent, was released in Minnesota in 1954. To date, more than 100 hard spring cultivars have Frontana in their pedigrees (http://www.wheatpedigree.net/), but the genetic contribution of Frontana to FHB resistance in these cultivars remains unknown.Different from Sumai 3, the FHB resistance in Frontana is mainly type I, resistance to initial infection [47]. Pyramiding type I with type II resistance(to fungal spread within a spike)from Sumai 3 may achieve a higher level of resistance.
2.2.2.Local sources of FHB resistance
Although some exotic materials had superior FHB resistance,these sources usually produced low yield and were poorly adapted to local environments;local sources of resistance,by contrast,were more easily used in breeding because of better yield and adaptation.Nationwide screening of locally adapted materials identified many resistant and MR US winter wheat cultivars, including SRW cultivars Massey (released in 1981),Roane (1999), Tribute (2002), USG 3555 (2007), and Jamestown(2008) released by Virginia Tech, and Ernie (1994), Truman(2004) and Bess (2005) released by the University of Missouri.These cultivars all have moderate FHB resistance and were used in wheat production in eastern USA[48,49].Jamestown is currently used as the main local source of FHB resistance in SRW wheat and serves as a MR check in the Southern Uniform Winter Wheat Scab Nursery(SUWWSN).Other cultivars,such as Freedom (1991), Goldfield (1999), NC-Neuse (2003), and INW0411 (2004) also have acceptable levels of resistance[25,50].
Among HRW wheat materials, Heyne, Everest, Overland and Lyman were reported to carry local source of resistance genes [25]. Everest was the leading cultivar in Kansas from 2013 to 2018 and was planted on 9.3%-15.8%of the wheat area in this state.Overland and Lyman represented approximately 13.5%of the total winter wheat area in South Dakota and 20%in Nebraska in 2016 [43]. Mapping QTL underlying resistance in these cultivars is in progress at the USDA Central Small Grain Genotyping Laboratory at Manhattan, Kansas.
2.2.3. Triticum dicoccoides
The North Dakota breeding program has,for a long time,used T.dicoccoides to incorporate grain protein content(GPC)genes from T.dicoccoides into the HRS wheat.The derivatives were also tested for FHB response to search for new sources of resistance.In 2004,Steele-ND with T. dicoccoides in its pedigree [51]was released as the first HRS cultivar with acceptable non-Sumai 3 FHB resistance. Subsequently, Howard [52]was released with the same source of FHB resistance. The 2005-released cultivar Glenn may have also inherited FHB resistance from Steele-ND.The presence of FHB resistance in these cultivars is strongly indicative of a contribution of T.dicoccoides to FHB resistance although it remains to be confirmed.
2.2.4. Regional genotyping laboratories and marker-assisted selection
To facilitate application of modern genomic technologies in public small grain breeding programs, the first USDA Small Grain Genotyping Laboratory was established at Manhattan KS in 2001. Three other regional small grain genotyping laboratories were set up subsequently at Raleigh NC, Fargo ND, and Pullman WA. The Manhattan laboratory serves hard winter wheat breeders in the US Great Plains, and the other three laboratories at Raleigh, Fargo, and Pullman serve soft winter wheat breeders in eastern USA,hard spring wheat and other small grain breeding programs in the northern Great Plains, and small grain breeding programs on the west coast,respectively.Improving FHB resistance is a major focus in the first three laboratories due to the severe FHB epidemics in the corresponding regions. For example, the Raleigh laboratory tests all entries of the Northern and Southern Uniform Winter Wheat Scab Nurseries (NUWWSN and SUWWSN) with more than ten molecular markers. Those markers are the best indicators for presence of QTL or genes on chromosomes 3BS(Fhb1),3BL(Massey),5A(Ernie),5A(Ning 7840),2DL(Wuhan 1/W14), 1BL (Jamestown), 1A/4A/6A (NC-Neuse), and 2B/3B(Bess) (https://scabusa.org/research_vdhr). The laboratory at Manhattan has cloned Fhb1 and developed diagnostic markers for Fhb1 [53,54]that has been used in many wheatbreeding programs worldwide [36]. Using marker-assisted backcrossing the laboratory transferred Fhb1 to 16 different locally adapted HRW wheat backgrounds, providing strong incentives for breeders to deploy Fhb1 in HRW wheat[25].The laboratory at Fargo routinely screens for presence of Fhb1 with molecular markers and assists breeders in improving the accuracy and efficiency for selecting this gene in HRS wheat.
In addition to use of limited numbers of markers in MAS,genomic selection based on estimated genome-wide breeding values (GEBV) is becoming increasingly important for selecting complicated traits such as FHB resistance. The potential of genomic selection to improve FHB resistance was evaluated in spring wheat of the Pacific Northwest [55],winter wheat in eastern USA and Canada [56], and winter wheat lines bred or collected by the University of Illinois [57].Moderate to high prediction accuracies were achieved for FHB response. However, release of an FHB resistant cultivar using this strategy has not been reported.
2.3. Canada
The prairie provinces of Saskatchewan,Alberta and Manitoba produce more than 90% of Canadian wheat with the remainder coming from British Columbia and eastern Canada. FHB epidemics in eastern Canada (1980) and Manitoba (1993)spurred FHB research and development of FHB resistant cultivars in Canada[58].
In the early stages of breeding for FHB resistance, cultivar Frontana was used as the primary source of resistance in Canadian spring wheat breeding [59]. The Canadian western red spring (CWRS) wheat cultivar Neepawa with lower FHB severity than most other cultivars under moderate disease conditions [60]may have inherited resistance from Frontana.Several Neepawa derivatives, including Stettler, Katepwa, AC Barrie and AC Cora have similar levels of FHB resistance as Neepawa, indicating the value of Frontana as a resistance source [61]. McCartney et al. [15]observed that cultivars CDC Bounty, AC Cadillac, Journey, Kane, McKenzie, 5500HR,5601HR,and 5602HR had intermediate levels of FHB resistance that was not derived from Asian sources.
North Dakota cultivars or elite breeding lines such as Alsen[44]and ND744 [62]were used as bridging parents to transfer resistance genes from Sumai 3 into Canadian wheat. At least five wheat cultivars grown in Manitoba,the province with the most severe FHB epidemics in Canada,were related to Sumai 3: AAC Brandon, AAC Elie, Cardale, AC Carberry and CDC VR Morris (Fig. 1). These cultivars ranked 1st, 2nd, 3rd, 5th, and 19th in the wheat planting area in Manitoba in 2018 and occupied 65.1%,8.6%,7.2%,2.1%,and 0.3%,respectively,of the spring wheat area. AAC Brandon was the most widely grown cultivar not only in Manitoba but also over the entire Canadian prairies in 2018 (https://www.masc.mb.ca/masc.nsf/sar_varieties_2018.pdf). Cultivars with Sumai 3 in their pedigrees appear to have superior FHB resistance compared to cultivars previously released in this area. The popularity of these cultivars suggests that FHB resistance is becoming an important criterion for choice of cultivars by producers.
Winter wheat in eastern Canada is mainly produced in the province of Ontario, which constitutes approximately 85%SRW, 10% HRW, and 5% soft white winter (SWW). FHB is a major threat to wheat production in this area. Doubled haploids and MAS is the preferred breeding method in breeding for FHB resistance and has been particularly effective in breeding local winter wheat genotypes that require a long period of vernalization. Local winter wheat cultivars AC Morley(MR)and Emerson(R)likely carry different QTL for FHB resistance. These cultivars are being characterized because transgressive segregants with higher resistance than either parent are common when they are used as parents[63].
3. Lessons learnt from the past
Germplasm exchange has set a foundation for global FHB resistance improvement in wheat. Asian germplasm, such as Sumai 3 and its derivatives, has been successfully used in North America, where many leading cultivars carry FHB resistance from that source (Fig. 1). European germplasm such as Italian cultivars were introduced into China and used either directly in wheat production or as donors for improvement of both grain yield and FHB resistance[27].The Brazilian cultivar Frontana has also contributed to some extent to the improvement of FHB resistance in North America. These successful stories clearly illustrate the significance of germplasm exchange for improvement of FHB resistance on a global scale.
Depending upon the frequency and severity of FHB epidemics, wheat breeding programs from different regions selected different sources of resistance and breeding approaches for trait improvement. In the case of hard spring wheat in the USA, FHB epidemics have been frequent and severe, thus FHB resistance is an essential trait in breeding thus justifying the use the most resistant germplasm such as Sumai 3 and its derivatives as the source of resistance.In the early stages of breeding, compromises between yield and superior resistance might be a necessary outcome exemplified as the North Dakota lines ND2603 and ND2710. These breeding lines with exotic resistance genes were mainly used as bridging parents in further crosses to high yielding parents for new cultivar development. Continued breeding and selection eventually produced cultivars such as Alsen and Glenn that combined both high grain yield and improved FHB resistance [64]. FHB tends to occur more sporadically in the HRW wheat regions although incidence appears to be increasing. To date, pyramiding of local sources of minor effect on FHB resistance in hard winter wheat has made significant progress. Cultivars such as Everest, Overland and Lyman have been leading cultivars with acceptable levels of FHB resistance. Availability of 16 Fhb1-carrying HWW lines developed by USDA Central Small Grain Genotyping Laboratory provides ideal bridging parents for more rapid deployment of Fhb1 in this region.Combining major resistance gene(s)such as Fhb1 from spring wheat or other genetic resources with minor genes from local sources will significantly enhance the level of FHB resistance in new cultivars.
Molecular markers and speed breeding are being integrated into traditional breeding programs.Breeders are able to select resistant plants even when FHB is absent in some environments. Doubled haploid breeding to quickly fix FHB resistance genes has been more frequently used in Canadian wheat breeding programs. Although these strategies and newly emerging tools such as genomic selection are becoming more common, accurate phenotypic evaluation of FHB resistance remains paramount for breeders and any predicted performance must be upheld in farmer's fields.
4. Future perspectives
FHB is the most important wheat disease in China and had caused the largest yield losses among all wheat diseases from 2010 to 2015 [65]. There is an increasing public concern on mycotoxins and food safety and security caused by FHB infection. Although FHB has attracted some attention from hard winter wheat breeders in China, FHB resistance is not a priority objective in most current breeding programs.Flexible policies should be adopted to facilitate the release of cultivars with superior FHB resistance in order to permit farmers to sacrifice yield potential for greater protection from FHB.Taking Chinese cultivar registration as an example, cultivars with MR or MS ratings for FHB could be released in the Northern Yellow and Huai River Valleys even when yield potential is slightly lower than high-yielding check.
The successful cloning of Fhb1 [53,66]and development of diagnostic markers [54]will greatly facilitate deployment of this important gene in breeding particularly in China because some previous markers were not diagnostic across Chinese germplasm [54]. The cloning of the gene will also provide opportunities for breeders to use new biotechnologies such as gene transformation and gene editing in breeding to create new sources of resistance and to accelerate the breeding process. It is expected that genetically modified (GM) FHB resistant wheat cultivars will be beneficial to farmers for FHB control once GM wheat is approved for production by government.
In conclusion,the currently available resistant germplasm,characterized resistance genes, reliable markers, and flexible policies on cultivar release should enable routine release of new cultivars with better yield potential and high FHB resistance in the very near future.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Acknowledgments
This work was financially supported by the National Key Research and Development Program of China(2017YFD0101000), International Scientific and Technological Cooperation Project (2016YFE0108600), and Agricultural Science and Technology Innovation Program of CAAS.
- The Crop Journal的其它文章
- The Crop Journal 作物学报(英文版) (Started in 2013, Bimonthly)
- Brief Introduction
- Integrated transcriptomics and metabolomics analyses provide insights into cold stress response in wheat
- Assessment of the individual and combined effects of Rht8 and Ppd-D1a on plant height,time to heading and yield traits in common wheat
- Diversity and sub-functionalization of TaGW8 homoeologs hold potential for genetic yield improvement in wheat
- Comparative FISH and molecular identification of new stripe rust resistant wheat-Thinopyrum intermedium ssp. trichophorum introgression lines

