Clostridium butyricum alleviates intestinal low-grade inflammation in TNBS-induced irritable bowel syndrome in mice by regulating functional status of lamina propria dendritic cells
Qin Zhao, Wen-Rong Yang, Xiao-Hong Wang, Gai-Qin Li, Lei-Qi Xu, Xiao Cui, Yang Liu, Xiu-Li Zuo
Abstract BACKGROUND Irritable bowel syndrome (IBS) is one of the most common functional gastroenterological diseases characterized by abnormal visceral sensitivity and lowgrade inflammation. The role of Clostridium butyricum (C. butyricum) in reducing intestinal low-grade inflammation via immune pathways has been well defined.However, the detailed mechanisms of the effects of C. butyricum on intestinal mucosal immunity, especially on immune cells of the lamina propria, remain unclear. Dendritic cells (DCs), which are important immune cells, secrete proinflammatory cytokines (IL-1β, IL-6, and others) and express T cell immunoglobulin and mucin domain-3 (TIM3), promoting proliferation and activation of DCs, and mediating Th1 and Th17 inflammatory responses.AIM To investigate the role of DCs in the development of IBS in a rat model and to understand the regulation of DCs after C. butyricum intervention.METHODS An IBS animal model was established using C57BL/6 mice, and C. butyricum was continuously administered via the intragastric route to simulate different intestinal immune states. Intestinal visceral hypersensitivity and histopathology were assessed using the abdominal withdrawal reflex (AWR) test and hematoxylin & eosin (H&E) staining, respectively. The expression of proinflammatory cytokines (IL-1β and IL-6) and TIM3 was analyzed by Western blot analysis and real-time PCR. Flow cytometry was applied to analyze the quantity, function, and membrane molecule TIM3 of the lamina propria dendritic cells (LPDCs). The regulatory effect of C. butyricum was verified in bone marrowderived dendritic cells by in vitro experiments.RESULTS The secretion of proinflammatory cytokines (IL-1β and IL-6) in mice with IBS was significantly increased compared with that of the control group, which suggested that the intestinal mucosa in mice with IBS was in a low-grade inflammatory state. The expression of CD11C+CD80+ and CD11c+TIM3+ in intestinal LPDCs in mice with IBS increased significantly. Meanwhile, the cytokines (IL-1β and IL-6)were significantly reduced after the intervention with probiotic C. butyricum. The amount and function of LPDCs and the TIM3 on the surface of the LPDCs were decreased with the alleviation of the intestinal inflammatory response.CONCLUSION The results suggest that C. butyricum regulates the amount and functional status of LPDCs in the intestinal mucosa of mice with IBS, and therefore modulates the local immune response in the intestine.
Key words:Clostridium butyricum; Irritable bowel syndrome; Lamina propria dendritic cells; T cell immunoglobulin and mucin domain-3; Proinflammatory cytokines
INTRODUCTION
Irritable bowel syndrome (IBS) is one of the most common functional gastroenterological diseases which affects approximately 15% of the population worldwide[1]. IBS is thought to result from the activation of the mucosal immune system and the disruption of the epithelial barrier by the intestinal microbiota[2-4].Previous studies have reported that the intestinal flora interacts with the intestinal immune system to maintain intestinal homeostasis[5,6]. Therefore, an intervention with probiotics may be salutary for IBS treatment.
The probiotic Clostridium butyricum (C. butyricum) is a type of Gram-positive anaerobic bacterium, which has been used for the treatment of IBS in clinical settings.It has been documented that the intragastric administration of C. butyricum significantly reduces intestinal inflammation and ameliorates the intestinal microbiota[7-9]. Besides, Clostridium species have been reported to affect the accumulation of intraepithelial lymphocytes (IELs) in the colon[10]and to increase the number of Treg cells, thereby inhibiting the expression of inflammatory cytokines(tumor necrosis factor [TNF]-α, interleukin [IL]-12, IFN-r, IL-1β, and IL-6) and upregulating the expression of inhibitory cytokines (IL-10)[11-15]. Other studies provided evidence that Clostridium species might suppress the intestinal inflammatory response via immune pathways[15-17].
The lamina propria dendritic cells (LPDCs) are important antigen-presenting cells in the intestines. They are now recognized to be essential for innate and acquired immunity[18,19]. LPDCs have a particular capacity to sample antigens from peripheral tissues and efficiently activate resting T cells and direct T cell bias (Th1, Th2, and Th17)[20]. In the steady state, LPDCs induce the differentiation of regulatory T cells to produce IL-10 and transform growth factor-beta (TGF-beta)[19,21]. Studies on LPDCs in IBS or IBD patients have revealed that LPDCs were significantly increased and the costimulatory molecules (CD80, CD86, and MHCII) were upregulated, the secretions of IFN-g, IL-1β, IL-6, IL-12, and TNF-α were increased, and the migration and proliferation of CD4+ T cells were enhanced. These regulations were found to be correlated with Th1 and Th17 inflammatory responses[22-24]. The immunoregulatory function of LPDCs is related to their subpopulation and the surface receptors[25]. The local microenvironment likely plays an important role in defining the phenotype and activation of dendritic cells (DCs).
Recently, detailed analyses of the effect of C. butyricum on LPDCs have been mainly focused on cytokines; e.g., C. butyricum was revealed to promot Treg cell generation in the intestine through the induction of TGF-β1 from LPDCs[16]. This strain negatively regulated the expression of IL-12/IL-23p40[26]. However, to date, there is no study that reveals the effect of C. butyricum on surface molecules of LPDCs . T cell immunoglobulin and mucin domain-3 (TIM3) is an immunoregulatory factor expressed on the surface of DCs[27,28]. It upregulates the secretion of the proinflammatory cytokines (TNF-α and IL-1β), promotes cell proliferation and activation,and eliminates various pathogens[29-32]. To our best known, the regulatory effect of C.butyricum on TIM3 has not been reported until now.
This study aimed to investigate the role of the probiotic C. butyricum in regulating the quantity and function of LPDCs and the membrane molecule TIM3 of the LPDCs in mice with IBS and to further elucidate the effects of the probiotic C. butyricum on the local immune response in the intestinal mucosa.
MATERIALS AND METHODS
Induction of PI-IBS in mice
Twenty-four C57BL/6 male mice (aged 6-8 wk) were randomly divided into four groups (normal control, IBS, IBS + C. butyricum intervention, and IBS + normal saline(NS) intervention (n = 6 per group)). Pentobarbitone (50 mg/kg intraperitoneal administration) was used to establish deep anesthesia in these mice[33]. A plastic catheter (outer diameter = 4 mm) was inserted into the descending colon of each mouse to a depth of 4-6 cm from the anus. 2,4,6-trinitrobenzenesulfonic acid (TNBS)(P2297, Sigma, Shanghai; 0.1 mL of 5% (w/v), diluted to 0.2 mL using 50% ethanol)was slowly instilled. In the control group, the clysis was replaced by an injection of NS. For the other groups, the mice were inverted for 10 min to prevent drug leakage after clysis. Four weeks after TNBS administration, C. butyricum (QingDao EastSea Pharmaceutical Co., Ltd., QinDao, China) was continuously administered via the intragastric route to 6 mice during 1 wk (200 μL/d; concentration of live bacteria concentration, 1 × 108CFU/mL). NS in the same amount was administered to the mice in the control group. Hematoxylin & eosin (H&E) staining and the abdominal withdrawal reflex (AWR) test were used to evaluate the degree of colon inflammation.
H&E staining
To evaluate the inflammatory grade of colon tissue in the four groups, formalin-fixed mouse colon tissue was embedded in paraffin, sectioned at 4 μm, and stained with H&E (Beijing Coribo Technology Co. Ltd., Beijing, China) for histological analysis to assess conditions such as epithelial erosion, crypt loss, and inflammatory infiltration with lymphocytes. A pathologist blindly assessed and assigned an inflammatory grade to each section.
AWR test
The AWR test was perfomed at day 28 to assess visceral hyperalgesia in response to colorectal distention (CRD)[34]. A disposable silicon balloon-urethral catheter for pediatric use (6 Fr, Terumo, Tokyo, Japan) was used. Mice were briefly anesthetized with ether. The balloon was inserted into the rectum until the catheter was positioned to the anus (2 cm distal from the end of the balloon). The catheter was fixed to the base of the tail to prevent detachment. After waking up and adapting for 1 hours,CRD was performed in a stepwise fashion. An ascending-limit phasic distention (0.25,0.35, or 0.50 mL) was applied in 30 s every 4 min. The AWR was semiquantitatively scored as previously described[35]. The AWR score was assigned as follows: 0 = no behavioral response to distension; 1 = brief head movements followed by immobility;2 = contraction of abdominal muscles without lifting of the abdomen; 3 = lifting of the abdomen; 4 = body arching and lifting of the pelvic structure.
Extraction of LPDCs
The mice were sacrificed by cervical dislocation, and their colon, rectum, fat, and mesentery were extracted. The colon was washed and cut into 5-mm sections,incubated in a solution containing 2% fetal calf serum (FCS) and 5 mmol/L EDTA(Sigma) for 30 min. The tissue fragments were filtered through a screen to remove the epithelial cells and were collected into a small bottle where they were washed, cut up,and incubated in RPMI-1640 containing 2% FCS, collagenase IV (4 mg/mL, Solarbio,Beijing, China), and DNase I (10 μg/mL, Solarbio) for 30 min. The suspension was stirred at 37°C, then the released cells were collected through a 300-mesh screen. The isolated cells were separated on a discontinuous 40%/75% Percoll (Sigma) gradient.The typical yield was 2-3 × 106lymphocytes/mouse.
Culture of bone marrow-derived DCs (BMDCs)
DCs were generated in vitro from bone marrow cells from 6- to 8-wk-old wild-type C57BL/6 mice[36]and were resuspended in 10% FCS medium (RPMI-1640: Gibco, Los Angeles, United States; GM-CSF: Biosciences, San Jose, CA, United States, [20 ng/mL]; IL-4: PeproTech, Rocky Hill, United States, [10 ng/mL]; penicillin [100 U/mL], and streptomycin [100 U/mL]). The cells were cultivated in a 6-well plate in an environment containing 5% CO2at 37 °C. A half volume of culture medium was replaced every other day. On the 7thday, penicillin-streptomycin was removed while replacing the medium. The collected DCs were separately co-cultured with 100 μL of the C. butyricum culture supernatant and 100 μL (concentration, 1 × 108CFU/mL) of the live bacterial suspension for 4 hours and then stimulated with 0.1 μg/mL of lipopolysaccharide (LPS; Sigma-Aldrich) for 3 hours. The DC cells were subsequently collected, and the supernatant was cultured for evaluation using flow cytometry and enzyme-linked immunosorbent assay (ELISA).
Flow cytometry
The colonic lamina propria cells were collected and suspended (1 × 106cells/mL) in PBS with 2% FCS. The DCs were marked with anti-CD11c- FITC, anti-CD80-PEcy5.5,and anti-TIM3-APC (eBioscience, CA, United States). The stained cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, USA), and the data were collected and analyzed with FlowJo software (Tree Star Inc., Stanford,United States).
Western blot analysis
The colon tissues were homogenized and ultrasonically treated on ice for cell lysis and protein extraction. After determining the protein content, the degeneration process was performed at 100 °C for 5 min. The proteins were separated using 10%SDS-PAGE and transferred to PVDF membranes (0.4-5 μm pore size; Millipore,United States). After blocking with 5% skimmed milk, the membranes were incubated with rabbit anti-TIM3 (1:2000), anti-IL-1β (1:2000), and anti-IL-6 (1:2000) antibodies(Abcam, Cambridge, UK) at 4 °C overnight and then incubated with secondary antibodies labeled with horseradish peroxidase (1:10000, Zhongshan Gold Bridge,Beijing, China) for 2 h at room temperature. The immunoblots were detected using an enhanced chemiluminescent substrate (Millipore) on the ChemiDoc MP system (Bio-Rad, United States). All of the detected protein bands were standardized against βactin. The semiquantification of each band was performed using ImageJ NIH software(National Institutes of Health, Bethesda, MD, United States).
ELISA
Different groups of DC culture supernatants were collected and centrifuged at 300 g for 10 min at 4 °C. The supernatants were collected and stored at -80 °C until use.According to the manufacturer's instructions, an ELISA kit (Tianjin Anoric Biotechnology CO, Ltd.) was used to measure the mucosal cytokine levels of IL-1β and IL-6. The results are expressed as total protein (pg/mL).
Quantitative real-time PCR
Total RNA was extracted using Trizol (Invitrogen, San Diego, CA, USA). The total RNA (1 μg) was reverse transcribed into cDNA using the ReverTra Ace®qPCR RT Kit(TOYOBO, Japan) in a Mastercycler thermal cycler (Eppendorf, German). Real-time PCR was performed using the SYBR Green reagent (Takara, Japan) in a fluorescence thermocycler (LightCycler; Roche Diagnostics, Mannheim, Germany). The relative mRNA expression was determined after normalizing to β-actin levels using the 2-ΔΔCTmethod with the following sequence-specific primers: TIM3 forward, 5′-GC TAC GTC AAC AGC CAG CAG-3′ and reverse, 5′-CCA ATG AGG TTG CCA AGT GA-3′; IL-1β forward, 5′-GAA CCT AGC TGT CAA CGT GTG G-3′ and reverse, 5′-GCA ATG TGC TGG TGC TTC AT-3′; IL-6 forward, 5′-GCT AAG GAC CAA GAC CAT CCA-3′ and reverse, 5′-GAC CAC AGT GAG GAA TGT CCA-3′.
Statistical analysis
The statistical analyses were performed using SPSS Statistics 17.0 software. All the values are expressed as the mean ± standard error of the mean (SEM). For multiple comparisons, one-way analysis of variance (ANOVA) was applied. The comparisons with P < 0.05 were considered statistically significant.
RESULTS
Histopathological findings of the colonic mucosa
As shown in Figure 1, histological examination showed no difference with regard to morphology (epithelium erosion, crypt loss, and inflammatory infiltration with lymphocytes) between the normal control and IBS groups in H&E staining.Furthermore, no difference in morphology was observed between the IBS + C.butyricum and IBS + NS groups.
C. butyricum alleviates intestinal visceral hypersensitivity in TNBS-induced IBS mice
To determine whether C. butyricum treatment could reduce intestinal visceral hypersensitivity in IBS, C. butyricum was continuously administered via the intragastric route to IBS groups during 1 week. The degree of colon pain threshold pressures was assessed by the AWR test. As shown in Figure 2, the AWR scores for the IBS group were significantly increased compared with those for the normal control group at distention volumes of 0.25, 0.35, and 0.5 mL (P < 0.05). Similarly, C.butyricum treatment significantly decreased the AWR scores at the same distention volumes (P < 0.05).
C. butyricum suppresses the production of proinflammatory cytokines in TNBSinduced IBS mice
We further investigated the effects of C. butyricum on the expression of IL-1β and IL-6 proteins and their corresponding mRNAs in mice with TNBS-induced IBS. As shown in Figure 3, inflammatory cytokines (IL-1β and IL-6) were found to be significantly higher in the colon of mice with IBS compared with those in the normal control group(n = 6, P < 0.01). However, treatment with C. butyricum significantly reduced the expression of IL-1β and IL-6 proteins and the corresponding mRNAs (n = 6, P < 0.05).These results suggested that the intestinal mucosa in mice with IBS was in a lowgrade inflammatory state. And C. butyricum might suppress the production of proinflammatory cytokines induced by TNBS stimulation.
In the absence of colonic mucosa histopathology, low-grade inflammation together with visceral hypersensitivity and increased inflammatory cytokines were observed in the PI-IBS animal model.
C. butyricum regulates the quantity and function of LPDCs in mice with TNBSinduced IBS
To evaluate the effects of C. butyricum on colonic LPDCs, LPDCs were extracted and analyzed using flow cytometry. Figure 4A-B shows that TNBS induced an increase in the number of CD11c+ LPDCs (IBS group: 56.90% ± 1.86% and IBS + NS group:52.70% ± 1.13%) compared with that of the normal control group (36.43% ± 1.67%) (n= 6, P < 0.001). After treatment with C. butyricum, the number of CD11c+ LPDCs markedly decreased (34.15±1.54%), which was similar to that of the normal control group (n = 6, P > 0.5). Simultaneously, the amount of CD11c+CD80+ LPDCs increased in the IBS group (48.23% ± 2.92%) and in the IBS + NS group (47.20% ± 3.10%)compared with that of the normal control group (29.32% ± 1.19%). However, the amount of CD11c+CD80+ LPDCs was restricted by the C. butyricum treatment (28.37%± 1.39%) (n = 6, P < 0.001).
Also, we investigated the level of an immunoregulatory molecule, TIM3, in LPDCs.Flow cytometry analysis was used to quantify the number of CD11c+TIM3+ LPDCs.Figure 4C shows that the levels of CD11c+TIM3+ LPDCs at the IBS low-grade inflammatory stage were significantly increased compared with that of the normal control group (normal control group: 25.60% ± 1.42%; IBS group: 38.52% ± 2.23%) (n =6, P < 0.01). C. butyricum reduced the increase in the number of CD11c+TIM3+ LPDCs of the IBS group (IBS + C. butyricum group: 26.52% ± 1.78% and IBS + NS group: 37.40± 1.53%) (n = 6, P < 0.01) to match the number of TIM3+CD11c+ LPDCs of the normal control group (n = 6, P > 0.05). The other two methods (real-time PCR and Western blot analysis) also confirmed this conclusion at the protein and RNA levels (Figure 4E and F).
Figure 1 Photomicrographs of hematoxylin and eosin staining. There was no difference with regard to morphology between control (A), IBS (B), IBS + C. butyricum (C), and IBS + NS (D) tissues in hematoxylin and eosin staining (original magnification, ×200) (n = 6 per group).
Collectively, based on our results, we concluded that C. butyricum not only reduced the number and function of LPDCs in TNBS-induced IBS mice but also downregulated the expression of the immunoregulatory molecule TIM3 in LPDCs.
C. butyricum alleviates the proinflammatory activation of BMDCs in vitro
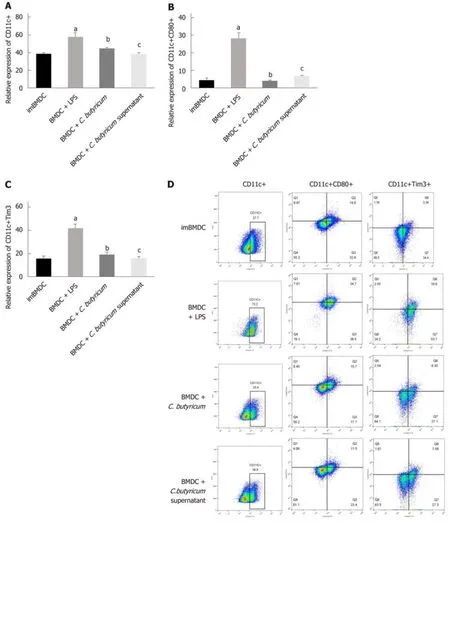
We examined the effect of C. butyricum on the BMDCs using flow cytometry analysis.Figure 5A-D shows that the C. butyricum treatment significantly decreased the number of CD11c+TIM3 + BMDCs (19.28% ± 1.57%) compared to that of the LPS group (43.33% ± 4.59%) (n = 6, P < 0.01). The number of CD11c+CD80+ DCs was also decreased in the BMDCs + C. butyricum group (n = 6, P < 0.001). The production of proinflammatory cytokines (IL-1β and IL-6) was also examined in BMDCs using ELISA. Figure 5E and F shows that C. butyricum markedly inhibited the LPS-induced secretion of IL-1β and IL-6 (n = 6, P < 0.05).
These results indicated that C. butyricum inhibited the proliferation and activation of BMDCs and reduced the secretion of pro-inflammatory cytokines, but downregulated the expression of the TIM3 on the membrane of BMDCs.
DISCUSSION
IBS is related to various physiological alterations such as changes in the composition of gut microbiota, immune activation, gastrointestinal (GI) dysmotility, hypersensitivity, and the gut mucosal barrier[2,4,37,38]. Although extensive studies have recently focused on the correalations between intestinal microenvironment and immune activation, associated mechanisms have been rarely reported.
Probiotic C. butyricum produces butyric acid, which plays an important role in intestinal function by enhancing the intestinal barrier, improving the intestinal microbiota, regulating the immune system, and promoting gastrointestinal motility[39,40]. In this study, we found that in TNBS clysis-induced PI-IBS mice, the intragastric administration of C. butyricum significantly alleviated intestinal visceral hypersensitivity and reduced low-grade mucosal inflammation. Some investigators have proposed chronic low-grade mucosal inflammation as a potential etiological factor[41]. Particularly, the changes in the cytokines IL-1β and IL-6 secreted by LPDCs contribute to the development of IBS[42,43]. Our results showed that the important proinflammatory cytokines IL-1β and IL-6 were significantly increased in a murine model of IBS (n = 6, P < 0.01). However, these two inflammatory cytokines were both significantly suppressed (n = 6, P < 0.01) after the intervention with C. butyricum. Such results provide evidence for the anti-inflammatory activity of C. butyricum[7-9].
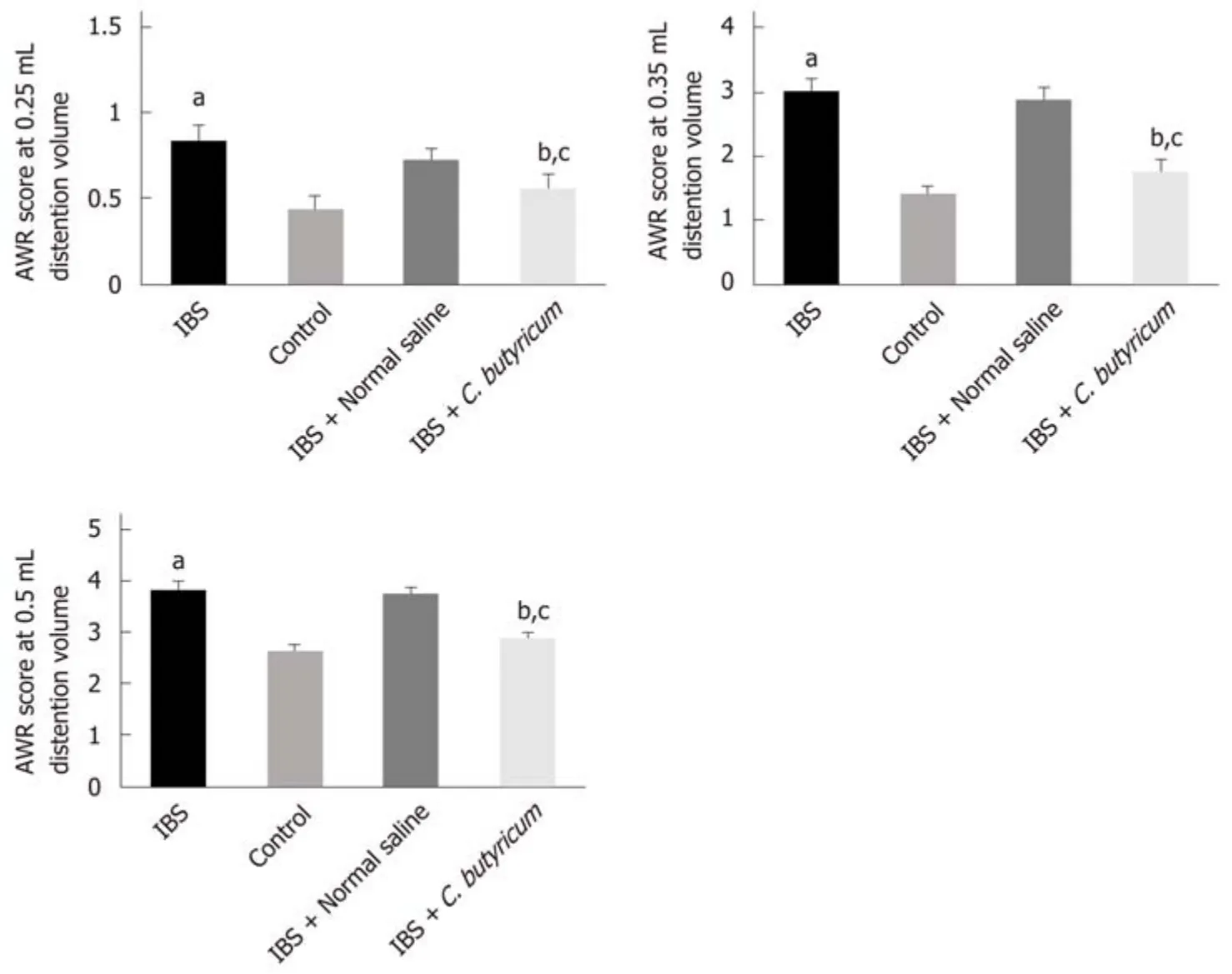
Figure 2 Effects of Clostridium butyricum on visceral hypersensitive in TNBS-induced irritable bowel syndrome mice. The abdominal withdrawal reflex(AWR) scores at 0.25 mL (A), 0.35 mL (B), and 0.50 mL (C) distension volumes were measured on day 28. Data are expressed as the mean ± SEM (n = 6). aP < 0.05,bP < 0.05, cP < 0.05 vs control group, IBS + normal saline group, and IBS group, respectively. IBS: Irritable bowel syndrome.
LPDCs are known to play a pivotal role in the regulation of the mucosal immune response[22]. Animal studies have highlighted the role of DCs in IBS pathogenesis based on the findings of induced visceral hypersensitivity and T cell activation[23,24]. It has been shown previously that the quantity of DCs in patients with IBS and IBD increased significantly, and the mature co-stimulatory molecules (CD80, CD86, and MHCII) were upregulated. Meanwhile, the secretion of the inflammatory cytokines was increased, and the migration and proliferation of CD4+ T cells were enhanced,resulting in the mediation of the Th1 and Th17 inflammatory responses[44-46]. In this study, we found that the expression of CD11c+ LPDCs and CD11c+CD80+ LPDCs in the colon of IBS mice was significantly higher than that of the normal control mice (n= 6, P < 0.001). These results suggested enhanced proliferation and activation of LPDCs in the local intestinal mucosa of IBS animals. Our results also demonstrated that LPDCs participated in and promoted the intestinal inflammatory immune response in PI-IBS. These findings are consistent with the results of previous studies[47].
In addition, we also found that after receiving C. butyricum intervention, the quantity of LPDCs was significantly decreased, and the co-stimulatory molecule CD80 was downregulated (n = 6, P < 0.001) in mice. These results indicate that C. butyricum alleviates the intestinal low-grade inflammation by regulating the functional status of LPDCs in mice with TNBS-induced IBS. Further investigation of the local microenvironment will be needed for the confirmation of DC phenotype and activation.
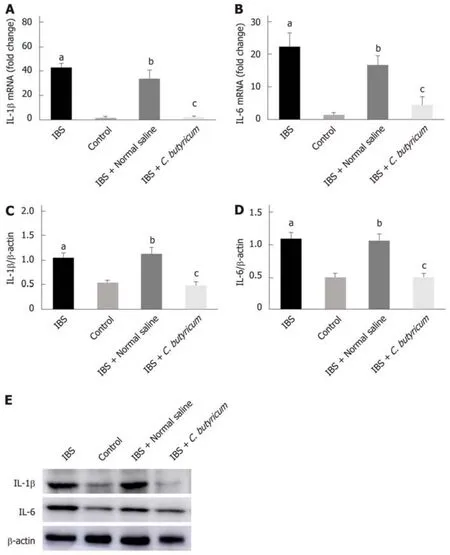
Figure 3 Effects of Clostridium butyricum on the production of proinflammatory cytokines in irritable bowel syndrome mice. Real-time PCR (A and B) and Western blot analysis(C-E) were used to measure the levels of proinflammatory cytokines IL-1β and IL-6. The results showed that IL-1β and IL-6 in the intestinal low-grade inflammatory state of IBS were significantly higher than those in the normal control group. The secretion of proinflammatory cytokines was significantly decreased after C. butyricum intervention. aP < 0.05, bP < 0.05, cP < 0.05 vs control group, IBS + normal saline group, and IBS group, respectively. IBS: Irritable bowel syndrome.
The immunoregulatory function of LPDCs is related to their subpopulation and surface receptors. TIM3 is an immune-regulatory factor. Recent studies have revealed that TIM3 expressed on macrophages and DCs upregulates the secretion of proinflammatory cytokines (TNF-α, IL-1β, IL-6 and so on) and promotes cell proliferation, activation, and phagocytosis, which result in enhanced elimination of different pathogens[29,31,32,43]. Anti-TIM3 mAb reduces the secretion of IL-12p70 in LPSinduced BMDCs and inhibits the upregulation of CD40, CD80, and CD86 expression in BMDCs[48]. The expression of TIM3 is upregulated after being stimulated to mature via the in vitro administration of IL-15 or IL-12 and IL-18 for immature natural killer cells[27,28,49]. Proinflammatory TIM3 is highly expressed in several inflammatory diseases[50,51]. TIM3/Gal9 induces the generation of the cytokines IL-1β, IL-6, and TNFα through caspase-1, and the induced IL-1β further promotes the generation and activation of other cytokines by autocrine feedback[30,52]. To our best known, this is the first study that reports the role of TIM3 in LPDCs in the pathogenesis of IBS and the regulatory effect of C. butyricum on TIM3. In this study, the results demonstrate that the expression levels of TIM3 on LPDCs are positively correlated with intestinal lowgrade inflammation according to the comparision among four different intestinal immune states. The levels of CD11c+TIM3+ LPDCs are significantly increased in the colon of mice with IBS. However, the CD11c+TIM3+ LPDCs are significantly reduced after the alleviation of the intestinal low-grade inflammation by C. butyricum intervention (n = 6, P < 0.01), but no significant difference is observed when compared to the normal control group (n = 6, P > 0.05).
Combined with previous studies, the regulatory effect of C. butyricum on DCs has been verified in the following cell tests. Compared with the BMDC + LPS group,through the stimulation of C. butyricum-treated BMDCs with LPS, the expression of CD11c+, CD11c+CD80+, and CD11c+TIM3+ LPDCs was also significantly downregulated. These results suggest that C. butyricum not only reduces the number of BMDCs and inhibits their functional status but also significantly reduces the expression of TIM3. Thus, we have reason to believe that DCs play an important regulatory role in the immune response and are regulated by the microenvironment.
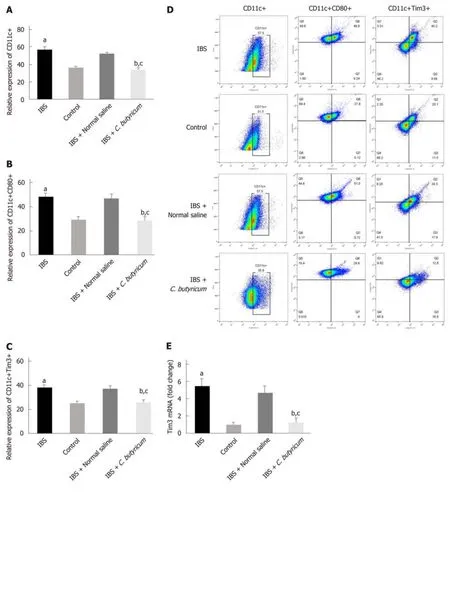
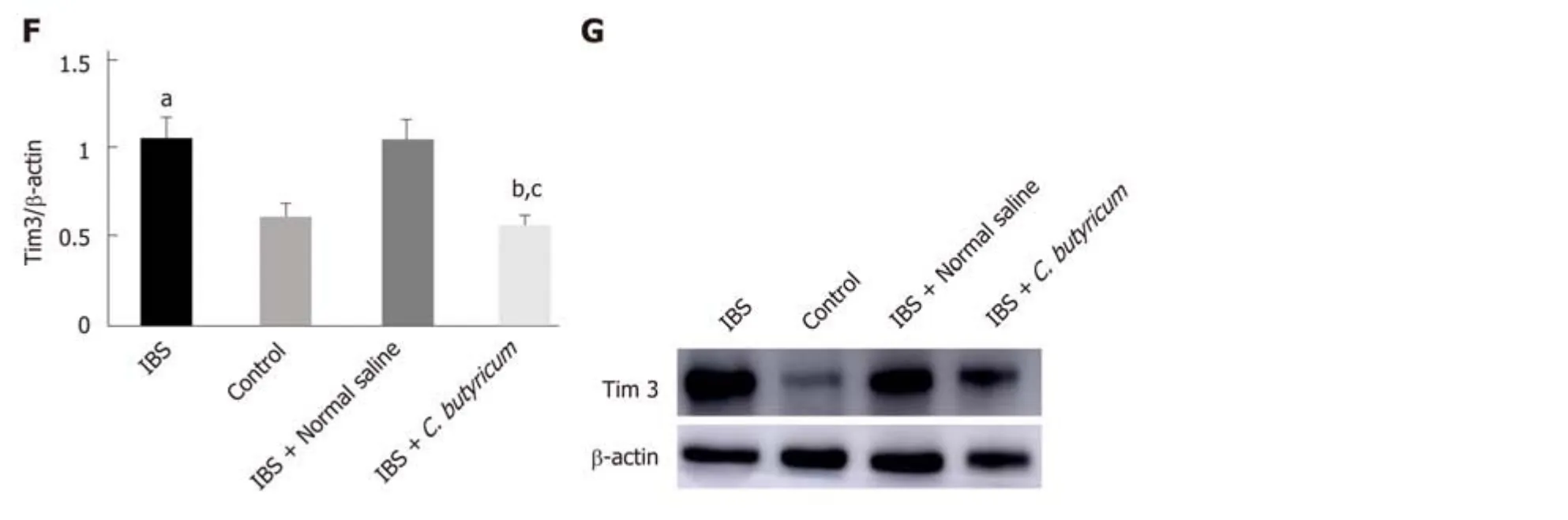
Figure 4 Effects of Clostridium butyricum on the quantity and functional status of the lamina propria dendritic cells. A: Flow cytometry of CD11c+ LPDCs expression; B: Flow cytometry of CD11C+CD80+ LPDCs expression; C: Flow cytometry of CD11c+Tim3+ LPDCs expression; D: Flow scatter plot of all test indicators;E: Tim-3 mRNA levels measured by real-time PCR; F: Tim3 protein expression measured by Western blot analysis. The results showed that the expression of CD11c+, CD11C+CD80+, and CD11c+Tim3+ LPDCs in the intestinal inflammatory state of IBS was significantly higher than in that in the normal control group, and the costimulatory molecule CD80 representing DC in the activated mature state was also significantly increased (P < 0.01). However, the above indicators were significantly decreased after C. butyricum intervention. Our experimental results suggest that the LPDCs participate in and promote the intestinal inflammatory immune response of IBS and C. butyricum suppresses intestinal inflammation by reducing the number, function, and immunoregulatory molecule Tim3 of LPDCs in IBS mice.aP < 0.05, bP < 0.05, cP < 0.05 vs control group, IBS + normal saline group, and IBS group, respectively. IBS: Irritable bowel syndrome; LPDCs: Lamina propria dendritic cells.
In conclusion, our results indicate that intestinal LPDCs play an important role in the pathogenesis of IBS, and C. butyricum can alleviate intestinal inflammatory immune responses by regulating the amount and function of LPDCs and the expression of TIM3 on the surface of LPDCs. Our research not only provides an indepth understanding of the local immune response mechanism in the intestinal mucosa of IBS patients, but also provides a newperspective for the application of probiotic C. butyricum in the treatment of IBS.
ARTICLE HIGHLIGHTS
Research background
Irritable bowel syndrome (IBS) affects 7% to 21% of the general population. It is a chronic diseases characterized by abnormal visceral sensitivity and low-grade inflammation. The role of Clostridium butyricum (C. butyricum) in reducing intestinal low-grade inflammation via immune pathways has been well defined. However, the mechanism has not been clearly elucidated.
Research motivation
To test the hypothesis that the function of dendritic cells (DCs) changes in the development of IBS and to understand the regulation of DCs after C. butyricum intervention.
Research objectives
We aimed to investigate the mechanism of DCs in the development of IBS in a mice model and to understand the regulation of DCs after C. butyricum intervention.
Research methods
An IBS animal model was established using C57BL/6 mice, and C. butyricum was continuously administered via the intragastric route to simulate different intestinal immune states. Intestinal visceral hypersensitivity and histopathology were assessed using the abdominal withdrawal reflex test and hematoxylin & eosin staining, respectively. The expression of proinflammatory cytokines (IL-1β and IL-6) and TIM3 was analyzed by Western blot analysis and real-time PCR.The flow cytometry was applied to analyze the quantity, function, and membrane molecule TIM3 of the LPDCs. The regulatory effect of C. butyricum was verified in BMDCs by in vitro experiments.
Research results
We found that the IBS mouse model has abundant expression of IL-1β, IL-6, and CD11C+CD80+and CD11c+TIM3+ LPDCs compared with the control group. Further investigation showed that probiotic C. butyricum reduced the expression of cytokines (IL-1β and IL-6). The amount and function of LPDCs and the membrane molecule TIM3 of the LPDCs were decreased with the alleviation of the intestinal inflammatory response.
Research conclusions
This study demonstrated that C. butyricum could induce the expression of various proinflammatory cytokines via regulating the amount and the functional status of LPDCs in the intestinal mucosa of mice with IBS.
Research perspectives
This research not only provides an in-depth understanding of the local immune response mechanism in intestinal mucosa of IBS humans, but also provides a new perspective for the application of probiotic C. butyricum in the treatment of IBS.
 World Journal of Gastroenterology2019年36期
World Journal of Gastroenterology2019年36期
- World Journal of Gastroenterology的其它文章
- Chinese guidelines on management of hepatic encephalopathy in cirrhosis
- Sexual health and fertility for individuals with inflammatory bowel disease
- High mobility group box-1 release from H2O2-injured hepatocytes due to sirt1 functional inhibition
- Zinc-α2-glycoprotein 1 attenuates non-alcoholic fatty liver disease by negatively regulating tumour necrosis factor-α
- CARMA3/NF-κB signaling contributes to tumorigenesis of hepatocellular carcinoma and is inhibited by sodium aescinate
- Laparoscopy-assisted pylorus-preserving gastrectomy for early gastric cancer: A retrospective study of longterm functional outcomes and quality of life
