Analysis of Internal Moisture Change of Camphor Seeds during Drying by Low-Field NMR
Yan XUAN Yilong XIANG Jing YANG Buhong GAO Ying TANG Feng LIN

Abstract In this study, lowfield nuclear magnetic resonance (LFNMR) was used to collect the transverse relaxation signals of camphor seeds that had been dried at different temperatures and for different durations. The transverse relaxation time of the hydrogen nucleus was obtained by SIRT (Simultaneous Iterative Reconstruction Technique) inversion, and the correlation between the moisture content on dry basis and the amplitude of NMR signal of camphor seeds sampled at different drying stages was analyzed. The results showed that water existed in three main forms strongly bound water, weakly bound water and free water in camphor seeds. During the drying process, the peak position of strongly bound water remained unchanged, but the peak positions of weakly bound water and free water fluctuated. Drying rate increased with drying temperature increasing. In addition, there was a very significant linear relationship between the total NMR signal amplitude and moisture content on dry basis, and the correlation coefficient reached 0.984 4.
Key words Nuclear Magnetic Resonance (NMR); Camphor seed; Water distribution; Relaxation time spectrum; Correlation
Camphor tree (Cinnamomum capmhora) is an evergreen broadleaved tree species belonging to the family Lauraceae. It has a dense crown, and thus is extensively used as ornamental, landscape, shade and shelter tree, and it is also widely grown in the south of the Yangtze River in China[1-4]. The strong aroma and volatile oils released by camphor trees have the functions of expelling insects and resisting corrosion. The roots, stems and fruits of camphor contain the active ingredient camphor oil[5-6], while its leaves contain several volatile organic compounds such as camphene, αpinene, βpinene, cineole, linalool and safrole[7]. Camphor fruit has certain pharmacological effects such as relieving pain, alleviating diarrhea, lowering blood lipid and cholesterol levels. Camphor seed contains more than 55% fat and oil, and decanoic acid and lauric acid are the main fatty acids[8-9]. In recent years, owing to urbanization and afforestation, camphor trees have been cultivated in many areas, so there is an urgent need to determine the optimal conditions for drying and storage of camphor seeds.
Lowfield nuclear magnetic resonance (LFNMR) can be used to study the distribution, migration and content of internal moisture and other properties associated with them in various organic materials by measuring the transverse and longitudinal relaxation time of the hydrogen nuclei under the action of radiofrequency (RF) pulse in a strong static magnetic field. It has become one of the important techniques for detecting moisture distribution of various materials, and now is extensively used in the study of water migration during the drying process of fruits, vegetables, meat, grain and oil crops[10-19].
In this paper, LFNMR was used to acquire the transverse relaxation time and signal amplitude of mature camphor seeds, to explore the changes of water forms and their contents during the drying process, and to provide a more scientific basis for optimizing the drying and storage conditions, and rapid detection of moisture content of camphor seeds.
Materials and Methods
Instruments
LFNMR spectrometer (proton resonance frequency 21 MHZ, magnet temperature 32 ℃, probe tube diameter 10 mm) was purchased from Shanghai Niumag Corporation, DHG9123A drying oven from Shanghai Jinghong Laboratory Instrument Co. Ltd., and BSA224S electronic balance from Sartorius Scientific Instruments (Beijing) Co., Ltd.
Methods
Materials
Camphor fruits were picked on the campus of Nanjing Forestry University in midNovember 2017 and stored at 4 ℃. Camphor fruits with intact skin were selected, rinsed with deionized water for three to five times. After pulp was removed, camphor seeds were dried superficially with absorbent paper. The average moisture content of the seeds was measured to be 46.3% before drying treatments.
Drying treatments
The selected camphor fruits were dried in an electric thermostatic blast drying oven. The drying temperature and duration are two key factors during this process. In this experiment, six different drying temperatures 60, 70, 80, 90, 100, and 110 ℃ were set. In brief, five replicates were prepared for each drying treatment, and there were 10 cleaned camphor seeds in each replicate, each of the seeds were weighed and numbered before they were dried. During the drying process, the seeds were sampled and measured for transverse relaxation time at 30min time interval, until the moisture content of the seeds dropped to 10%.
Data acquisition and inversion
The NMR signals were detected with a CarrPurcellMeiboomGill (CPMG)[20-21]sequence, and then using an iterative method, the signal attenuation curves were substituted into was obtained by SIRT (Simultaneous Iterative Reconstruction Technique) algorithm to invert T2 spectra of the samples. NMR spectroscopy parameters were set as follows: spectrometer frequency (SF) 21 MHz, receiver bandwidth 200 kHz, sampling time 0.15 ms, gain 20 dB, 90° pulse width 4 μs, 180° pulse width 9 μs, number of data points 320 264, repetition time 4 000 ms, scanning number 16, echo time 0.4 ms, echo count 4 000, and digital gain 3. Each sample was detected repeatedly for five times.
Results and Analysis
Transverse relaxation time of fresh camphor seeds
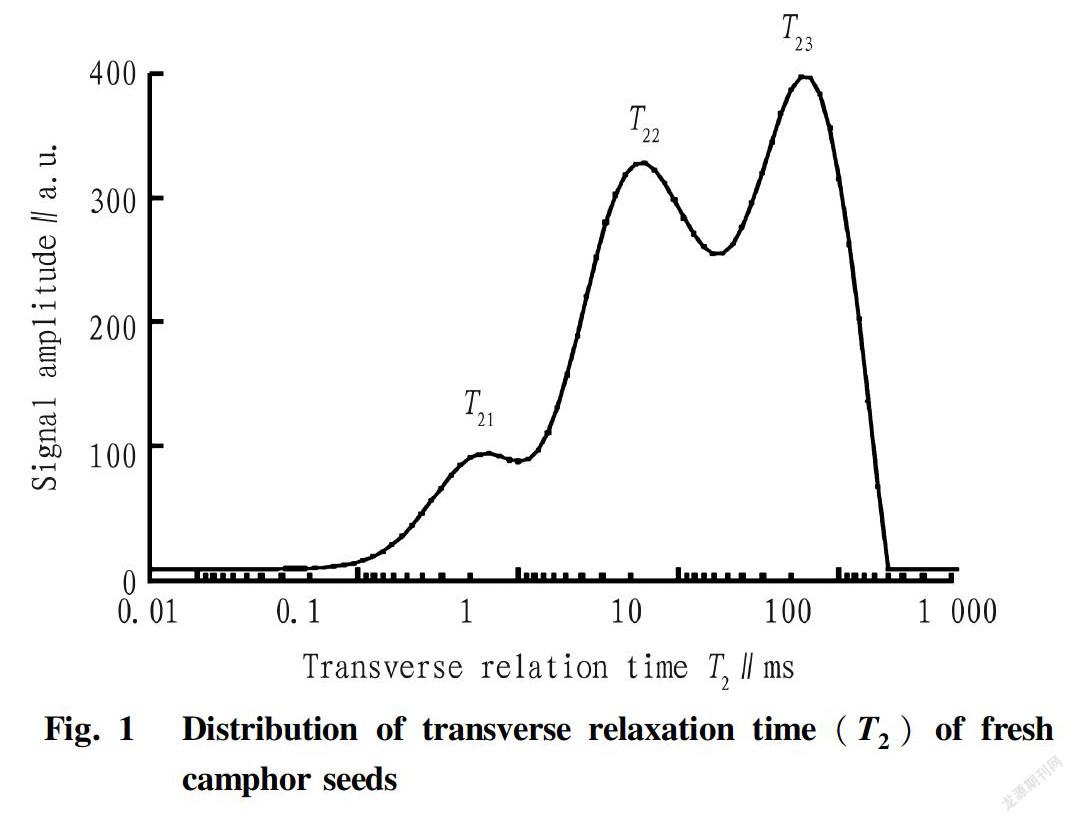
The spectrum of transverse relaxation time T2 of the freshly picked camphor seeds was obtained by SIRT inversion, by plotting transverse relaxation time T2 on xaxis, and signal amplitude on yaxis(Fig. 1).
Water exists in three different states in plants, according to previous literatures[22-26]. The main substances contained in camphor seeds are fat, protein, carbohydrate and water. Due to the different physical or chemical environments, hydrogen nuclei of these components produced different relaxation time peaks in NMR spectrum. As shown in Fig. 1, water in fresh camphor seeds has three different forms: strongly bound water, weakly bound water and free water represented by T21, T22and T23respectively. T21(1.32 ms) corresponds to the hydrogen nucleus of water that tightly combines to other substances in the cells, T22(12.33 ms) corresponds to the hydrogen nucleus of water that weakly binds to other substances, while T23(114.98 ms) corresponds to hydrogen nuclei of free water and fat. Longer transverse relaxation time T2 of the samples indicates greater freedom degree of the hydrogen nucleus, and smaller impact of environmental constraints, and the hydrogen nucleus binds more weakly to other substances, so it is easier to remove this form of water from camphor seeds. Conversely, shorter transverse relaxation time T2 suggests that the hydrogen nucleus binds more strongly to other substances, and it is harder to remove the water from camphor seeds.
Effect of drying temperature on internal moisture content of camphor seed
For camphor seeds dried at the same temperature, there was a certain correlation between moisture content and drying duration. The influence of drying temperature on moisture content on dry basis was evaluated according to the weight and NMR relaxation time of the seeds measured at 30min interval during the drying process, as shown in Fig. 2. For fresh camphor seeds, the moisture content on dry basis was about 46.3%. For the seeds dried at different temperatures, moisture content gradually reduced with drying duration increasing. Within the first 50 min of drying, moisture content decreased rapidly, suggesting a high drying rate, and began to drop slowly after 100 min of drying. When the drying temperature was increased from 60 to 110 ℃, water evaporated faster due to accelerated molecular movement, so moisture content in camphor seeds dropped faster and the curves became steeper.
Effect of drying duration on water state in camphor seeds
Figure 3 shows the T2 spectrum of camphor seeds dried at 60 ℃and sampled at 30min interval. There were three peaks in each curve of camphor seeds sampled at different drying stages. And the intensity of all the three peaks of fresh camphor seeds was stronger, indicating higher contents of strongly bound water, weakly bound water and free water in fresh seeds. With drying duration increasing, the intensity of the three peaks became weaker, indicating that during the drying process, the internal moisture of the seeds was gradually removed, so the moisture content gradually reduced. After the seeds were dried at 60 ℃ for 5 h, only the peak at 170 ms remained, For the seeds that were completely dry, this peak still existed, corresponding to camphor oil, indicating that it was the peak of oil in camphor seeds.
The peak positions and relative contents of strongly bound water, weakly bound water and free water in camphor seeds dried at 60, 80 and 100 ℃ were shown in Fig. 4. The results showed that the peak position of strongly bound water in camphor seeds changed little during the drying process, because water of this form binds closely to other substances in seeds. The peak position of weakly bound water increased at first and decreased subsequently with drying duration increasing, suggesting that weakly bound water converted to free water, then migrateed outward and finally evaporated during the drying process. The peak position of free water increased at first, decreased and kept unchanged until the end of the drying process. As the contents of both free water and weakly bound water reduced during the drying process, the original water balance was broken and the whole seed was gradually dried, and camphor oil and strongly bound water in the cells were finally left in seeds.
Correlation between total relaxation signal amplitude and moisture content on dry basis of camphor seeds
The correlation between total relaxation signal amplitude and moisture content on dry basis of camphor seeds that had been dried at different temperatures and for different durations is shown in Fig. 5. The results indicated that there was a significant linear relationship between total signal amplitude and moisture content on dry basis, and the linear equation was y=137.30x+3 882.84,
and the correlation coefficient R2 reached 0.984 4, indicating that total relaxation signal had a great influence on moisture content on dry basis. By using this equation, moisture content on dry basis of camphor seeds can be quickly calculated according to NMR relaxation peak area (total relaxation signal).
Conclusions
NMR technique can be used to determine the distribution and migration of internal moisture in camphor seeds during the drying process. The internal moisture of camphor seeds exists in three main forms, strongly bound water, weakly bound water and free water. The drying treatment changed the distribution of the three forms of water in camphor seeds, and the relaxation time of strongly bound water did not change much, while weakly bound water and free water were quickly lost. The total NMR signal of camphor seeds has a significant correlation with moisture content on dry basis, and correlation coefficient reaches 0.984 4, so the LFNMR technique can be used to calculate the moisture content of camphor seeds dried at different temperatures and for different durations according to the total signal of transverse relaxation time.
References
[1] LIU J F, DENG L, WANG M, et al. Lipase catalyzed synthesis of mediumchain biodiesel from Cinnamonum camphora seed oil[J]. Chinese Journal of Chemical Engineering, 2014, 22(11-12): 1215-1219.
[2] CHEN Y, DAI G. Antifungal activity of plant extracts against Colletotrichum lagenarium, the causal agent of anthracnose in cucumber[J]. Journal of the Science of Food & Agriculture, 2012, 92(9) : 1937-1943.
[3] YANG F, LONG E, WEN J, et al. Linalool, derived from Cinnamomum camphora (L.) Presl leaf extracts, possesses molluscicidal activity against Oncomelania hupensis, and inhibits infection of Schistosoma japonicum[J]. Parasites & Vectors, 2014, 7 (1): 1-13.
[4] ZUO Z, WANG B, YING B, et al. Monoterpene emissions contribute to thermotolerance in Cinnamomum camphora[J]. Trees, 2017, 31(6): 1-13.
[5] GENG JZ, LIU JH. Extraction of Cinnamomum Camphora seed oil by SFECO2 and antioxidation[J]. Journal of the Chinese Cereals and Oils Association, 2014, 29(2): 57-61.
[6] RAN XM, LI ZH, FU XJ, et al. Advancement of active compositions in leaves and seeds of camphor tree[J]. Food & Nutrition in China, 2010(8): 27-30.
[7] ZHOU X, MO JG, XIE YX, et al. Study on the chemical constituents of essential oil from linalool type of Cinnamomum camphora stem in Guangxi[J]. Food Science and Technology, 2011, 36(1): 282-285.
[8] HU JN, ZHANG B, ZHU XM, et al. Characterization of mediumchain triacylglycerol (MCT) enriched seed oil from Cinnamomum camphora (Lauraceae) and its oxidative stability[J]. Journal of Agricultural $ Food Chemistry, 2011, 59(9): 4771.
[9] LI QY, LU LY, YIN YC, et al. Preparation of triglyceride (TG) from camphor tree seed oil[J]. Journal of the Chinese Cereals and Oils Association, 1988(s2): 35-39.
[10] ZHANG XK, ZHU SS, HUANG JH, et al. Analysis on internal moisture changes of carrot slices during drying process using lowfield NMR[J]. Transactions of the Chinese Society of Agricultural Engineering, 2012, 28 (22): 282-287.
[11] WANG XY, GAO K, CHEN QQ, et al. Water diffusion characteristics of apple slices during short and mediumwave infrared drying[J]. Transactions of the Chinese Society of Agricultural Engineering, 2015, 31(12): 275-281.
[12] LI B, YIN Q, YIN LJ, et al. Studies on Characteristics and Mechanism of Hot AirMicrowave Fluidized Drying of Lentinusedodes[J]. Journal of Chinese Institute of Food Science and Technology, 2015, 15(5): 134-139.
[13] SHI F, XIAO XN, YANG YX, et al. Characterization of moisture transfer inrehydration process for dried mushroom (Lentinus edodes) by different drying methods[J]. Food and Fermentation Industries, 2017, 43(10): 144-149.
[14] LIU ZB, ZHANG ZYM LI DJ, et al. Analysis of moisture change during farinfrared drying of Agaricus bisporus[J]. Food Science, 2016, 37(9): 82-86.
[15] REN GY, ZENG FL, DUAN X, et al. Analysis of internal moisture changes in corn dry process investigated by low fieldNMR[J]. Journal of the Chinese Cereals and Oils Association, 2016, 31(8): 95-99.
[16] YU X, WANG Z, ZHANG Y, et al. Study on the water state and distribution of Chinese dried noodles during the drying process[J]. Journal of Food Engineering, 2018, 233: 81-87.
[17] DU J, CHENG L, HONG Y, et al. Enzyme assisted fermentation of potato pulp: An effective way to reduce water holding capacity and improve drying efficiency[J]. Food Chemistry, 2018, 258: 118.
[18] XU F, JIN X, ZHANG L, et al. Investigation on water status and distribution in broccoli and the effects of drying on water status using NMR and MRI methods[J]. Food Research International, 2017, 96: 191.
[19] WANG L, XU B G, WEI B X, et al. Low frequency ultrasound pretreatment of carrot slices: Effect on the moisture migration and quality attributes by intermediatewave infrared radiation drying[J]. Ultrasonics Sonochemistry, 2018, 40(Pt A) : 619-628.
[20] CARR HY, PRCELL EM. Effects of diffusion on free procession in NMR experiments[J]. Physical Review, 1954, 94(3) : 630-638.
[21] MEIBOOM S, GILL D. Modified spinecho method for measuring nuclear relaxation times[J]. Review of Scientific Instruments, 1958, 29: 688-691.
[22] SONG ZP, WEI S, HE F, et al. Analysis of moisture migration and drying characteristics of tobacco during fluecuring by low field NMR[J]. Acta Tabacaria Sinica, 2017, 23(4): 50-55.
[23] WANG HO, XIE HX, CHEN SJ, et al. Effect of different drying methods on drying characteristics and qualities of lemon slices[J]. Transactions of the Chinese Society of Agricultural Engineering, 2017, 33(14): 292-299.
[24] LI D, TAN SM, CHEN CY, et al. LFNMR study on variations of different moisture states during the process of rice drying[J]. Journal of the Chinese Cereals and Oils Association, 2016, 31(7): 1-5.
[25] CAO X, ZHANG F, ZHAO D, et al. Effects of freezing conditions on quality changes in blueberries[J]. Journal of the Science of Food & Agriculture, 2018, 98( 12): 4673-4679.
[26] WANG J, MUJUMDAR A S, Deng L Z, et al. Highhumidity hot air impingement blanching alters texture, cellwall polysaccharides, water status and distribution of seedless grape[J]. Carbohydrate Polymers, 2018, 194: 9-17.
Editor: Qingqing YIN Proofreader: Xinxiu ZHU
- 农业生物技术(英文版)的其它文章
- Advances in the Coenzyme Q10 Biosynthesis Pathway in Rhodobacter sphaeroides and the Enha
- Differences in Chlorophyll Fluorescence Parameters Yield and Its Components Between Different Genotypes of Wheat Under Waterlogging Conditions at Anthesis
- Breeding of a Natural Green Cocoon Quaternary Hybrid Combination Xiangcailu No.1 for Spring Reari
- Bioinformatics and Expression Analysis of CaERF Gene in Capsicum
- A Review on the Protection Mechanism of Trehalose on Plant Tissues and Animal Cells
- Genetic Diversity Analysis of Cherry Tomato Core Collection Based on Genotypic Values

