慢性皮肤溃疡间充质干细胞治疗及中医药调节作用的研究进展
蒙玉娇 陈朝霞 王燕 赵京霞 底婷婷 张金超 林燕 李萍
基金项目:国家自然科学基金项目(81774328)作者简介:蒙玉娇(1989.01—),女,博士研究生在读,研究方向:中医药治疗炎性反应性皮肤疾病,E-mail:meng.yu.jiao@163.com通信作者:李萍(1965.04—),女,博士,研究员,博士研究生导师,研究方向:中医药治疗炎症性皮肤疾病,E-mail:liping411@163.com
摘要 慢性皮肤溃疡是外科常见病及多发病,治疗周期长且临床治疗效果不理想,寻求有效的治疗药物和方法是研究热点。间充质干细胞由于低免疫原性、多项分化潜能、来源广泛等优势,其通过旁分泌/自分泌效应和向类皮细胞分化等功能参与创面修复的多个环节,为促进难愈性创面的愈合提供了新的手段,在临床得到广泛应用和研究。作者对间充质干细胞在慢性皮肤溃疡创面愈合中的作用及中医药的调节功能进行综述,为临床和基础研究提供证据。
关键词 慢性皮肤溃疡;创面愈合;间充质干细胞;旁分泌/自分泌;多项分化;中医药;疗效;综述
Research Progress Study on Mesenchymal Stem Cells Treatment on Chronic Skin Ulcer and Regulation of Traditional Chinese Medicine
Meng Yujiao1,2,Chen Zhaoxia1,Wang Yan1,Zhao Jingxia1,Di Tingting1,Zhang Jinchao1,Lin Yan1,Li Ping1
Abstract Chronic skin ulcer is a common and frequently-occurring disease.The treatment period is long and the clinical treatment effect is not ideal.It is a research hotspot to seek effective therapeutic drugs and methods.Mesenchymal stem cells(MSCs)participate in multiple aspects of wound repair through the functions of low immunogenicity,multiple differentiation potentials,and wide sources,etc.,through the functions of paracrine/autocrine effect and differentiation to epithelial cells,in order to promote refractory wounds.The healing provides a new means of clinical application and research.The authors reviewed the role of MSCs in the healing of chronic skin ulcer wounds and the regulatory functions of Traditional Chinese Medicine,providing evidence for clinical and basic research.
Key Words Chronic skin ulcer; Wound healing; MSCs; Paracrine/autocrine; Differentiation; Traditional Chinese medicine; Efficacy; Review
中圖分类号:R277.5文献标识码:Adoi:10.3969/j.issn.1673-7202.2019.11.060
慢性皮肤溃疡属于中医“疮疡”的范畴,是糖尿病、周围血管病、微生物感染、放疗等常见的并发症,是外科常见病及多发病,因其病因复杂、迁延不愈、易复发等特点,一直是外科领域颇为棘手的难题。目前临床上针对创面主要采用清创、换药、植皮、激光、高压氧、干细胞移植等方法,并配合系统的维持治疗,临床疗效不理想。国内外众多学者都在积极探讨影响慢性皮肤溃疡愈合的因素,寻求有效的治疗药物和方法。随着细胞和分子生物学技术研究的不断深入,来源于发育早期中胚层的间充质干细胞作为创面修复的种子细胞,越来越受到广泛关注[1]。
1 间充质干细胞在慢性皮肤溃疡愈合中的作用
创伤修复是个复杂而又有序的病理生理过程,可划分为3个阶段:炎性反应期、增生期和重建期。在每个时期都有大量的细胞、细胞外基质、细胞因子的参与,任何影响创面修复过程的因素都可能最终导致创面难以愈合[2]。在参与创伤修复的众多细胞中,间充质干细胞具有调节免疫功能、低免疫原性、自我复制和更新及多向分化潜能等优点,是国内外学者研究的重点和热点[3]。
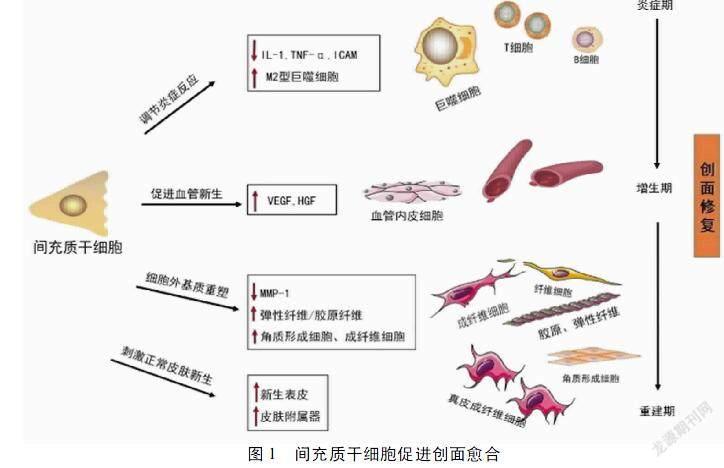
1.1 间充质干细胞是创面愈合过程的“核心” 间充质干细胞(Mesenchymal Stem Cells,MSCs)是一种具有自我更新和多向分化潜力、并且已被证实能加速皮肤伤口愈合的成体多能干细胞[4]。在创面愈合过程中,内源性MSCs通过募集趋化、旁分泌/自分泌和转分化效应调控创面愈合的整个过程[4],包括1)促进血管生成因子分泌和血管生成;2)促进肉芽组织形成;3)诱导M2型巨噬细胞极化;4)促进创面炎性反应消退;5)调节内源性干/祖细胞募集归巢、细胞外基质重塑;6)刺激具有正常结构和功能的皮肤再生等,具有治疗皮肤溃疡的潜在价值[3]。基于间充质干细胞对整个愈合过程不可替代的调控作用,目前间充质干细胞已被定义为创面愈合过程的“核心”[4-6]。见图1。
1.2 慢性皮肤溃疡愈合迟缓与MSCs的募集、增殖及趋化迁移等功能受损密切相关 大量临床及基础实验表明,慢性创面愈合延迟与内源性MSCs的募集、存活、增殖及趋化、迁移等功能受损密切相关。与普通伤口比较,在患者和动物的慢性难愈合伤口中MSCs处于数量缺乏和功能缺陷的状态[7-9]。并且,糖尿病引起的创面延迟愈合与MSCs向受伤部位的迁移和募集减少具有密切关系[10-12],有文献报道,MSCs损伤是糖尿病的主要并发症之一[13]。动物研究证实,在实验性糖尿病动物模型中MSCs的细胞活力降低、增殖减少,细胞骨架组织改变,并且多向分化潜能偏向于脂滴形成,释放细胞因子和抗凋亡能力明显受损,形成细胞集落的能力差且出现生长抑制的时间早[14-16]。骨髓是MSCs的重要来源,骨髓中的MSCs含量最为丰富且增殖能力强[17]。db/db糖尿病小鼠骨髓MSCs增殖活力較正常小鼠显著降低[12]。慢性难愈性创面患者的干细胞募集反应检测结果显示,内源性骨髓MSCs数量较少并且存在募集、迁移等功能失调[18]。同时,在难愈合创面中超极化的巨噬细胞无法向亲愈合的M2表型转换,与MSCs功能障碍也有关[19]。因此,改善创面微环境、提高MSCs活性,促进其增殖和迁移等能力,可以加速创面愈合。
1.3 慢性难愈合性溃疡创面局部MSCs的存活能力及旁分泌/自分泌功能降低 基于MSCs促进血管内皮细胞增殖和血管新生的功能,目前外源性MSCs在慢性难愈合伤口的植入成为慢性皮肤溃疡治疗的方法之一。然而多项研究表明,在慢性创面中,尤其是糖尿病创面处植入的MSCs增殖和存活率很低[20],并且在愈合过程中持续减少,需要多次重复注射或者加入MSCs外泌体或诱导剂才能保持其存活率,但仍达不到理想的临床疗效[21]。究其原因,在难愈合性皮肤溃疡中,尤其中医属“阴疮”的溃疡,开放性的创面呈现一种持续的低反应的炎性反应状态,各种炎性因子、活性氧、基质金属蛋白酶及各种代谢产物导致创面微环境复杂,均可导致外源性MSCs的存活率较低及MSCs分泌功能降低[22]。
一方面,在难愈合创面中,创面M1型巨噬细胞及其分泌的高浓度的TNF-α、活性氧可显著减少MSCs数量,增加MSCs的凋亡[23];而MSCs数量的减少及旁分泌/自分泌功能的降低,又使M1型巨噬细胞无法向M2型转换,并且影响血管内皮细胞和成纤维细胞的募集[24],如此形成恶性循环;并且,MSCs向内皮细胞的成功分化也依赖于M2型巨噬细胞来源的VEGF[25]。因此,除了调节创面微环境之外,动员自身的MSCs向伤口迁移,提高伤口局部MSCs的分泌功能,保留内源性MSCs并最大化提高其功能和再生能力,可成为中医药治疗“阴疮”的作用靶点之一。
1.4 SDF-1/CXCR4信号轴贯穿MSCs迁移、扩增及分泌的过程,介导组织修复 基质细胞衍生因子1(Stromal Derived Factor-1,SDF-1)是一种炎性趋化因子,系统命名为CXCL12(CXC Chemokine Ligand 12,CXCL12)。SDF-1是唯一能与CXC趋化因子受体(CXC Chemokine Receptor,CXCR)4结合并激活的天然趋化因子,SDF-1与CXCR4特异性结合称之为SDF-1/CXCR4轴。SDF-1/CXCR4轴在间充质干细胞的动员、迁移、增殖和存活中起着至关重要的作用[26-29]。在创伤愈合过程中,研究表明,SDF-1/CXCR4轴在MSCs向损伤部位迁移中具有重要作用,因为当SDF-1信号受损时,迁移活动将无法进行[30]。在SDF-1的作用下,MSCs表达30多种差异基因,其中11种都参与了细胞运动[31]。同时,SDF-1/CXCR4信号轴诱导MSCs迁移到创伤部位,并诱导其分泌多种生长因子如血管内皮生长因子、促纤维生长因子和转化生长因子β等,形成许多血管网,参与伤口的修复,而阻断CXCR4后这种作用减弱[26]。
在组织修复过程中,SDF-1主要由MSCs和损伤组织分泌,其受体广泛表达于内皮细胞、巨噬细胞、干细胞及损伤组织等[32,33]。MSCs来源的SDF-1具有抗细胞凋亡、促进干细胞转分化、诱导迁移、募集内皮细胞和巨噬细胞的作用,并且MSCs来源的SDF-1通过自分泌的方式可显著提高创面局部MSCs的存活和分泌功能。而损伤部位来源的SDF-1可募集和加速MSCs向损伤部位的迁移,进一步放大MSCs的旁分泌效应[34]。因此,调节SDF-1、CXCR4蛋白表达,调控SDF-1/CXCR4信号轴是促进MSCs迁移、扩增及分泌,介导组织修复的途径之一。
2 中医药调控MSCs功能,促进创面修复
中医药治疗慢性皮肤溃疡历史悠久,疗效颇佳。研究发现,中药对MSCs的迁移、增殖等功能均具有调节作用。麝香的有效成分麝香酮、淫羊藿的有效成分淫羊藿苷,可调节SDF-1/CXCR4信号通路,促进骨髓MSCs的存活、增殖和迁移[35]。吴刚[36]等对补肾活血汤联合骨髓MSCs治疗大鼠膝关节炎的研究中发现,补肾活血汤促进骨髓MSCs体外迁移与定向分化。朱磊[37]在健脾补肾、清肠化湿治疗溃疡性结肠炎的研究中发现,健脾补肾清肠化湿方可以促进骨髓MSCs归巢方,减轻炎性反应、修复受损黏膜。补肾类中药巴戟天、杜仲、鹿茸等含有的活性物质,可以促进骨髓MSCs增殖和定向分化[38]。回阳生肌方是临床治疗难愈性创面的临床有效方,目前复方对MSCs作用的研究尚无报道,然而方中组成药物对MSCs的调节作用,也已有大量文献证实。李高申等对活血生肌汤联合干细胞移植干预糖尿病足的研究中发现,活血生肌汤可以促进干细胞移植后的增值和分化,达到促加速血管新生、建立侧支循环的作用。补益类中药黄芪、丹参等可促进MSCs的增殖。牛膝提取物能够促进骨髓MSCs增殖并抑制其凋亡[39]。
3 结语
在创面修复进程中,MSCs的活性、增值、迁移和分化,均是创面修复的关键,中药可通过调节MSCs功能,减轻局部炎性反应,为创面修复提供良好的生物环境。因此,调节MSCs的功能也可作为难愈合性创面治疗及中医药干预的作用环节。可见使用传统中医药,刺激间充质干细胞的动员、迁移、分泌、分化等功能在临床具有广阔的应用前景。
參考文献
[1]Kanji S,Das H.Advances of Stem Cell Therapeutics in Cutaneous Wound Healing and Regeneration[J].Mediators Inflamm,2017,2017:5217967.
[2]Ko SH,Nauta A,Wong V,et al.The role of stem cells in cutaneous wound healing:what do we really know?[J].Plast Reconstr Surg,2011,127(1):10S-20S.
[3]Lee DE,Ayoub N and Agrawal DK.Mesenchymal stem cells and cutaneous wound healing:novel methods to increase cell delivery and therapeutic efficacy[J].Stem Cell Research & Therapy,2016,37(7):193-201.
[4]Julianto I,Rindastuti Y.Topical Delivery of Mesenchymal Stem Cells "Secretomes" in Wound Repair[J].Acta Med Indones,2016,48(3):217-220.
[5]Motegi SI,Ishikawa O.Mesenchymal stem cells:The roles and functions in cutaneous wound healing and tumor growth[J].J Dermatol Sci,2017,86(2):83-89.
[6]Jackson WM,Nesti LJ,Tuan RS.Concise review:clinical translation of wound healing therapies based on mesenchymal stem cells[J].Stem Cells Transl Med,2012,1(1):44-50.
[7]Ennis WJ,Sui A,Bartholomew A.Stem Cells and Healing:Impact on Inflammation[J].Adv Wound Care(New Rochelle),2013,2(7):369-378.
[8]Cianfarani F,Toietta G,Di RG,et al.Diabetes impairs adipose tissue-derived stem cell function and efficiency in promoting wound healing[J].Wound Repair Regen,2013,21(4):545-553.
[9]Rodriguez-Menocal L,Salgado M,Ford D,et al.Stimulation of skin and wound fibroblast migration by mesenchymal stem cells derived from normal donors and chronic wound patients[J].Stem Cells Transl Med,2012,1(3):221-229.
[10]Frykberg RG,Banks J.Challenges in the Treatment of Chronic Wounds[J].Adv Wound Care(New Rochelle),2015,4(9):560-582.
[11]van de Vyver M,Niesler C,Myburgh KH,et al.Delayed wound healing and dysregulation of IL6/STAT3 signalling in MSCs derived from pre-diabetic obese mice[J].Mol Cell Endocrinol,2016,426:1-10.
[12]Shin L,Peterson DA.Impaired therapeutic capacity of autologous stem cells in a model of type 2 diabetes[J].Stem Cells Transl Med,2012,1(2):125-135.
[13]Silva JC,Sampaio P,Fernandes MH,et al.The Osteogenic Priming of Mesenchymal Stem Cells is Impaired in Experimental Diabetes[J].J Cell Biochem,2015,116(8):1658-1667.
[14]Jin P,Zhang X,Wu Y,et al.Streptozotocin-induced diabetic rat-derived bone marrow mesenchymal stem cells have impaired abilities in proliferation,paracrine,antiapoptosis,and myogenic differentiation[J].Transplant Proc,2010,42(7):2745-2752.
[15]Liu H,Tang W,Li C,et al.CdSe/ZnS Quantum Dots-Labeled Mesenchymal Stem Cells for Targeted Fluorescence Imaging of Pancreas Tissues and Therapy of Type 1 Diabetic Rats[J].Nanoscale Res Lett,2015,10(1):959.
[16]高冬蘊,谷城威,张振中,等.正常和糖尿病来源间充质干细胞生物学特性及创面修复效果差异的比较研究[J].感染、炎症、修复,2014,15(1):13-17.
[17]Tongers J,Roncalli JG,Losordo DW.Role of endothelial progeniror cells during is chemia-induced vasculogenesis and collateralformation[J].Micovasc Res,2010,79(3):200-206.
[18]Wicks K,Torbica T,Mace KA.Myeloid cell dysfunction and the pathogenesis of the diabetic chronic wound[J].Semin Immunol,2014,26(4):341-353.
[19]Cerqueira MT,Pirraco RP,Marques AP.Stem Cells in Skin Wound Healing:Are We There Yet?[J].Adv Wound Care(New Rochelle),2016,5(4):164-175.
[20]Gnecchi M,Danieli P,Malpasso G,et al.Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair[J].Methods Mol Biol,2016,1416:123-146.
[21]Ko KI,Coimbra LS,Tian C,et al.Diabetes reduces mesenchymal stem cells in fracture healing through a TNFα-mediated mechanism[J].Diabetologia,2015,58(3):633-642.
[22]Jianguo W,Tianhang L,Hong Z,et al.Optimization of culture conditions for endothelial progenitor cells from porcine bone marrow in vitro[J].Cell Prolif,2010,43(4):418-426.
[23]Cao J,Wang L,Du ZJ,et al.Recruitment of exogenous mesenchymal stem cells in mandibular distraction osteogenesis by the stromal cell-derived factor-1/chemokine receptor-4 pathway in rats[J].Br J Oral Maxillofac Surg,2013,51(8):937-941.
[24]Hu C,Yong X,Li C,et al.CXCL12/CXCR4 axis promotes mesenchymal stem cell mobilization to burn wounds and contributes to wound repair[J].J Surg Res,2013,183(1):427-434.
[25]Dong F,Harvey J,Finan A,et al.Myocardial CXCR4 expression is required for mesenchymal stem cell mediated repair following acute myocardial infarction[J].Circulation,2012,126(3):314-324.
[26]Lee DE,Ayoub N,Agrawal DK.Mesenchymal stem cells and cutaneous wound healing:novel methods to increase cell delivery and therapeutic efficacy[J].Stem Cell Res Ther,2016,7:37.
[27]Li N,Yang YJ,Qian HY,et al.Intravenous administration of atorvastatin-pretreated mesenchymal stem cells improves cardiac performance after acute myocardial infarction:role of CXCR4[J].Am J Transl Res,2015,7(6):1058-1070.
[28]Togha M,Jahanshahi M,Alizadeh L,et al.Rapamycin Augments Immunomodulatory Properties of Bone Marrow-Derived Mesenchymal Stem Cells in Experimental Autoimmune Encephalomyelitis[J].Mol Neurobiol,2017,54(4):2445-2457.
[29]Yu X,Chen D,Zhang Y,et al.Overexpression of CXCR4 in mesenchymal stem cells promotes migration,neuroprotection and angiogenesis in a rat model of stroke[J].J Neurol Sci,2012,316(1-2):141-149.
[30]Kitaori T,Ito H,Schwarz EM,et al.Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model[J].Arthritis Rheum,2009,60(3):813-823.
[31]Hocher B,Sharkovska Y,Mark M,et al.The novel DPP-4 inhibitors linagliptin and BI 14361 reduce infarct size after myocardial ischemia/reperfusion in rats[J].Int J Cardiol,2013,167(1):87-93.
[32]Xu X,Zhu F,Zhang M,et al.Stromal cell-derived factor-1 enhances wound healing through recruiting bone marrow-derived mesenchymal stem cells to the wound area and promoting neovascularization[J].Cells Tissues Organs,2013,197(2):103-113.
[33]Luo Q,Zhang B,Kuang D,et al.Role of Stromal-Derived Factor-1 in Mesenchymal Stem Cell Paracrine-Mediated Tissue Repair[J].Curr Stem Cell Res Ther,2016,11(7):585-592.
[34]Ghadge SK,Mühlstedt S,Ozcelik C,et al.SDF-1α as a therapeutic stem cell homing factor in myocardial infarction[J].Pharmacol Ther,2011,129(1):97-108.
[35]王佃亮.干細胞治疗现状、策略与前景展望[J].转化医学杂志,2018,7(6):329-333.
[36]吴刚,童培建.补肾活血汤含药血清干预体外培养大鼠骨髓间充质干细胞成软骨分化及补肾活血汤联合骨髓间充质干细胞治疗大鼠膝骨关节炎的实验研究[J].中医正骨,2018,30(1):6-11.
[37]朱磊.健脾补肾、清肠化湿方促进骨髓间充质干细胞归巢重建溃疡性结肠炎肠黏膜屏障的研究[D].南京:南京中医药大学,2016.
[38]陈谊敬.补肾中药有效成分对骨髓间充质干细胞成骨分化的影响[D].沈阳:辽宁中医药大学,2013.
[39]岳宗进,于露,刘汝银,冯仲锴,王新立,王西彬,鲁花.牛膝提取物对大鼠骨髓间充质干细胞向髓核样细胞增殖与分化的影响[J].中成药,2018,40(12):2635-2639.
(2018-12-27收稿 责任编辑:王明)

