Effect of temperature on antioxidant defense and innate immunity in Brandt’s voles
De-Li Xu,Meng-Meng Xu,De-Hua Wang,*
1 College of Life Sciences,Qufu Normal University,Qufu Shandong 273165,China
2 State Key Laboratory of Integrated Management of Pest Insects and Rodents,Institute of Zoology,Chinese Academy of Sciences,Beijing 100101,China
ABSTRACT Ambient temperature is an important factor influencing many physiological processes, including antioxidant defense and immunity. In the present study, we tested the hypothesis that antioxidant defense and immunity are suppressed by high and low temperature treatment in Brandt’s voles(Lasiopodomys brandtii). Thirty male voles were randomly assigned into different temperature groups(4, 23, and 32 °c, n=10 for each group), with the treatment course lasting for 27 d. Results showed that low temperature increased gross energy intake(GEI) and liver, heart, and kidney mass, but decreased body fat mass and dry carcass mass.With the decline in temperature, hydrogen peroxide(H2O2) concentration, which is indicative of reactive oxygen species (ROS) levels, increased in the liver,decreased in the heart, and was unchanged in the kidney, testis, and small intestine. Lipid peroxidation indicated by malonaldehyde (MDA) content in the liver, heart, kidney, testis, and small intestine did not differ among groups, implying that high and low temperature did not cause oxidative damage.Similarly, superoxide dismutase (SOD) and catalase(cAT) activities and total antioxidant capacity (TAOc) in the five tissues did not respond to low or high temperature, except for elevation of cAT activity in the testis upon cold exposure. Bacteria killing capacity, which is indicative of innate immunity, was nearly suppressed in the 4 °c group in contrast to the 23 °c group, whereas spleen mass and white blood cells were unaffected by temperature treatment. The levels of testosterone, but not corticosterone, were influenced by temperature treatment, though neither were correlated with innate immunity,H2O2 and MDA levels,or SOD,cAT,and TAOc activity in any detected tissues. Overall, these results showed that temperature had different influences on oxidative stress, antioxidant enzymes,and immunity, which depended on the tissues and parameters tested. Up-regulation or maintenance of antioxidant defense might be an important mechanism for voles to survive highly variable environmental temperatures.
Keywords: Antioxidant defense; Brandt’s voles(Lasiopodomys brandtii);Immunity;Temperature
INTRODUCTION
Ambient temperature is an important factor influencing many physiological processes in animals, including antioxidant defense and immune function (King, 2004; Marnila & Lilius,2015). While antioxidant defense can eliminate reactive oxygen species (ROS), oxidative stress can occur when ROS production overwhelms antioxidant capacity (Dickinson &chang, 2011; Selman et al., 2013). Oxidative stress can cause oxidative damage to biomolecules (i.e., lipids, proteins,and DNA) and is deleterious to the structure and function of cells and tissues (Marri & Richner, 2015; Raut et al., 2012).The immune system, which protects animals from infection and pathogens, plays a key role in survival and fitness(Owens & Wilson, 1999; Sheldon &Verhulst, 1996). Therefore,both antioxidant capacity and immune function are crucial for animals to maintain health and survival; however, both are markedly influenced by environmental temperature (carroll et al.,2012;Metcalfe&Alonso-Alvarez,2010;Zhou et al.,2015).
Several researchers have investigated the impact of temperature on antioxidant defense. For instance, cold exposure in rats has been shown to increase lipid peroxidation in the brain but decrease it in the liver (Lomakina,1980) and enhance superoxide dismutase (SOD) activity in the heart and kidney but suppress it in the lungs and pancreas(Vasilijević et al., 2007; Yuksel et al., 2008). Moreover, cold stress is reported to increase protein oxidation in the liver and muscle but not affect brown adipose tissue in short-tailed field voles (Microtus agrestis) (Selman et al., 2000, 2002, 2008).Lipid peroxidation, total antioxidant capacity (T-AOc), and glutathione peroxidase activity in brown adipose tissue has been shown to increase under low temperature but decrease under high temperature in striped hamsters (Cricetulus barabensis)(Zhou et al.,2015).Furthermore,high temperature exposure has been shown to cause oxidative damage in broiler chickens (Tan et al., 2010) and decrease SOD activity in rat testes(Kanter et al.,2013).
Bacteria killing capacity, which entails phagocytes,opsonizing proteins, and natural antibodies acting against specific pathogens, has been used to evaluate innate immunity in mammals (Demas et al., 2011; Tieleman et al.,2005). Immune organs and total white blood cells (WBc) are indirect parameters indicative of immune function (calder &Kew, 2002). A larger spleen is representative of a stronger immune system (Smith & Hunt, 2004), and adipose tissue is no longer regarded as a simple passive energy reserve but also as an important endocrine and immune organ (Ahima &Flier, 2000; Fantuzzi, 2005; Trayhurn, 2005). Although investigators have examined the impact of temperature on immunity in laboratory animals, including mice (Xu et al.,1992) and rats (Kozyreva & Eliseeva, 2000, 2004), research on wild rodents remains scarce or contradictory. For instance,both cellular immunity and bacteria killing capacity are reported to be unaffected by low and high temperature in female Mongolian gerbils (Meriones unguiculatus) (Yang et al.,2013).
Stressful conditions, such as cold or hot temperatures, can stimulate the hypothalamic-pituitary-adrenal axis, and hence secretion of glucocorticoids such as corticosterone, which is related to oxidative damage and immunity (Kim et al., 2013;Sapolsky et al., 2000). Moreover, testosterone incurs oxidative costs such as increased production of ROS according to the oxidation handicap hypothesis (Alonso-Alvarez et al., 2007,2008). Testosterone also has suppressive effects on immune function in many species, including mammals and birds(Trigunaite et al.,2015).
Brandt’s voles (Lasiopodomys brandtii) are primarily distributed in the grasslands of Inner Mongolia in china as well as the Republic of Mongolia and the Baikal region of Russia (Li et al., 2010; Walker, 1968). They are strictly herbivorous and feed mainly on grass (Wang et al., 2003).The climate is arid and characterized by warm, dry summers(maximum 42.6 °c) and cold winters (minimum -47.5 °c)(chen, 1988; Zhao & Wang, 2006). Thus, this species experiences considerable seasonal fluctuations in temperature, photoperiod, and food availability (Wang et al.,2000; Zhang & Wang, 1998). Previous research has shown that resting metabolic rate (RMR) and uncoupling protein 1(UcP1) content increase but body fat mass decreases in voles upon cold exposure (Liu et al., 2009; Wang, 2007). Both immune responses (Demas, 2004; Martin et al., 2003) and oxidative stress come at a cost (Dowling & Simmons, 2009)and life-history trade-off (Hall et al., 2010; Martin et al., 2007;Monaghan et al., 2009). Therefore, we expect that low and high temperature exposure should cause oxidative stress and immunosuppression in voles.
MATERIAL AND METHODS
Animals and experimental design
Animals used in this study were the offspring of a captive colony trapped on the Inner Mongolian grasslands in May 1999 and brought to the animal facility at the Institute of Zoology, chinese Academy of Sciences, Beijing, china. All animal procedures were licensed under the Institutional Animal care and Use committee of the Institute of Zoology,chinese Academy of Sciences (approval number:QFNUDW2012016; approval date: 20120628). The animals were housed individually after weaning in plastic cages (30 cm×15 cm×20 cm) with sawdust as bedding under a constant photoperiod (16 h:8 h light-dark cycle) and temperature (23±1 °c). Rabbit pellet chow and water were provided ad libitum.Thirty male voles (aged 4-6 months) were randomly assigned into the cold (4±1 °c) (n=10), warm (23±1 °c) (n=10), and hot groups (32±1 °c) (n=10) (hereafter referred to as the 4 °c,23°c,and 32°c groups).The treatment course lasted for 27 d.One vole escaped after 13 d of cold treatment and another vole died after 16 d of cold treatment. Therefore, these two voles were not included in subsequent statistical analyses.
Energy intake
Body mass was recorded every 3 d and energy budgets were determined at 3-d intervals over the course of the study. Food intake was measured in metabolic cages, as described previously (Xu & Wang, 2011; Xu et al., 2011). Food was provided quantitatively. Food residue and feces were collected from each subject over 3 d before acclimation began and during the course of treatment and were separated after they were dried at 60 °c to a constant mass (Liu et al., 2003).Energy content of the food and feces was determined using a Parr 1281 oxygen bomb calorimeter (Parr Instrument, USA).Gross energy intake (GEI) was calculated according to Grodzinski & Wunder (1975) and Liu et al. (2003) using the following equation:

Organ and body composition
At the end of the experiment, animals were sacrificed by cO2asphyxiation,after which trunk blood was collected for later measurement of WBc. Blood samples were allowed to clot for 1 h and centrifuged at 4 °c for 30 min at 4 000 r/min. Sera were collected and stored in polypropylene microcentrifuge tubes at -80 °c for later hormone (i.e., corticosterone and testosterone) and bacterial killing capacity assays. Organ mass was measured as described previously (Xu & Wang,2011; Xu et al., 2011). In brief, visceral organs, including the heart, liver, kidneys, testes, small intestine, and spleen, were dissected on an ice box and weighed (±1 mg). The length of the small intestine was measured by extending the organ to its unstressed length along a ruler (±1 mm) (Pei et al., 2001).The small intestine was then opened and rinsed with saline to eliminate all gut contents, blotted dry on tissue paper, and weighed. The heart, liver, kidneys, testes, and small intestine were stored at -80 °c for later antioxidant enzyme assays.The carcass was dried in an oven at 60 °c to a constant mass, and then weighed again to obtain dry mass. The difference between the wet and dry carcass mass was the water mass of the carcass. Total body fat was extracted from the dried carcass by petroleum ether extraction in a Soxhlet apparatus, and body fat content was calculated as the proportion of total body fat mass divided by wet carcass mass(Xu&Wang,2010).
Oxidative stress marker assays
Lipid peroxidation, which is indicative of oxidative damage,was examined as described previously (Yang et al., 2013).Specifically, lipid peroxidation was evaluated by quantifying malonaldehyde (MDA) (Del Rio et al., 2005) using a thiobarbituric acid reactive substances (TBARS) assay kit(Nanjing Jiancheng, Nanjing, china) following the manufacturer’s instructions. The absorbance of the eluent was monitored spectrophotometrically at 532 nm (BioTek Synergy 4 Hybrid Microplate Reader, BioTek, Winooski,Vermont, USA). The intra- and inter-assay coefficients of variation for this assay were <1.5% and <3.32%, respectively.Lipid peroxidation was expressed as nmol MDAper mg protein.ROS levels were measured in the tissues by examining hydrogen peroxide (H2O2) levels (Zhou et al., 2015). The H2O2levels were analyzed using a commercial kit (Nanjing Jiancheng, Nanjing, china) in accordance with the manufacturer’s instructions. Levels of H2O2were expressed as μmol/g protein.
Antioxidant enzymes
The activities of antioxidant enzymes, including SOD and cAT,and total antioxidant capacity (T-AOc) were also determined using commercial kits (Nanjing Jiancheng, Nanjing, china)according to the manufacturer’s instructions. One unit of SOD was defined as the amount of enzyme that caused 50%inhibition of superoxide radical produced by the reaction between xanthine and xanthine oxidase at 37 °c; one unit of cAT activity was defined as the decomposition of 1 μmol H2O2per min; one unit of T-AOc was defined as the extent to which optical density increased by 0.01 per milligram protein per min(chen et al.,2014).
Immunological parameters
WBc count was determined as described previously (Xu &Wang, 2010). In brief, 20 μL of whole blood was diluted immediately in a 0.38 mL solution containing 1.5% glacial acetic acid and 1% crystal violet (Sigma). The leukocytes were then counted in an improved Neubauer chamber using a microscope. Total number of WBc was determined by counting all leucocytes in the four large corner squares of the Neubauer chamber and multiplying the raw data by 5×107to obtain final values(109cells/L)(Yang,2004).
Serum bacterial killing capacity, which is indicative of innate immunity, was performed in a sterile laminar flow cabinet to assess the functional response by the animal’s innate immune system against a relevant pathogen, Escherichia coli(Demas et al., 2011; Tieleman et al., 2005; Yang et al., 2013).Briefly, serum samples were diluted 1:20 in a cO2-independent medium (Gibco no. 18045, carlsbad, GA, USA).A standard number of colony-forming units (cFUs) of E. coli(ATcc no. 8739, Microbial culture collection center of Guangdong Institute of Microbiology, china) was added to each sample at a ratio of 1:10, after which the mixture was allowed to incubate at 37 °c for 30 min to induce bacterial killing. After incubation, 50 μL of each sample was added to tryptic soy agar plates in duplicate. All plates were covered and left to incubate upside down at 37 °c for 24 h. After this,total cFUs were counted and bactericidal capacity was calculated as 100% minus the mean number of cFUs for each sample divided by the mean number of cFUs for the positive controls (containing only medium and standard bacterial solution), i.e., the percentage of bacteria killed relative to the positive control.
Serum corticosterone assays
Serum corticosterone concentrations were determined using a rat corticosterone ELISA kit (cat. No. HR083, RapidBio Lab.calabasas,california,USA).The lowest level of corticosterone that could be detected by this assay was 1.0 nmol/L. All procedures were in accordance with the manufacturer’s instructions. Inter- and intra-assay variabilities for corticosterone were <1.1%and 7.5%,respectively.
Serum testosterone
Serum testosterone concentrations were assessed using a rat testosterone ELISA kit (cat. No. HR083, RapidBio Lab.calabasas, california, USA) following the manufacturer’s instructions. The tested range of testosterone was 0.13-25.6 ng/mL. Intra- and inter-assay variabilities for testosterone were <9.0%and 11.0%,respectively.
Statistical analysis
Data were analyzed using SPSS 13.0 software (SPSS Inc.,chicago, IL, USA). Prior to all statistical analyses, data were examined for normality and homogeneity of variance using Kolmogorov-Smirnov and Levene tests, respectively. changes in body mass and GEI during the experiment were analyzed with the Repeated Measure of General Linear Model (GLM).Differences in body mass at any time point, MDA and H2O2levels, SOD and cAT activity, T-AOc, WBc, innate immunity,and corticosterone and testosterone concentrations in different groups were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc tests. Group differences in wet organ mass and GEI, with body mass as the covariate, at any time point were analyzed by a one-way analysis of covariance (ANcOVA) followed by Bonferroni posthoc tests. Significant group differences were further evaluated by GLM multivariate analysis followed by Bonferroni post hoc tests. Pearson correlation analysis was performed to determine the correlations of corticosterone, testosterone, and antioxidant parameters. Results are expressed as means±SE,with P<0.05 considered to be statistically significant.
RESULTS
Body mass and energy intake
Body mass among the 4 °c, 23 °c, an d 32 °c groups did not differ significantly before the experiment began (day 0, F2,25=0.650, P=0.531) (Figure 1A). Based on repeated measure ANOVA, body mass changed significantly over the 27 d acclimation to low and high temperature (F9,225=40.915, P<0.001). Body mass among the three groups show no significant differences from day 3 (F2,25=1.477, P=0.248) to day 12 (F2,25=2.822, P=0.078), whereas body mass differed significantly from day 15 (F2,25=4.600, P=0.020) to day 27(F2,25=4.203, P=0.027) (Figure 1A). At the end of the experiment, body mass in the 4 °c, 23 °c, and 32 °c groups increased by 16.2%, 17.4%, and 32.4%, respectively,compared with body mass on day 0 (Table 1). There was no difference in GEI among the three groups on day 0 (F2,24=0.251, P=0.780) (Figure 1B); however, GEI changed significantly over the course of the experiment (F8,200=54.430,P<0.001). GEI was significantly influenced by temperature from day 3 to day 27 and was significantly higher in the 4 °c group and lower in the 32 °c group relative to the 23 °c group(day 3, F2,24=10657.8, P<0.001; day 27, F2,24=22.7, P<0.001)(Figure 1B).
Body composition and organs
Body composition (i.e., wet carcass, dry carcass, fat free dry carcass, body fat mass, and fat content) decreased, whereas organ mass (i.e., heart, liver, and kidney) and small intestine length increased, and spleen, testis, and small intestine mass remained unchanged with the decline in temperature (Table 1). Body fat mass was 37.9% lower in the 4 °c group and 62.1% higher in the 32 °c group in contrast with the 23 °c group.
Oxidative stress
H2O2levels showed significant differences among the various tissues in all three treatment groups (F4,135=58.61, P<0.001,Figure 2A). In the 4 °c group, H2O2levels were significantly higher in the small intestine than in the liver, heart, kidney, or testis (post hoc, P<0.05). Temperature had a significant effect on H2O2levels in the liver,which were 46.7%higher in the 4°c group and 27.9% lower in the 32 °c group compared to the 23 °c group (F2,25=4.584, P=0.020). H2O2levels in the heart were also affected significantly by temperature and were 44.3% and 102.9% higher in the 4 °c and 32 °c groups,respectively, compared to the 23 °c group (F2,25=7.949, P=0.002). However, temperature had no effect on H2O2levels in the kidney (F2,25=1.506, P=0.241), testis (F2,25=0.166, P=0.848),or small intestine(F2,25=1.649,P=0.212)(Figure 2A).

Table 1 Effect of temperature on body composition and wet organ mass in Brandt’s voles

Figure 1 Effect of temperature on body mass (A) and gross energy intake (B) in Brandt’s voles acclimated to 4 °C, 23 °C, and 32°C

Figure 2 Effect of temperature on H2O2 (A) and MDA levels (B) in Brandt’s voles acclimated to 4°C,23°C,and 32°C
MDA levels differed significantly among the various tissues(F4,135=59.29, P<0.001, Figure 2B), and were significantly higher in the kidney and small intestine than that in the liver,heart, or testis in the 4 ° c group (post hoc, P<0.05).Temperature had no influence on MDA content in the liver(F2,25=0.247, P=0.783), kidney (F2,25=1.816, P=0.184), testis(F2,25=2.039, P=0.151), or small intestine (F2,25=0.356, P=0.704). However, MDA levels in the heart were 31.5% lower in the 32 °c group than that in the 23 °c group (F2,25=3.391, P=0.050)(Figure 2B).
Antioxidant enzymes
A significant difference in total SOD activity was found among the five tissues (F4,135=42.64, P<0.001, Figure 3A), with higher activity detected in the small intestine compared with the other four tissues (post hoc, P<0.05). There were no significant differences in total SOD activity in the liver (F2,25=0.531, P=0.594), heart (F2,25=0.076, P=0.927), kidney (F2,25=0.523, P=0.599), and small intestine (F2,25=0.184, P=0.833) among the 4 °c, 23 °c, and 32°c groups. Total SOD activity in the testis was 18.8% and 19.9% higher in the 4 °c and 32 °c groups,respectively, compared to the 23 °c group (F2,25=3.239, P=0.056)(Figure 3A).

Figure 3 Effect of temperature on total SOD activity (A), CAT activity (B), and total antioxidant capacity (T-AOC) (C) in Brandt’s voles
cAT activity exhibited significant differences among the five tissues (F4,135=31.17, P<0.001, Figure 3B), and was higher in the kidney than the other four tissues in the 4 °c group (post hoc, P<0.05). cAT activity in the testis was significantly increased in the 4 °c (304.6%) and 32 °c groups (87.3%),respectively, compared to the 23 ° c group (Figure 3B).However, temperature had no significant impact on cAT activity in the liver, heart, kidney, or small intestine (liver, F2,25=1.165,P=0.328;heart,F2,25=0.016,P=0.984;kidney,F2,25=1.299,P=0.291;small intestine,F2,25=1.110,P=0.345)(Figure 3B).
T-AOc differed significantly among the five tissues (F4,135=59.44, P<0.001, Figure 3c), and was higher in the small intestine than that in the other four tissues in the 4 °c group(post hoc, P<0.05). No significant differences in T-AOc were observed in the liver (F2,25=2.313, P=0.120), heart (F2,25=0.683, P=0.514), kidney (F2,25=0.437, P=0.651), testis (F2,25=2.368, P=0.114), and small intestine (F2,25=0.068, P=0.934)among the groups (Figure 3c). However, significant positive correlations were found between MDA levels and SOD, cAT,and T-AOc activities in the heart and small intestine and between H2O2levels and MDA, SOD, and T-AOc activities in the small intestine, with some significant correlations found in the liver,kidney,and testis(Table 2).
Immunological parameters
No statistically significant differences were observed in wet spleen mass (F2,25=0.748 P=0.484) (Figure 4A), WBc (F2,25=0.504, P=0.610) (Figure 4B), or bacteria killing capacity (F2,25=2.822, P=0.079) (Figure 4c). Innate immunity was 118.0%and 35.5% lower in the 4 °c and 32 °c groups, respectively,compared to the 23 °c group. Innate immunity was negatively correlated with liver H2O2levels and kidney MDA content, but not with body fat mass, H2O2levels, or MDA content in other tissues(Table 3).

Figure 4 Effect of temperature on spleen wet mass(A),white blood cell count(B),and bacteria killing capacity(C)in Brandt’s voles
Serum corticosterone
corticosterone concentration did not differ among the groups(F2,25=1.818, P=0.183) (Figure 5A). Furthermore,corticosterone concentration was not correlated with H2O2levels, MDA content, or SOD, cAT, and T-AOc activities in the liver, heart, kidney, testis, or small intestine, except for a negative correlation between corticosterone and SOD activity in the testis (Table 2). Moreover, no significant correlation was found between innate immunity and corticosterone concentration(r=0.206,P=0.293)(Table 3).
Serum testosterone
Testosterone concentration was significantly influenced by temperature and was 25.8% and 53.1% higher in the 4 °c and 32 °c groups,respectively,compared to the 23°c group (F2,25=4.403, P=0.023) (Figure 5B). Testosterone concentration was not correlated with H2O2levels, MDA content, or SOD, cAT,and T-AOc activities in the liver, heart, kidney, testis, or small intestine, except for a positive correlation with testis SOD(Table 2). Testosterone concentration was negatively correlated with corticosterone concentration (Table 2) but was not correlated with innate immunity (r=-0.119, P=0.546)(Table 3).
DISCUSSION
Ambient temperature is one of the most important factors affecting physiological processes. contrary to our expectation,we found that temperature exerted different influences on oxidative stress, antioxidant enzymes, and immunity depending on the tissues and parameters tested. With the decline in temperature, ROS levels increased in the liver but did not change in the kidney, testis, or small intestine. ROS levels in the heart were higher in the 32 °c group than in the 23 °c group. compared to the 23 °c group, cold exposure elevated cAT activity in the testis but not in the liver, heart,kidney, or small intestine. MDA content and SOD and T-AOc activities in the five tissues showed no response to low or high temperature. Bacteria killing capacity was nearly suppressed in the 4 °c group compared with that in the 23°c group,whereas spleen mass and WBc were unaffected by temperature treatment.
Body mass,body composition,and organs
To satisfy the augmentation in energy requirements during cold temperature, animals usually increase energy intake(Hammond & Wunder, 1995). In the present study, we also found that gross energy intake increased in the voles with the decline in temperature. However, the body masses in the 4 °c and 32 °c groups at the end of the experiment were 7.5%lighter and 14.8% heavier, respectively, than that in the 23 °c group. This may be due to an increase in energy expenditure with the decrease in temperature (chi & Wang, 2011;Hammond & Wunder, 1995). Moreover, voles also mobilized energy reserves, including fat free carcass and body fat mass,to meet the elevated energy requirements with the decrease in temperature. In general, an increase in expensive metabolic organ mass (i.e., liver, heart, small intestine, and kidneys) and small intestine length during cold acclimation are adaptive responses to elevated energy needs and food processing capability (Daan et al., 1990; Hammond & Wunder, 1995;Konarzewski & Diamond, 1995). In our study, the liver, heart,and kidney mass and small intestine length also increasedsignificantly with the decline in temperature, consistent with previous studies (chi & Wang, 2011; Zhang & Wang, 2006).These changes in metabolic organs would satisfy the increased energy requirements and food processing capability under cold exposure.

Table 2 Pearson’s correlation coefficients between H2O2 and MDA levels,SOD,CAT,T-AOC activity,and corticosterone and testosterone in Brandt’s voles
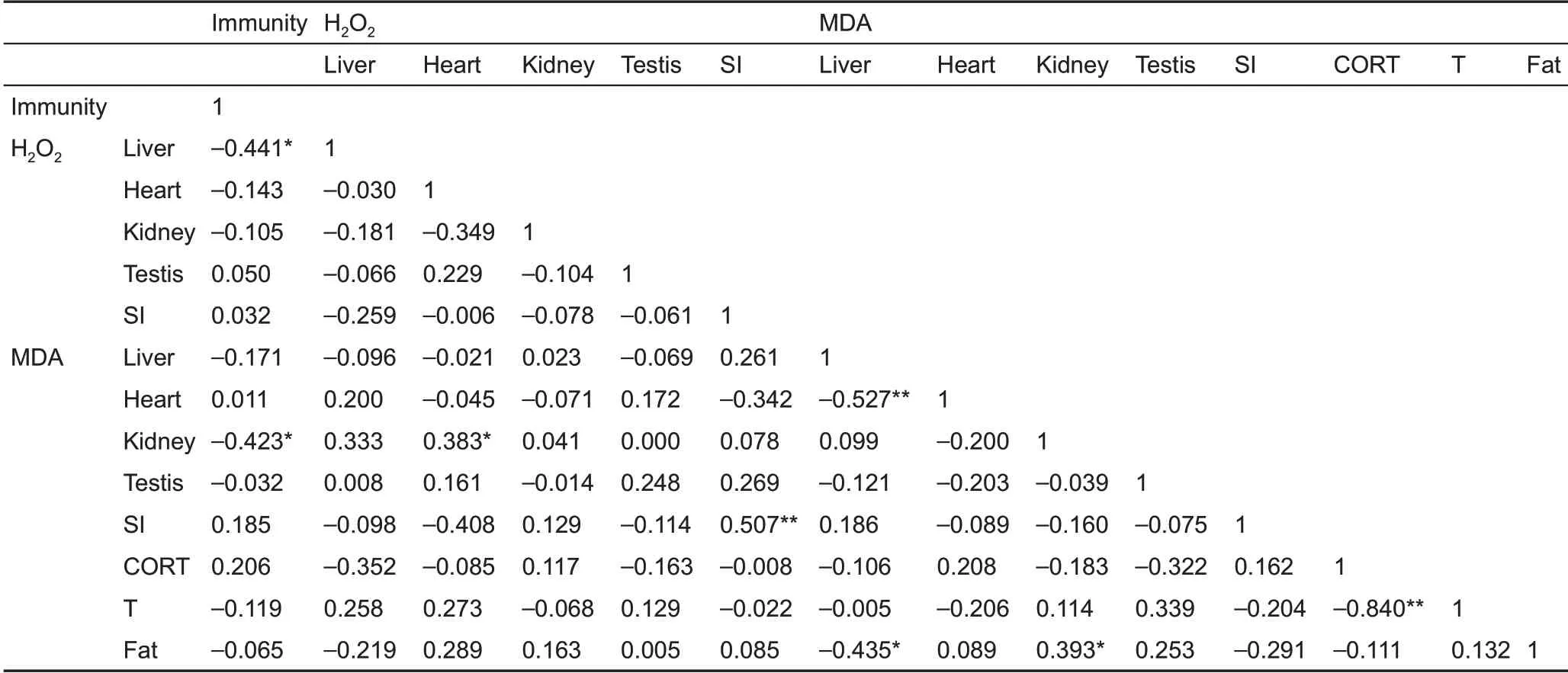
Table 3 Pearson’s correlation coefficients between innate immunity, H2O2 and MDA levels, corticosterone, testosterone, and body fat mass in Brandt’s voles
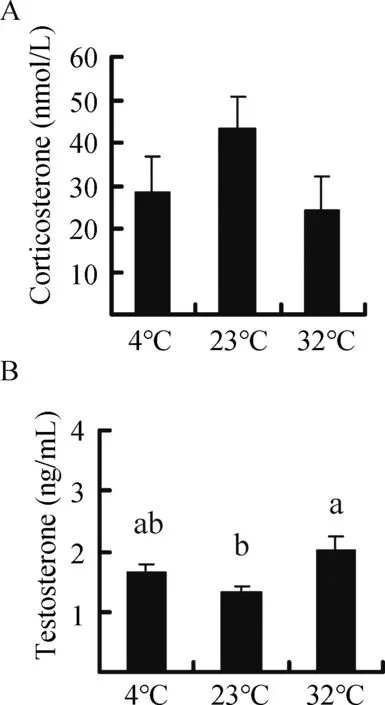
Figure 5 Effect of temperature on the concentration of corticosterone (A) and testosterone (B) in Brandt’s voles acclimated to 4°C,23°C,and 32°C
Oxidative stress and gross energy intake
Energy requirements usually increase across many species in cold environments (chi & Wang, 2011; Liu et al., 2009; Zhou et al., 2015). Thus, animals adopt different adaptive strategies, such as increased food and gross energy intake,as observed in the voles in our study.The increase in GEI with the decline in temperature also indicated an elevation in the metabolic rate of voles, although resting metabolic rate was not examined in our study. Previous research has shown that cold exposure significantly increases metabolic rate in voles(Li et al., 2001; Liu et al., 2009; Zhang & Wang, 2006).According to the rate of living-free radical hypothesis, higher metabolic rates, which are achieved by enhancing mitochondrial oxidative phosphorylation, should increase the production of free radicals (i.e., ROS) (Harman, 1956; Pearl,1928; Selman et al., 2013; Speakman et al., 2004). In the current study, the increase in ROS production with the decrease in temperature, as indicated by H2O2levels in the liver, was consistent with this hypothesis, whereas the reduction of ROS in the heart and unchanged ROS in the kidney, testis, and small intestine were not compatible with this hypothesis. The reason of these disparate results might lie in the fact that an elevated metabolic rate during cold acclimation does not necessarily result in greater ROS production (costantini, 2008; Hulbert et al., 2007). In light of the“uncoupling to survive”hypothesis, increased uncoupling proteins in response to cold temperature can decrease ROS production by lowering the potential of the inner mitochondrial membrane (Brand, 2000; Speakman et al., 2004). Several studies have also demonstrated that an increased metabolic rate is not associated with elevated ROS levels in skeletal muscle or certain organs, including the liver, heart, lungs,spleen, kidneys, and digestive tract (chen et al., 2014;Selman et al., 2002; Stier et al., 2014). The increased UcP1 content in voles upon cold exposure in previous studies might account for the ROS results in our experiment (Liu et al.,2009;Zhang&Wang,2006).
In the present study, high temperature led to an increase in oxidation, as indicated by the H2O2levels in the vole heart,which is consistent with previous research showing increased levels of oxidation at high temperature in broiler chickens (Tan et al., 2010). In light of the oxidation handicap hypothesis,testosterone may elevate the levels of ROS (Alonso-Alvarez et al., 2007, 2008). The increased ROS levels in voles under high temperature may also be caused by higher testosterone concentration, although no significant correlation was detected between H2O2and testosterone levels.
In general, excessive ROS production can lead to oxidative damage (Burton & Jauniaux, 2011; costantini, 2008; Finkel &Holbrook, 2000; Hulbert et al., 2007). In the present study,however, MDA content in the liver, heart, kidney, testis, and small intestine, which did not respond to high or low temperature treatment, was not related to ROS (H2O2) levels.This result agrees with several studies in which MDA content in the liver and serum of female Mongolian gerbils (Yang et al., 2013) or in the liver, heart, muscle, and brain of striped hamsters (Zhou et al., 2015) was not affected by high or low temperature treatment. This may be because the voles were able to maintain or increase SOD, T-AOc, and cAT activity in the five tissues, even under high or low temperature environments,which removed excessive free radicals.
Antioxidant defense and hormone profiles
In the present study, cAT activity in the testis increased upon cold exposure but did not change under high temperature.conversely, testosterone levels increased under high temperature but did not change with cold exposure. Thus, the effects of high temperature on the voles were different from those of low temperature.
Secretion of stress hormones such as corticosterone usually increase in animals facing stressful conditions,including high or low ambient temperature (Bligh-Tynan et al.,1993; Kim et al., 2013; Sapolsky et al., 2000). In the current study, however, corticosterone concentration in voles was not affected by high or low temperature, which is inconsistent with other studies showing increased corticosterone levels under cold stress (Adels et al., 1986; Shu et al., 1993). This discrepancy may be due to the difference in cold exposure duration. Secretion of corticosterone usually increases upon acute cold exposure, whereas its levels can recover to baseline under chronic cold exposure (Bligh-Tynan et al.,1993).
Several studies have shown that exogenous corticosterone administration can increase oxidative stress and decrease SOD activity in rats (Dhanabalan et al., 2010) and birds(Alonso-Alvarez et al., 2007; Lin et al., 2004). Here, we found that corticosterone concentration did not respond to high or low temperature treatment, and it was not correlated with ROS and MDA levels or SOD, cAT, and T-AOc activities in the liver, heart, kidney, testis, and small intestine (except for a negative correlation with SOD activity in the testis). Thus, it appears that corticosterone could not fully explain the impact of hot and cold temperature on oxidative stress and antioxidant capacity in voles.
The oxidation handicap hypothesis holds that testosterone incurs oxidative costs (Alonso-Alvarez et al., 2007, 2008).Although the testosterone concentration was significantly affected by temperature in the tested voles, it was not correlated with H2O2levels,MDA content,or SOD,cAT,and TAOc activities in the five tested tissues (i. e., liver, heart,kidney, testis, and small intestine), with the exception of a positive correlation with SOD activity in the testis. This result is consistent with earlier research demonstrating that increased testosterone does not cause oxidative damage to DNA but enhances total antioxidant capacity in yellowthroat warblers (Geothlypis trichas) (Taff & Freeman-Gallant, 2014).Several authors have argued that detection of oxidative costs is both tissue and assay-dependent (Yang et al., 2013),suggesting that additional parameters and tissues may have identified certain direct oxidative costs of testosterone in the present study.
Immunity,body fat,and hormone profiles
Innate immunity, as indicated by bacteria killing capacity, was lower in the 4 °c group than in the 23 °c group. This may be due to the reduction of body fat in voles upon cold exposure.Adipose tissues are not only considered as endocrine and immune organs (Ahima & Flier, 2000; Trayhurn, 2005), but also as energy depots for expensive physiological processes,including immune function (Demas et al., 1997; Demas, 2004;Moret & Schmid-Hempel, 2000). Previous research has demonstrated that animals with low energy reserves choose to allocate less energy to immune defense than animals with higher reserves (Houston et al., 2007). Therefore, reductions in body fat can impair immunity (chandra, 1996; Demas et al.,2003). In the face of energy demanding conditions, including cold environments, trade-offs may occur among different physiological processes. Voles might divert energy from less critical physiological functions such as immunity to more important processes such as thermogenesis for immediate survival during cold exposure (Liu et al., 2009; Zhang & Wang,2006). However, further research is required to clarify whether trade-offs occur between thermogenic capacity and immune function under cold environments.
Both corticosterone and testosterone have suppressive effects on immunity (Marketon & Glaser, 2008; Sapolsky et al.,2000; Trigunaite et al., 2015). In the present study, however,we found no correlation between corticosterone and testosterone and innate immunity. Thus, these two hormones could not account for the variation in immunity observed in voles. Recently, several authors have shown that oxidative stress may also impair immune function (costantini & Møller,2009; Sordillo &Aitken, 2009). We found that innate immunity was negatively correlated with H2O2levels in the liver and MDA content in the kidney. Suppression of innate immunity in the 4 °c group compared to the control may be caused by oxidative stress in voles.
In the current study, voles exhibited several adaptive strategies to cope with high and low temperature. Voles increased gross energy intake and active metabolic organs (i.e., heart, liver, kidneys) and mobilized energy reserves with the decline in temperature. However, voles may maintain or increase antioxidant defense at the cost of innate immunity under cold temperature. Therefore, up-regulation or maintenance of antioxidant defenses may be crucial for voles to survive under dramatically fluctuating ambient temperatures from the perspective of oxidative ecology(Boonstra,2013).
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’CONTRIBUTIONS
D.L.X. and D.H.W. designed the study. D.L.X. and D.H.W. supervised the analyses. D.L.X. and M.M.X examined the body mass and gross energy intake of the study. D.L.X. performed the parameters of antoxidant enzymes and oxidative stress. D.L.X. wrote the manuscript with the direction of D.H.W.. D.L.X. revised the manuscript. All authors read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
We are grateful to Xue-Ying Zhang from Institute of Zoology, chinese Academy of Sciences for her help in the experiment. We are also grateful for the valuable suggestions provided by the anonymous reviewers and the carefully editing of the editors of Zoological Research.
- Zoological Research的其它文章
- Transcription profiles of skin and head kidney from goldfish suffering hemorrhagic septicemia with an emphasis on the TLR signaling pathway
- A new cave-dwelling blind loach,Тriplophysa erythraea sp.nov.(cypriniformes:Nemacheilidae),from Hunan Province,china
- Leukocyte cell-derived chemotaxin 2 inhibits development of atherosclerosis in mice
- Allele-specific expression and alternative splicing in horse×donkey and cattle×yak hybrids
- Natural history of Valentin’s rock lizard(Darevskia valentini)in Armenia
- On the road to Mandalay:contribution to the Microhyla Tschudi,1838(Amphibia:Anura:Microhylidae)fauna of Myanmar with description of two new species

