One-year outcomes of a NeoHexa sirolimus-eluting coronary stent system with a biodegradable polymer in all-comers coronary artery disease patients: Results from NeoRegistry in India
Rajagopal Jambunathan, Dinesh Basavanna, Preeti Vani, Malte Neuss, Prashant Janbandhu
Rajagopal Jambunathan, Dinesh Basavanna, Cauvery Heart and Multispecialty Hospital,Mysore, Karnataka 570011, India
Dinesh Basavanna, Department of Cardiology Mysore Medical College and Research Institute,Mysore, Karnataka 570001, India
Preeti Vani, Malte Neuss, Prashant Janbandhu, Medical Division, Sahajanand Laser Technology Ltd., Gandhinagar, Gujarat 382027, India
Malte Neuss, Manemed Research and Development, Roeckumstr, Bonn 53123, Germany
Abstract
Key words: Sirolimus; Drug-eluting stent; Myocardial infarction; Thrombosis; Coronary artery disease
INTRODUCTION
The prevalence of cardiovascular diseases, particularly coronary artery disease(CAD), is ever-increasing in India and has reached epidemic proportions[1].Approximately 17% of total deaths were attributed to coronary heart disease in 2001-2003, which increased to 23% in 2010-2013 in India[1,2].Percutaneous coronary intervention (PCI) is one of the most commonly performed cardiac procedures aimed at improving symptoms and quality of life of patients with CAD[3-5].
The unceasing research over decades in the field of PCI has led to improved devices and treatment strategies.Bare-metal stents (BMS) are able to reduce the rates of restenosis and acute occlusion compared with balloon angioplasty.Subsequently,the advent of drug-eluting stents (DES) has further decreased the rates of restenosis.Of note, first-generation DES were durable polymer DES, and delayed reendothelialization due to the polymer raised concerns regarding late and very-late stent thrombosis (ST).Despite several efforts to reduce the ST rates of durable polymer DES such as alteration of stent platforms to increase tissue compatibility,modification of the outer layer of the stent surface, and using effective antiproliferative drugs and appropriate polymer carriers, the issue of inflammatory response still persists.Therefore, biodegradable polymer drug-eluting stents (BP-DES)were introduced with anticipation to reduce ST[6-8].As expected, long-term clinical evidence has demonstrated superiority of BP-DES in reducing very-late ST events compared with durable polymer DES[9-11].
NeoHexa is one of such BP-DES designed with the aim to reduce rates of late ST and was launched in July 2015.The present study investigated the 1-year clinical outcomes of patients who had received this new DES in real clinical practice.
MATERIALS AND METHODS
Study design and patient selection
Data obtained from a single-center cohort of patients who had received NeoHexa stents as part of routine treatment for CAD between July 2015 and July 2016 at the Cauvery Heart and Multispecialty Hospital, Mysore, were retrospectively investigated in January 2017.The study was conducted in accordance with the Helsinki Declaration and was approved by an independent ethics committee.Verbal informed consent was obtained before collecting data from patients who were contacted to participate in this study.This investigator initiated trial was registered with Clinical Trial Registry of India (CTRI/2018/03/012522)
Description of device
NeoHexa is a cobalt-chromium sirolimus-eluting coronary stent system.It is a premounted, balloon expandable DES with a persistent coating of BP carrier, loaded with 1.0 μg/mm2sirolimus in a slow-release formulation.It is mounted on a rapid exchange percutaneous transluminal coronary angioplasty balloon catheter.It has two radiopaque markers beside the mounted stent for accurate placement.It is available in diameters of 2.25, 2.5, 2.75, 3.0, 3.5, 4.0, and 4.5 mm and in stent lengths of 7, 10, 13, 15,17, 20, 24, 28, 33, 38, 42, and 45 mm.
Study procedure and data collection
This was an all-comer study, and the indications for the angioplasty procedure and technique of stent implantation were as per the discretion of the treating physician.All patients were advised to receive dual antiplatelet therapy with clopidogrel and aspirin.Patients who were not pretreated received a bolus dose of 300-600 mg of clopidogrel or 60 mg of prosugrel and ≥ 100 mg of soluble aspirin just before the procedure.
Data were sourced from clinical notes, including inpatient progress notes and outpatient notes and letters, angiogram reports, and procedural angiographic images.Case report forms were completed for all patients, and data were stored in a secure,off-site database.Follow-up data were collected using either clinical visits or telephonic interactions by using structured questionnaires developed for this study to determine endpoint status at 1 mo, 6 mo, and 1 year after the index procedure.Supporting clinical documents were sought when necessary.Patients with incomplete clinical notes or who were noncontactableviatelephone were excluded from the analysis.
Endpoint definitions
The primary endpoint of the study was the rate of major adverse cardiac events(MACEs) defined as the composite of death, myocardial infarction (MI), and target lesion revascularization (TLR) during the follow-up period after the index procedure.Deaths were categorized as cardiac or noncardiac.Stent thrombosis was evaluated according to the Academic Research Consortium criteria[12].Procedural success was defined as successful stent placement at the desired position with < 30% residual stenosis.
Sample size and statistical analysis
A random sample size of 129 patients was calculated based on the primary endpoint of the study.Categorical data are presented as numbers and percentages.Continuous variables are presented as the mean ± SD.All data were processed using the statistical analysis software SPSS, version 21 or higher (SPSS Inc., Chicago, IL, United States).
RESULTS
Baseline demographic and clinical characteristics
In total, 129 patients with 172 lesions were enrolled in the study.Baseline demographics and clinical characteristics are summarized in Table1.Mean age of patients was 56.57 ± 11.73 years, and the majority were men (76.74%).The most common comorbid conditions were hypertension (49.61%), followed by diabetes mellitus (39.53%).Over 70% of patients presented with angina class II and above.
Lesion and procedural characteristics
Most lesions were located in LAD (42.44%), RCA (32.56%), and LCx (21.51%), and a majority of them were positioned proximal (48.26%), mid (31.39%), or distal (12.79%)(Table2).Approximately 72% of patients had a lesion length ranging 20-40 cm.The average stenosis rate was 88.12%.Bifurcation and thrombotic lesions comprised approximately 11% of all lesions.Approximately 95% lesions were moderate- to highrisk lesions as per ACC/AHA criteria, and most (96.51%) lesions had a TIMI flow grade below 3.The average length and diameter of the stent was 27.30 ± 9.20 and 2.98± 0.69, respectively.Average stent per patient was 1.34 ± 0.53, and pre- and postdilation was performed in 97.67% and 26.16% of patients, respectively.Procedural success was achieved in all patients, and no in-hospital MACE was reported.
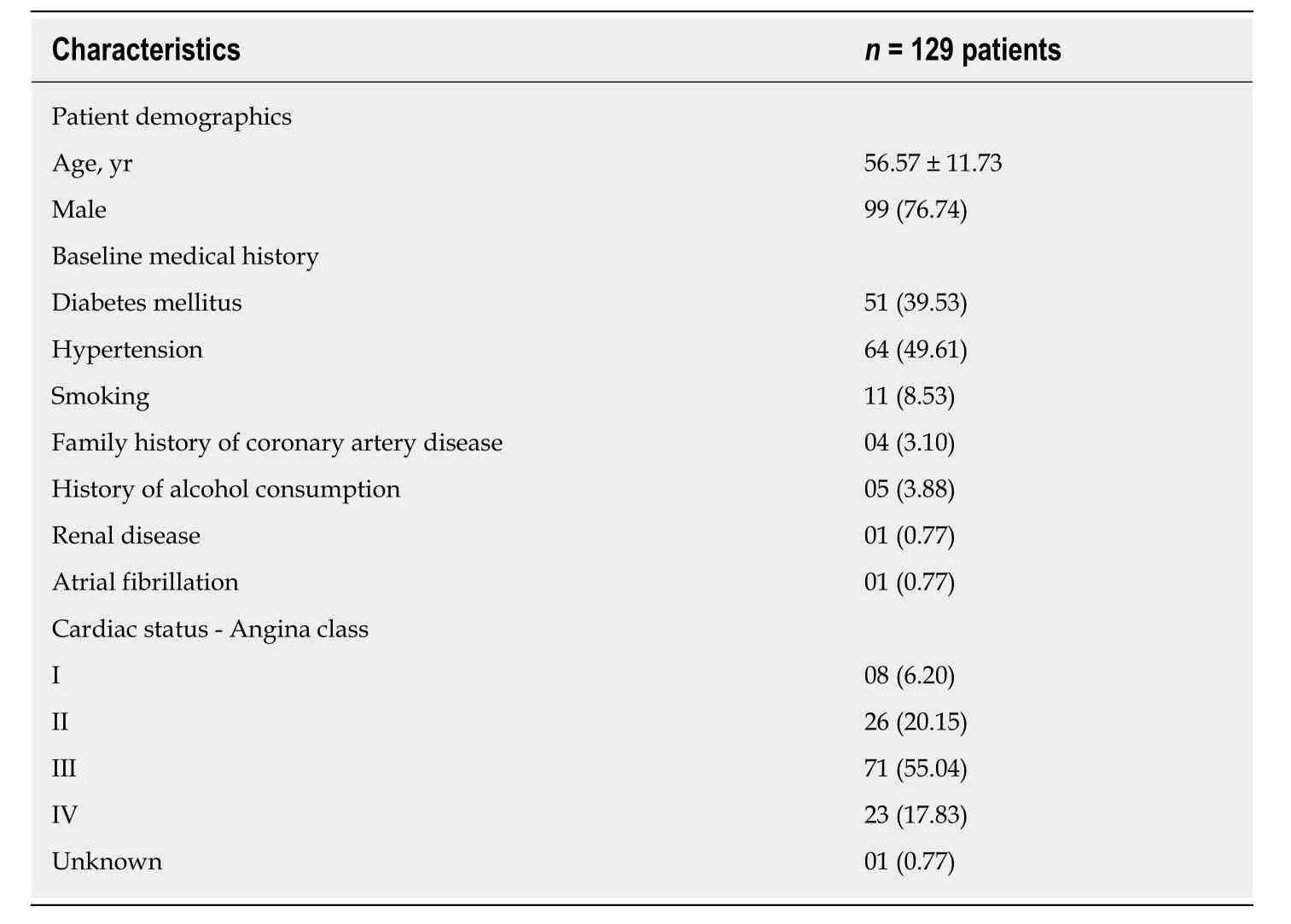
Table1 Demographic and baseline clinical characteristics
Clinical outcomes during follow-up
The incidence of composite of MACE at 30 d was 0.78% with one cardiac death.MACE rates during the follow-up duration are depicted in Table3.In brief, MACEs were reported in 6 (4.87%) patients at 1 year, consisting of 2 cardiac deaths, one noncardiac death, one (0.81%) MI, and 2 (1.63%) TLR events.Both TLR events were PCI, and the patients recovered after treatment.As shown in Table4, the cumulative rate of ST was 1.55% (2/129) at 1 year and late ST was ARC-possible ST.
DISCUSSION
The present postmarketing surveillance study was conducted to support the safety of NeoHexa stents for treatment of coronary artery lesions in real-world clinical practice.One-year follow-up results demonstrated the favorable safety and performance of the stent with low rates of MACE and ST of 4.87% and 1.55%, respectively.
We evaluated real-world data of NeoHexa in an unselected clinical practice population with diverse clinical profiles, which included diabetes (39.53%),hypertension (49.61%), bifurcation and thrombotic lesions (11.04%), and ACC/AHA type B and C lesions (94.77%).The presentation of patients was similar to that reported in studies of other similar stents[13,14].The NeoHexa stent is designed to have thin struts (60 μm) on a cobalt-chromium platform with a unique and innovative “s”link and an alternate “C” link, which provides high radial strength and no foreshortening, making it ideal for all lesion locations including ostial lesions.
The first-generation DES were built on bulky stent platforms, making deliverability quite challenging[15]; however, the thin struts and growth of 8% from nominal pressure to rated burst pressure of this new-generation NeoHexa DES offer good deliverability and conformability, thereby allowing complete deployment and good wall apposition.The design leads to a minimal balloon overhang, minimizing the risk of edge dissection/injury, which is a common procedural complication of PCIs.The finding that procedural success was achieved in 100% of patients in this study supports these claims.
Compared with BMS, first-generation DES with a durable polymer have reduced the rate of restenosis but are associated with higher late ST[11].Delayed endothelial healing secondary to a hypersensitivity reaction to the durable polymer could be responsible for the observed high rate of ST with such DES[16-18].BP-DES were developed to address this potential limitation of durable polymer DES.The drug encapsulated in polymer is completely released within 3-9 mo, and the polymer also gradually degrades into carbon dioxide and water molecules.Therefore, BP-DESinitially provide antiproliferative benefits similar to durable polymer DES and later behave like BMS once drug delivery and polymer biodegradation are complete[19].Given the importance of ST in evaluating the overall performance of DES, we estimated the ST rate in our study.The rates of both possible and probable late ST was 0.78% in the present study, which are comparable to those of other standard BP-DES such as sirolimus-eluting Orsiro stents (0.4%), biolimus-eluting Nobori stents (1.2%),and Biolimus A9 stents (0.2%) at 1-year follow-up[14,20].The low rate of ST observed in our study could be attributed to complete wall apposition of the NeoHexa stent and appropriate endothelial healing over the 1-year period.

Table2 Lesion and procedural characteristics (n = 129 patients and 172 lesions)
Although there is no scientific difference between indigenously developed DESvsthose developed and marketed by global manufacturers, cost effectiveness remains a key factor in the decision-making process for patients and health care providers in India[21].The most promising results of this retrospective study are 100% procedural success rate and low rates of MACE (4.87%).MACE rates in our study are comparable to previously reported incidence rates for other BP-DES: Endeavor stent (12.9%),NOBORI stent (11%), and Metafor SES (1.6%)[13,22,23].Moreover, our results are comparable to the rate observed in the SPIRIT II trial (7.2%)[7].
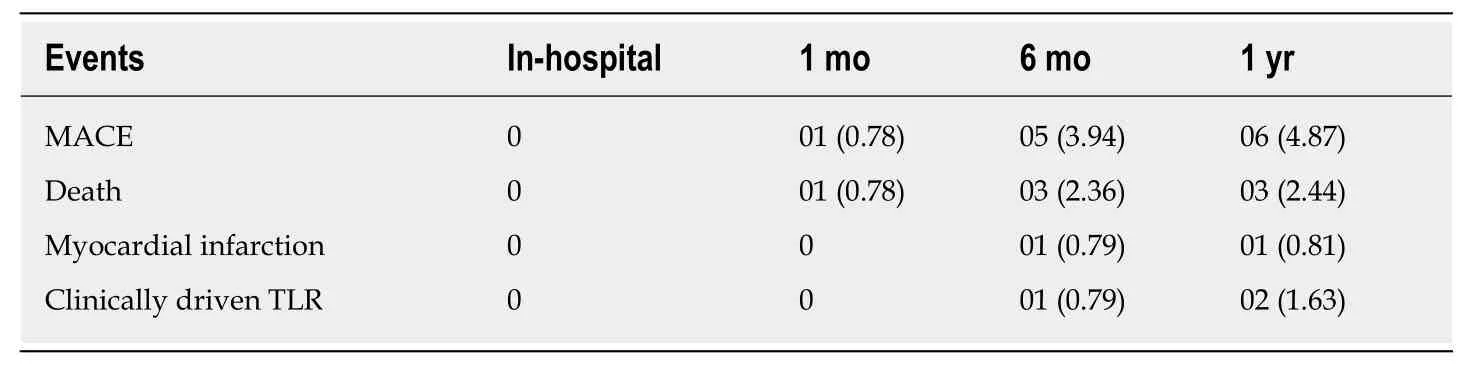
Table3 Mortality, morbidity, and major adverse cardiac event, n (%)
A major limitation of the present study is the observational design and retrospective analysis of data.However, observational data allow true representation of all-comer population unlike randomized trials with restricted enrollment criteria.In addition, a 1-year follow-up period might not be adequate to evaluate the safety and performance of NeoHexa DES.Therefore, our results must be further substantiated in well-designed studies with longer follow-up duration.
In conclusion, the relatively low rates of MACE and ST in this cohort of patients after 1 year of follow-up support the favorable safety and performance of NeoHexa stents.Product characteristics such as advanced sent design with the use of biodegradable polymer that provides high radial strength, minimal balloon overhang,low recoil, and uniform scaffolding could be responsible for these results.NeoHexa could be suggested as an effective alternative to other contemporary stents available in the market for the treatment ofde novolesions in native coronary arteries.
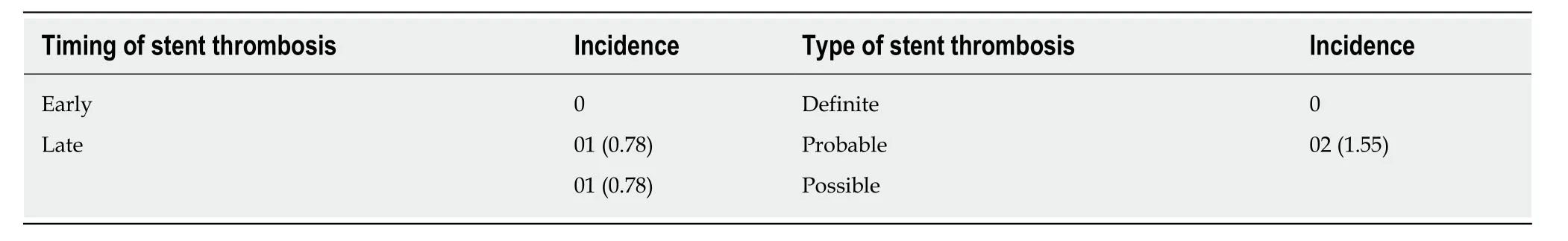
Table4 Stent thrombosis, n (%)
ARTICLE HIGHLIGHTS
Research background
Biodegradable polymer drug-eluting stents have been shown to reduce restenosis rates and have low rates of stent thrombosis.Thus, this post-marketing surveillance assessing outcomes after 1 year of treatment shows the real implications of biodegradable drug eluting stents.
Research motivation
Proving the real-life reduced restenosis rates of biodegradable stents was the motivation behind this study.Key problems were the rates of major adverse cardiac events (MACEs) myocardial infarction, and target lesion revascularization.Solving this would increase patient survival rate.
Research objectives
The main objective was to identify the rate of MACE during the follow-up period at 1 mo, 6 mo,and 1 year after the procedure completion.
Research methods
This was a retrospective analysis of a single-centre cohort of patients who had received NeoHexa stents as part of routine treatment for CAD.
Research results
Procedural success was achieved in all patients, and no in-hospital MACE was reported.The incidence of composite MACE at 30 d, 6 mo, and 1 year was 0.78%, 3.94%, and 4.87%,respectively.
Research conclusions
Relatively low rates of MACE and stent thrombosis in this study support the safety and performance of NeoHexa stents, suggesting that it is an effective alternative for treatment ofde novolesions.
Research perspectives
Our results must be further substantiated in well-designed studies with longer follow-up duration.
ACKNOWLEDGEMENTS
Authors would like to thank CBCC Global Research for providing medical writing assistance in the preparation of this manuscript which was funded by Sahajanand Laser Technology Ltd., India.
