Frequency, localization, and types of gastrointestinal stromal tumorassociated neoplasia
Johanna Waidhauser, Anne Bornemann, Martin Trepel, Bruno Märkl
Abstract BACKGROUND In recent years, increasing evidence of second neoplasms associated with gastrointestinal stromal tumors (GIST) has been found. Numerous case reports,mostly retrospective studies and a few reviews, have been published. To our knowledge, however, no systematic review or meta-analysis of the existing data has been performed so far.AIM To prepare a compilation, as complete as possible, of all reported second tumor entities that have been described in association with GIST and to systematically analyze the published studies with regard to frequency, localization, and types of GIST-associated neoplasms.METHODS The MEDLINE and EBSCO databases were searched for a combination of the keywords GIST/secondary, synchronous, coincident/tumor, neoplasm, and relevant publications were selected by two independent authors.RESULTS Initially, 3042 publications were found. After deletion of duplicates, 1631 remained, and 130 papers were selected; 22 of these were original studies with a minimum of 20 patients, and 108 were case reports. In the 22 selected studies,comprising a total number of 12050 patients, an overall rate of GIST-associated neoplasias of 20% could be calculated. Most second neoplasias were found in the gastrointestinal tract (32%) and in the male and female urogenital tract (30%). The specific risk scores of GISTs associated with other tumors were significantly lower than those without associated neoplasias.CONCLUSION In this first systematic review, we could confirm previously reported findings of a more than coincidental association between GIST and other neoplasias. The question whether there is an underlying causal association will need further investigation. Our data suggest that even GIST with a very low risk of disease progression should prompt screening for second neoplasia and subsequent frequent controls or extended staging.
Key words: Gastrointestinal stromal tumor; Associated; Secondary; Neoplasia; Tumorupon this work non-commercially,and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:http://creativecommons.org/licen ses/by-nc/4.0/
INTRODUCTION
Gastrointestinal stromal tumors (GIST) are the most common mesenchymal tumors of the digestive tract. Yearly incidence rates vary from 4.3 to 22 per million in different geographic regions, which may at least in part be caused by changing and improving diagnostic criteria and a lack of established GIST registries. In most studies, however,the yearly incidence is indicated as 10 to 15 per million[1], fulfilling the criterion of a rare disease. Median age at diagnosis is reported to be between 60 and 69 years in most studies[1,2]. GIST are considered to develop from interstitial cells of Cajal (ICC),which play an important role in autonomous gastrointestinal movement[3]. The most common localization of GIST is the stomach, followed by other gastrointestinal tract localizations[2,4].
Several driver mutations have been identified as playing an essential role in the development of GIST. The most frequent mutation can be found in the tyrosine kinase receptor c-kit (c-KIT), which accounts for 70%-80% of GIST[5-7]and is nowadays the most important target of medical tumor therapy in GIST patients. Other relevant mutations can be observed in the platelet-derived growth factor receptor alpha(PDGFR-α) in 5%-10%[5-7]and, in rare cases, in other genes such as neurofibromin 1(NF1), succinate dehydrogenase (SDH), or BRAF[6,8].
GIST can occur in the setting of genetic syndromes such as neurofibromatosis 1[9],Carney triad[10], or familial GIST[11]and, in these cases, frequently come along with other benign or malignant neoplasias. In recent years, though, there has been increasing evidence of second neoplasia in patients with sporadic GIST[12-15]. Several retrospective studies and case series have been published on this topic, complemented by many case reports and a few reviews, but so far to our knowledge, no metaanalysis or systematic reviews have been conducted. The aim of this first systematic review is to prepare a compilation, as complete as possible, of all reported second tumor entities that have been described in association with GIST and to systematically analyze the published studies with regard to frequency, localization, and types of GIST-associated neoplasms.
MATERIALS AND METHODS
Literature research
We performed a literature search in the MEDLINE and EBSCO databases, using the keywords GIST/secondary, synchronous, coincident/tumor, neoplasm. All results were transferred to the citation manager Endnote®and duplicates were deleted. The remaining results were screened by two authors with regard to suitable topic,language, and publication standard. Discrepancies were resolved after discussion with a senior third author. Case reports and case series/studies in English or German were included. Only data that was fully published was eligible. Syndromic settings as familial GIST or neurofibromatosis were excluded as well as cases involving children.Studies had to include at least 20 patients, and studies that investigated only one specific kind of second neoplasm were excluded. Second neoplasms were considered regardless of the time frame between their occurrence and the occurrence of GIST.Malignant as well as benign second neoplasms were selected. In addition, the bibliographies of all selected papers that were published between 2016 and 2019 were screened for suitable references, as were the six published reviews on this topic.
Statistical analysis
For statistical calculations, we used SigmaPlot 13.0 (Systat, Erkrath, Germany) and Microsoft Excel (Microsoft Office 16). The chi-squared test was performed for testing the relationship between two categorical variables. AaP-value of < 0.05 was considered significant.
P ROSPERO registration
Before starting the literature research, a registration of this systematic review in the international Prospective Register of Systematic Reviews (PROSPERO, registration number CRD42019122784) was performed.
RESULTS
The literature search revealed a total of 3042 publications before February 2019. After deletion of duplicates, 1631 papers remained. Screening by the two authors(Waidhauser J and Bornemann A) resulted in 126 eligible papers. In addition, one study on 188 GIST patients that was performed at our institute by Mayr et al[15]and had not been published by the time of the literature search was included. Of the 130 selected publications, 108 were case reports and 22 were case series or retrospective and prospective studies (Figure 1).
All additional neoplasms that were reported in the case reports are listed in Table 1.The most frequent types were gastric and colorectal adenocarcinomas.
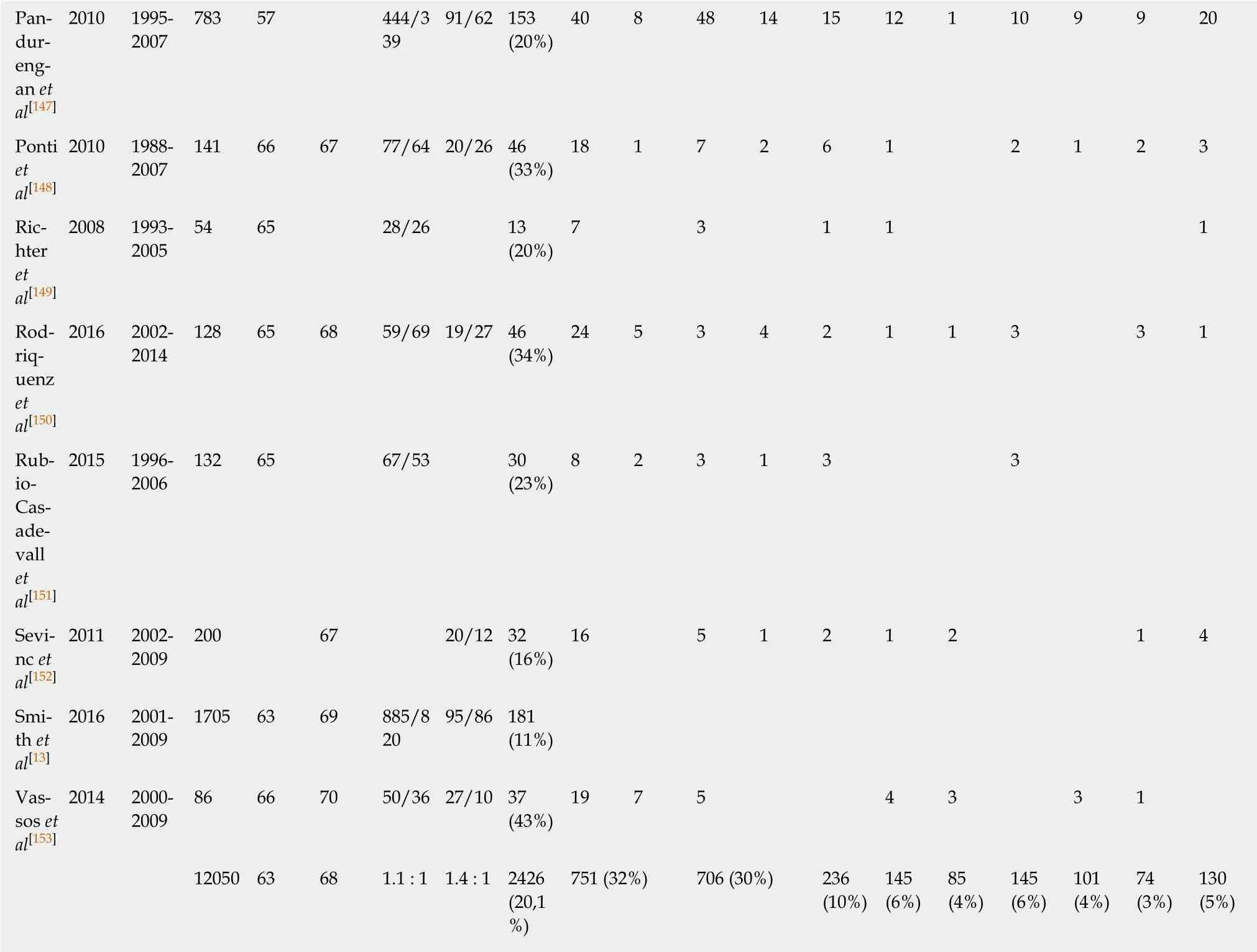
Among the 22 retrospective and prospective studies, a total of 12050 patients were included. Basic information on these studies is summarized in Table 2. The number of patients in which an additional tumor to the GIST diagnosis was found was 2426(20.1%). The median age at the diagnosis of GIST was 63 years in the total study population and 68 years in those patients with an additional tumor. The male-tofemale ratio was 1.1:1 in the total population and 1.4:1 in the GIST with secondary neoplasia group. The chi-squared test revealed a significant difference for the sex distribution of P < 0.001 with a predominance of male gender in cases with associated neoplasia (Table 3).
Of 2248 patients, for whom the respective data were available, 253 benign (11%)and 1995 (89%) malignant neoplasias were reported, with the restriction that in some studies, only patients with malignant second neoplasias were included. Chronological considerations revealed that 50% (366 of 732) of second neoplasias occurred synchronously to GIST, 26% (187 of 732) occurred before GIST, and 24% (179 of 732)were diagnosed after GIST. Focusing on synchronous second neoplasias, a rate of 6%(366 of 5131) was detected among all GIST patients. Of these synchronous second neoplasias 77% (177 of 230) occurred in the GI-Tract and 7% (16 of 230) in the male and female urogenital tract. The distribution of different histological subtypes(spindle vs epithelioid vs mixed) revealed no differences between the GIST-only patients and the patients with another neoplasm (spindle: 78% vs 80%; epithelioid: 8%vs 6%; mixed: 14% vs 14%) (Table 4).
Figure 2 gives an overview of the different localizations of the GIST-associated neoplasias. The most common manifestation was seen in the gastrointestinal tract(32%), followed by urogenital and female genital tract (30%); 10% of additional tumors were found in the breast and 6% each in the lung and in the blood and lymphatic system.
Regarding the risk scores for disease progression or recurrence of GIST, there was a significantly (P < 0.001) higher proportion of very low- and low-risk GIST in patients with an additional tumor (65%) compared to the GIST-only group (35%), whereas in this latter group, the portion of intermediate and high-risk patients was higher (69%vs 31%). For calculation, we used the risk scores as they were applied in the different studies, which were most frequently those according to Fletcher et al[4]or Miettinen et al[16](Table 5).
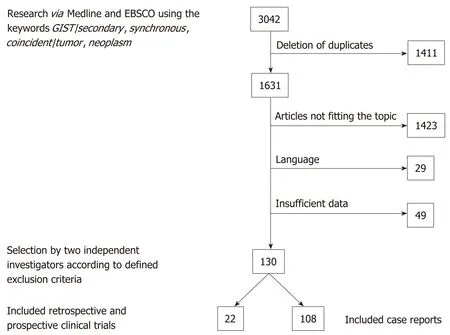
Figure 1 Flow of analysis based on the retrieved literature. GIST: Gastrointestinal stromal tumors.
The mutational status of driver genes in patients with GIST and associated neoplasias was reported in only four of the 22 studies, with a total of 167 patients.These patients with GIST and second tumors showed mutations in exon 11 of the KIT gene in 69%, non-exon-11 mutations of the KIT gene in 6%, mutations in the PDGFR-α gene in 13%, and a “wildtype” status in 13%.
Data on follow-up was very heterogeneously reported or not available in most of the included studies, which is why even a descriptive analysis was not feasible.
DISCUSSION
In our systematic review, we detected a rate of 20.1% of second neoplasias in GIST patients, with the most frequent localization of associated tumors in the gastrointestinal tract and in the urogenital and female genital tract. Previously described rates of GIST-associated neoplasias varied between 11% and 50%[13,17]. The general probability of being diagnosed with cancer twice in a lifetime is estimated between 2% and 17% (syndromic settings or familial predisposition included)[18]or in other words with a chance of 1:9[12,19]. Compared to this number the rate of second neoplasias we found in GIST patients is obviously higher than expected. Several reasons can be considered accountable for the development of multiple tumors in one patient, for example, similar risk factors, environmental factors, or genetic predisposition, but also the higher likelihood of detection of another tumor within the examinations for staging or follow-up. In cases of sporadic GIST there are no definitely confirmed intrinsic risk factors or environmental factors. Genetic factors play a role in syndromes such as neurofibromatosis type I or Carney triad, but these patients were excluded in our study, and only patients with sporadic GIST were included. The occurrence of GIST-specific mutations such as in the c-KIT or PDGFRα gene that we found in the group of patients with GIST and second neoplasms were similar to those reported for GIST in general before[5-7].
The localization of GIST-associated neoplasias with the highest frequency in the gastrointestinal tract, the urogenital, and female genital tract is consistent with previously reported findings[20,21]. In addition to a possible common underlying predisposition, the probability of detecting even small GIST during staging examinations for gastrointestinal tract tumors might be higher than in cases of, for example, lung or head and neck tumors. This is might also be the explanation for the high rate of 77% of GI-tract localization in synchronous second neoplasias.
The median age of the total study population compared to the group of patientswho developed a second tumor showed a difference of five years at the time-point of GIST diagnosis, with the higher median age in the GIST with associated neoplasm group. A possible explanation for this finding could be the age-dependent risk increase for cancer in general[14]. Previously performed reviews on the topic of GIST and associated neoplasms mostly concentrated on the occurrence rate and localization of the associated tumors and, in some cases, on the outcome and follow-up[20-24].Analysis of age or sex distribution have not been performed on larger numbers of patients. We found a significantly higher number of male patients who were diagnosed with GIST and a second neoplasia than in the total population of GIST patients. An equal sex distribution for GIST patients in general has been reported in the literature before[1]. Regarding the overall incidence of cancer worldwide, the sex distribution of patients diagnosed with cancer in 2018 is estimated at a male-to-female ratio of 1.1:1 (9.5 million new cases in men and 8.6 million new cases in women).Among gastrointestinal neoplasias, which were the most frequent GIST-associated neoplasias in our review, there is a difference in the worldwide incidence between men and women with a higher rate of gastrointestinal (GI) tumors diagnosed in men in 2018 (2.7 million cases in men vs 1.4 million cases in women)[25]. Whether there is a difference of distribution of the second neoplasias between sexes in our review population remains unclear. In most analyzed studies, the reported data was not sufficient to answer this question.

Table 1 Case reports: Overview of tumor entities

IEN: Intraepithelial neoplasia; SCC: Squamous-cell carcinoma; NET: Neuroendocrine tumor; DLBCL: Diffuse large B cell lymphoma; MALT: Mucosa associated lymphatic tissue; NSCLC: Non-small cell lung cancer.
Several risk classification systems are used to assess the risk of disease progression or recurrence of GIST[4,16]. Most of them use the size, localization, and mitotic rate of GIST and are therefore, at least in part, comparable. By summarizing the risk categories in two groups (very low and low vs intermediate and high), we could find a significant difference between the GIST-only patients and those having GIST and another neoplasia by using the chi-squared test. The patients with GIST-associated neoplasias had lower risk scores, which might be due to a higher detection rate of even small GISTs with low risk scores in the setting of another neoplastic disease.
Since the establishment of targeted therapies for GIST with imatinib or second-line tyrosine kinase inhibitors, the prognosis even of advanced GIST has significantly improved[26]. On the other side, there is growing interest in the question of elevated risk of developing secondary neoplasia under the treatment with imatinib. Phan et al.found a higher rate of secondary tumors among GIST patients in the imatinib era than in the pre-imatinib era[27], although the most likely reason for this is the prolonged survival even of patients with advanced GIST, as it has been described by different authors[28]. Another point that relativizes the impact of imatinib on the development of secondary tumors can be seen in our review: About 75% of associated neoplasias were diagnosed either before GIST or synchronously with GIST; furthermore, not all patients with a GIST diagnosis in advance were treated with imatinib.
In summary, in this, to our knowledge first, systematic review on the topic of secondary neoplasia in patients with GIST, we confirm the previously described elevated number of associated neoplasms and the most common localizations of this neoplasms. We found a higher median age in the GIST with second neoplasia group and significantly more male patients who developed associated tumors, whereas the risk scores of GIST in this group were significantly lower. We conclude that even very low- and low-grade GISTs should be a reason to consider frequent controls or extended staging for early detection of second neoplasias, especially in the gastrointestinal and urogenital tract. To understand whether there is an underlyingr
genetic cause for the elevated rates of GIST-associated neoplasias, further studies will be needed.
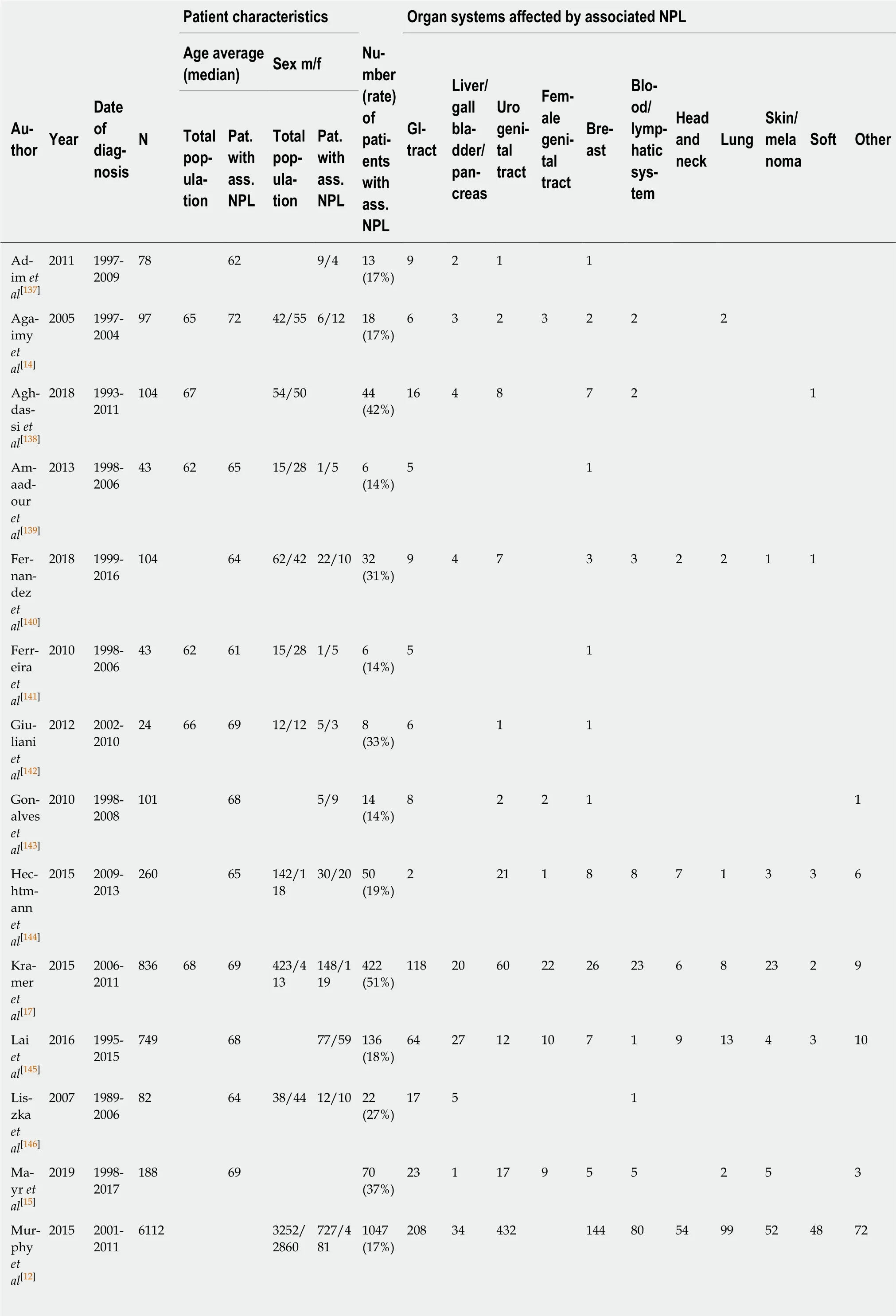
Table 2 Retrospective and prospective studies used for this investigation
?

Table 3 Age and sex distribution
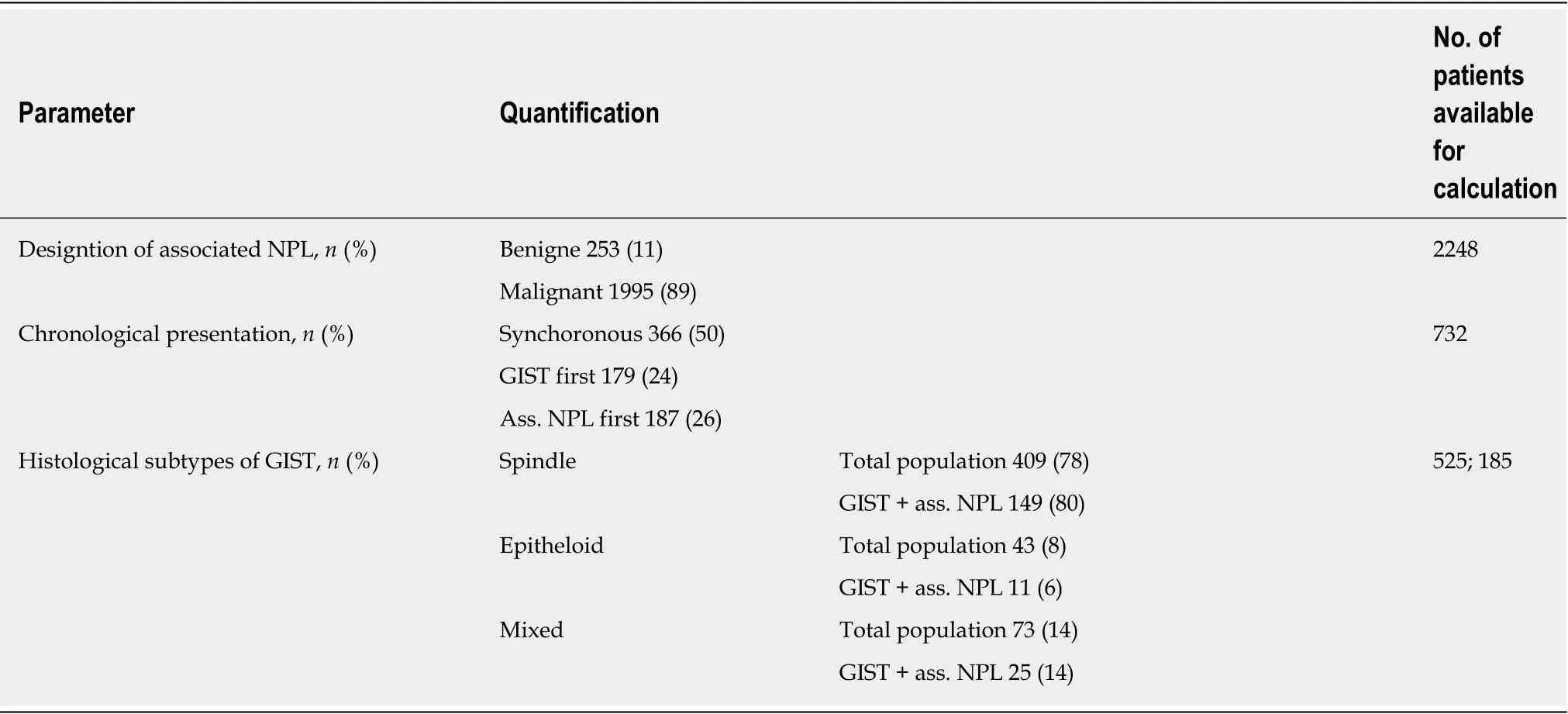
Table 4 Characteristics of gastrointestinal stromal tumors and associated neoplasias

Table 5 Distribution of risk scores, n (%)
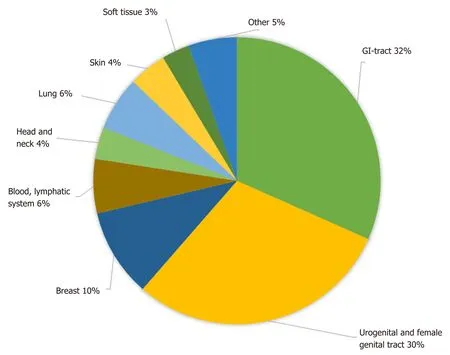
Figure 2 Localization of gastrointestinal stromal tumor-associated neoplasias. GI: Gastrointestinal.
ARTICLE HIGHLIGHTS
Research background
In recent years, numerous case reports, mostly retrospective studies and a few reviews on the topic of second neoplasias associated with gastrointestinal stromal tumors (GIST) have been published. To our knowledge, however, this is the first systematic review of the existing data.
Research motivation
The aim of this review was to prepare a compilation, as complete as possible, of all reported second tumor entities that have been described in association with GIST, and to systematically analyze the published studies with regard to frequency, localization, and types of GISTassociated neoplasms.
Research objectives
The main focus of this review was on frequency, localization, dependence of gender, age and risk classification of GIST associated neoplasias. Summarizing the data of a large number of patients could especially help in the daily clinical work with GIST patients.
Research methods
The MEDLINE and EBSCO databases were searched for a combination of the keywords GIST/secondary, synchronous, coincident/tumor, neoplasm, and relevant publications were selected by two independent authors. All case reports were summarized according to the reported tumor entity and included clinical studies were analyzed with regard to the previously mentioned topics.
Research results
Of the initially found 3042 publications, 130 papers were selected; 22 of these were original studies, and 108 were case reports. In the 22 selected studies, comprising a total number of 12050 patients, an overall rate of GIST-associated neoplasias of 20% could be calculated. Most second neoplasias were found in the gastrointestinal tract (32%) and in the male and female urogenital tract (30%). The male-to-female ratio revealed a predominance of male gender in cases with associated neoplasia. The specific risk scores of GISTs associated with other tumors were significantly lower than those of GIST without associated neoplasias. The question if there are specific genetic mutations that occur with a higher frequency in GIST patients with second tumors could not be answered and would be an interesting topic for future research.
Research conclusions
GISTs are associated with other neoplasias with a rate of 20% and occur most frequently in the gastrointestinal and urogenital tract. This confirms previous findings on a larger number of patients. GIST associated neoplasias occur with a higher likelihood in older, male patients with low grade GIST. 50% of GIST associated neoplasias are detected synchronously. Our findings should be a reason to consider frequent controls or extended staging for early detection of second neoplasias, especially in the gastrointestinal and urogenital tract.
Research perspectives
If there is a causal relation between GIST and second tumors remains unclear. As data on genetic mutations of the GIST were reported very heterogeneously focusing on this topic could be an interesting point for future research.
 World Journal of Gastroenterology2019年30期
World Journal of Gastroenterology2019年30期
- World Journal of Gastroenterology的其它文章
- Letter to the editor: Diagnosis of erythropoietic protoporphyria with severe liver injury - a case report
- Quantitative risk of positive family history in developing colorectal cancer: A meta-analysis
- Inflammatory bowel disease patient profiles are related to specific information needs: A nationwide survey
- Natural history of children with mild Crohn’s disease
- Bone morphogenetic protein-7 represses hepatic stellate cell activation and liver fibrosis via regulation of TGF-β/Smad signaling pathway
- Novel magnetic compression technique for establishment of a canine model of tracheoesophageal fistula
