Study on immunological characteristics of T lymphocyte in peripheral blood from patients with Sjogren's syndrome
Rong Bi, Sheng-Ping Zeng, Bo Wen, Chun-Bo Wang, Ji-Hong Tan
Study on immunological characteristics of T lymphocyte in peripheral blood from patients with Sjogren's syndrome
Rong Bi1, Sheng-Ping Zeng2, Bo Wen3, Chun-Bo Wang4, Ji-Hong Tan5
1Affiliated Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China.2Affiliated Hospital of Chengdu University of Traditional Chinese Medicine/Chengdu Gaoxin Huisheng Rheumatism Hospital, Chengdu, China.3Beijing Traditional Chinese Medicine Hospital Affiliated to Capital Medical University, Chengdu, China.4Affiliated Hospital of Liaoning University of Traditional Chinese Medicine, Chengdu, China.5Chengdu Yingdu District Hospital of Traditional Chinese Medicine, Chengdu, China.
: To study on in immunological characteristics of T lymphocyte in peripheral blood from patients with Sjögren's syndrome (SS), provide the theory basis for the further research of Traditional Chinese medicine (TCM) targeted therapy.: T lymphocyte subsets in peripheral blood from SS, RA and normal control groups were tested with various monoclonal antibodies and multicolor flow cytometry. T lymphocyte subsets were tested in peripheral blood from SS patients of different TCM Syndromes. The relationship between T lymphocyte subsets and disease activity was measured by erythrocyte sedimentation rate (SR)and serum IgG.: The percentage of CD4+T cells and the ration of CD4+/ CD8+T cells in peripheral blood from SS patients decreased significantly, and the percentage of CD8+T cells increased than that in control groups (< 0.05). Compared with patients in inactive period, the percentage of CD4+T cells in peripheral blood from SS patients in the active stage increased (> 0.05), the percentage of CD8+T cells decreased (> 0.05), and the ration of CD4+/ CD8+T cells increased (< 0.05). Compared with control groups, the percentage of CD69 expressed on T cells in peripheral blood from SS patients decreased, and the percentage of CD95's expression increased (< 0.05). The percentage of CD69CD95's expression also increased (>0.05). There were no differences among CD4+T cells, CD8+T cells and CD4+/ CD8+in peripheral blood from SS patients of different TCM Syndromes (> 0.05). CD69 and CD95's expression on SS patients of different TCM Syndromes was no significantly different (> 0.05). T cell receptor Vβ (TCR Vβ) subfamilies gene in peripheral blood from SS patients and control groups were expressed in all. Compared with control groups, Vβ 5.3, Vβ 13.1, Vβ 12, Vβ 7.2 had significant differences (< 0.05). Comparison of different TCM Syndromes in SS group: Vβ 13.1 and Vβ 14 were significantly different (< 0.05).: There was dysimmunity on T lymphocyte subsets in peripheral blood of SS patients, which was related to disease activity; apoptosis after activation of autoreactive T cell may be induced by Chinese medicine; TCR Vβ subfamilies of different TCM syndromes were different, which noted that different autoreactive T cells of abnormal activation tended to result in different expression of TCM Different syndromes.
Sjogren's syndrome, T lymphocyte subsets, Flow cytometry, Monoclonal antibody, T cell receptor Vβ gene
There was dysimmunity on T lymphocyte subsets in peripheral blood of Sjogren's syndrome patients, which was related to disease activity; apoptosis after activation of autoreactive T cell may be induced by Chinese medicine; TCR Vβ subfamilies of different TCM syndromes were different, which noted that different autoreactive T cells of abnormal activation tended to result in different expression of TCM different syndromes.
Introduction
T and B lymphocytes in Sjgren syndrome (SS) patients are abnormal in function, and the activation of autoreactive T lymphocytes and the autoimmune reactions mediated thereby are the central link in the occurrence of SS [1]. Professor Zeng Shengping, a famous doctor of traditional Chinese medicine (TCM) in Sichuan Province, makes good use of warm yang and dehumidifying drugs with large dose of Aconitum carmichaeli as the main ingredient to treat difficult connective tissue diseases such as SS and rheumatoid arthritis, which has enabled some patients with definite diagnosis and prominent symptoms to obtain long-term relief after treatment. Some patients did not insist on medication for a long time, and the disease did not recur. Its curative effect is obviously superior to that of western medicine alone. Professor Zeng Shengping [2] inferred from this that the mechanism of action of warm yang and dehumidifying drugs mainly containing large dose of Aconitum carmichaeli is different from immunosuppressive agents, which may be related to inducing apoptosis after activation of self-reactive T lymphocytes. TCM induces autoimmune tolerance so as to achieve the purpose of treating autoimmune diseases.
This experiment combines flow cytometry and monoclonal antibody technology to analyze the immunological characteristics of peripheral blood T lymphocytes of SS patients treated by team Chinese medicine led by Professor Zeng Shengping, which is innovative. Taking rheumatoid arthritis patients and normal people as control group, the TCR Vβ gene in peripheral blood of SS patients was analyzed, and whether there were differences in restrictive access of TCR Vβ gene of different syndromes was compared. At the same time, the relevant immune phenotypes (CD3, CD4, CD8) of T cells and the surface markers (CD69, CD95) related to activation and apoptosis are detected to lay a foundation for further research on gene targets where effective components of TCM play a role and provide a theoretical basis for subsequent research and development of new drugs.
Methods
Research object
The SS group selected 8 patients diagnosed in the outpatient and inpatient departments of the Affiliated Hospital of Chengdu University of TCM and Chengdu Gaoxin Huisheng Rheumatism Hospital, 8 patients in the RA case group and 8 patients in the normal control group. Laboratory Cooperation Unit: west china hospital Stem Cell Biology Laboratory.
Inclusion criteria
(1) SS group met the 2002 International Classification (Diagnostic) Standard for Sjogren's Syndrome [3]; (2) The patients were between 20 and 80 years old; (3) Western medicine diagnostic criteria for RA: 2009 RA classification criteria and scoring system-developed by -ACR and European Anti-Rheumatism Alliance (EULAR); (4) The SS group conforms to the TCM syndrome differentiation type as follows: Blood stasis arthralgia syndrome [4], Phlegm-dampness block syndrome, Non-phlegm and Non-stasis Syndrome; (5) Stop using hormone or immunosuppressant for more than half a year; TCM treatment has lasted for more than half a year; (6) Assessment of disease activity of SS and RA: Detection of erythrocyte sedimentation rate and serum IgG or RF level. If the above indexes are higher than the laboratory reference value, it is determined that SS patients are in the active stage of illness [6-7].
Exclusion criteria
Combined with gastric and duodenal ulcer or other serious diseases such as heart, liver and kidney; Pregnant or lactating women and mental patients.
General information
This study is divided into three groups, namely SS group, RA group and control group, with 8 people in each group. The age of each group was between 32 and 67, with no statistical difference (> 0.05).
Experimental method
Using optimized multi-color immunofluorescence scheme, monoclonal antibody and flow cytometer.
Specimen requirements
(1) Heparin anticoagulated peripheral blood 2ml, fasting, no blood clots. (2) Fresh: Transport at constant temperature to avoid oscillation. It is best within 2 hours, not more than 4 hours at most. (3) Specimen data: recent examination of related diseases, blood routine, liver and kidney function, erythrocyte sedimentation rate, Ig quantification, RF, anti-SSA, anti-SSB and other immunological indexes on the same day.
Titration experiment steps (1) 300μl of human peripheral blood is taken to a 15ml centrifuge tube, 6ml of split red liquid is added, and red blood cells are split for 10 to 20 minutes at room temperature. (2) 1600rpm, room temperature, 6min. (3) Remove supernatant and resuspend with 1000 ul PBS. (4) 1600rpm, room temperature, 6min. (5) Remove the supernatant, resuspend with a proper amount of Binding buffer, and adjust the cell concentration: 2×107/ml, 50ul for each tube to 6 tubes. (6) Add corresponding antibodies according to the set antibody concentration, and incubate at room temperature and away from light for 20min. (7) Once 1ml Binding buffer is added to each tube, after mixing, 1600rpm, room temperature, 6min. (8) remove supernatant and add 100ul Binding buffer for resuspension. (9) Computer testing.
Specific dyeing steps of the experiment (1) The collected cells were resuspended with appropriate Binding buffer, and the cell concentration was adjusted to 2×107 / ml. (2) Add CD3, CD4, CD8, CD69, CD95 and Annexin V mixed antibodies to a total of 6X ul (determined during titration), and add 300ul Binding Buffer flow tube. (3) Immediately according to the (300+6X) / 8 equal parts into 7 tributary type pipe, a total of 8 pipes, named A-H respectively. (4) TCR antibody was added to 8 flow tubes respectively, and stained at room temperature and away from light. (5) When dyeing for 15min, DAPI shall be added to each tube, and dyeing shall be done at room temperature and away from light for 5min. (6) Add 1ml Binding buffer per tube, 1600rpm, 6min. (7) Once the supernatant was removed, about 100ul was retained for testing.
Statistical analysis
The experimental data were expressed as "", SPSS19.0 was used for statistical analysis, one-way anova was used for comparison among groups, and non-parametric test (Kruskal-Wallis Test or Jonckheere-Terpstra Test) was used for non-uniformity of variance, with< 0.05 showing that the difference was statistically significant.
Results
CD4+T lymphocyte, CD8+T lymphocyte and CD4+/CD8+ratio in peripheral blood of SS patients are abnormal, which is related to disease activity, suggesting immune dysfunction. (Table 1,< 0.05).
Intra-group comparison of SS patients: compared with inactive patients, CD4+T cells in active ss patients increased and CD8+T cells decreased, but there was no statistical significance (> 0.05). The ratio of CD4+/CD8+increased (< 0.05). See table 2. Compared with the control group, the expression ratio of CD69 decreased and the expression ratio of CD95 increased, with statistical significance (< 0.05). The number of T cells expressing CD69, CD95 at the same time increased (> 0.05). The reason may be that there are more cases in inactive period in the group, which may indicate that after treatment with TCM, apoptosis increases after activation of self-reactive T cells (Table 3).
Table 1 Comparison of CD4+T lymphocytes, CD8+T lymphocytes, CD4+ / CD8+ in peripheral blood between SS patients and control group ()
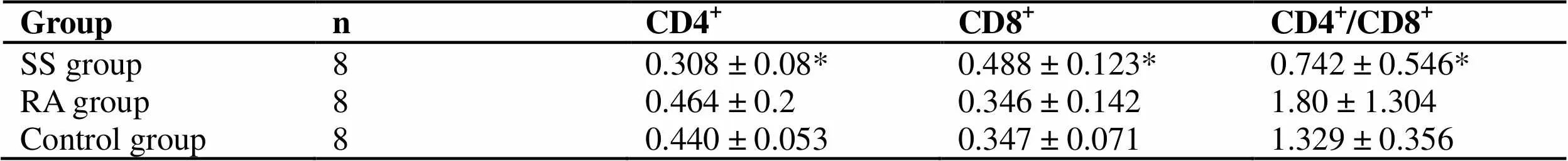
Table 1 Comparison of CD4+T lymphocytes, CD8+T lymphocytes, CD4+ / CD8+ in peripheral blood between SS patients and control group ()
Groupn CD4+CD8+CD4+/CD8+ SS group80.308 ± 0.08* 0.488 ± 0.123*0.742 ± 0.546* RA group80.464 ± 0.2 0.346 ± 0.1421.80 ± 1.304 Control group80.440 ± 0.053 0.347 ± 0.0711.329 ± 0.356
Note: Compare with control group, *< 0.05, **< 0.01.
Table 2 Comparison of CD4+T lymphocytes, CD8+T lymphocytes, CD4+ / CD8+ in peripheral blood of active and inactive SS patients ()
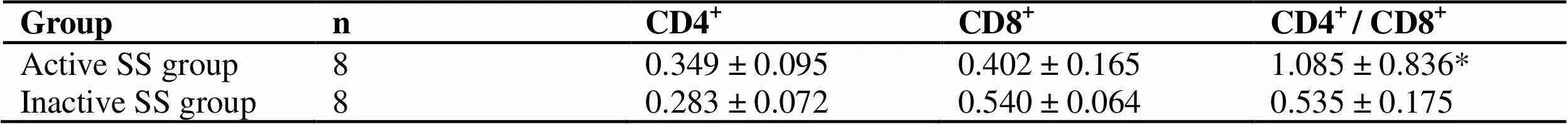
Table 2 Comparison of CD4+T lymphocytes, CD8+T lymphocytes, CD4+ / CD8+ in peripheral blood of active and inactive SS patients ()
Groupn CD4+CD8+CD4+ / CD8+ Active SS group80.349 ± 0.095 0.402 ± 0.1651.085 ± 0.836* Inactive SS group80.283 ± 0.072 0.540 ± 0.0640.535 ± 0.175
Note: Compare with inactive SS group, *< 0.05.
Table 3 T cell subsets of expression activation and apoptosis markers (CD69 and CD95) in peripheral blood of SS patients and controls were compared ()
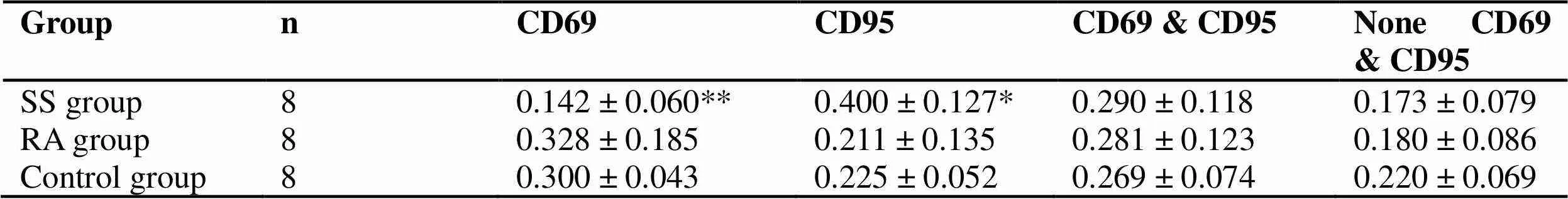
Table 3 T cell subsets of expression activation and apoptosis markers (CD69 and CD95) in peripheral blood of SS patients and controls were compared ()
Groupn CD69 CD95CD69 & CD95None CD69 & CD95 SS group80.142 ± 0.060** 0.400 ± 0.127*0.290 ± 0.1180.173 ± 0.079 RA group80.328 ± 0.185 0.211 ± 0.1350.281 ± 0.1230.180 ± 0.086 Control group80.300 ± 0.043 0.225 ± 0.0520.269 ± 0.0740.220 ± 0.069
Note: Compare with control group, *< 0.05, **< 0.01.
The expressions of CD4+, CD8+, CD4+/ CD8+, CD69 and CD95 in different TCM syndromes were not different (> 0.05). This is determined by the nature of the disease.
TCR Vβ subfamily: (1) T cells in peripheral blood of SS patients and control group all express all TCRVβ subfamily genes. Compared with the control group, there were significant differences in Vβ5.3, Vβ13.1, Vβ12 and Vβ7.2 (< 0.05). Among them, Vβ5.3 and Vβ7.2 have the highest expression ratio in SS, and Vβ13.1 and Vβ12 have the highest expression ratio in RA (Table 4). (2) The expression levels of different TCR Vβ are not balanced, and there are dominant subpopulations. Dominant subgroup is defined as TCR Vβ expression level in peripheral blood exceeding 10% [14]. (3) In SS peripheral blood, the expression balance between the Vβ subfamilies is the worst, with Vβ3 (4 cases), Vβ20 (2 cases), Vβ5.1 (1 case), Vβ12 (1 case), Vβ1 (1 case), Vβ14 (2 cases), and Vβ11 (1 case) as its dominant subpopulation, with Vβ14 reaching up to 23.5%; The highest expression level of RA was Vβ5.1 in peripheral blood, and 35.6 % in one case. The highest expression level in normal control group was only 13.5 %. (4) Compared with different TCM syndrome types in SS group, there were significant differences in Vβ13.1 and Vβ14 (< 0.05). Patients with non-phlegm and non-stasis type expressed Vβ13.1 and Vβ14 highly (Table 5). This indicates that the autoimmune reactions mediated by different abnormally activated autoimmune T cells may tend to show different TCM syndromes.
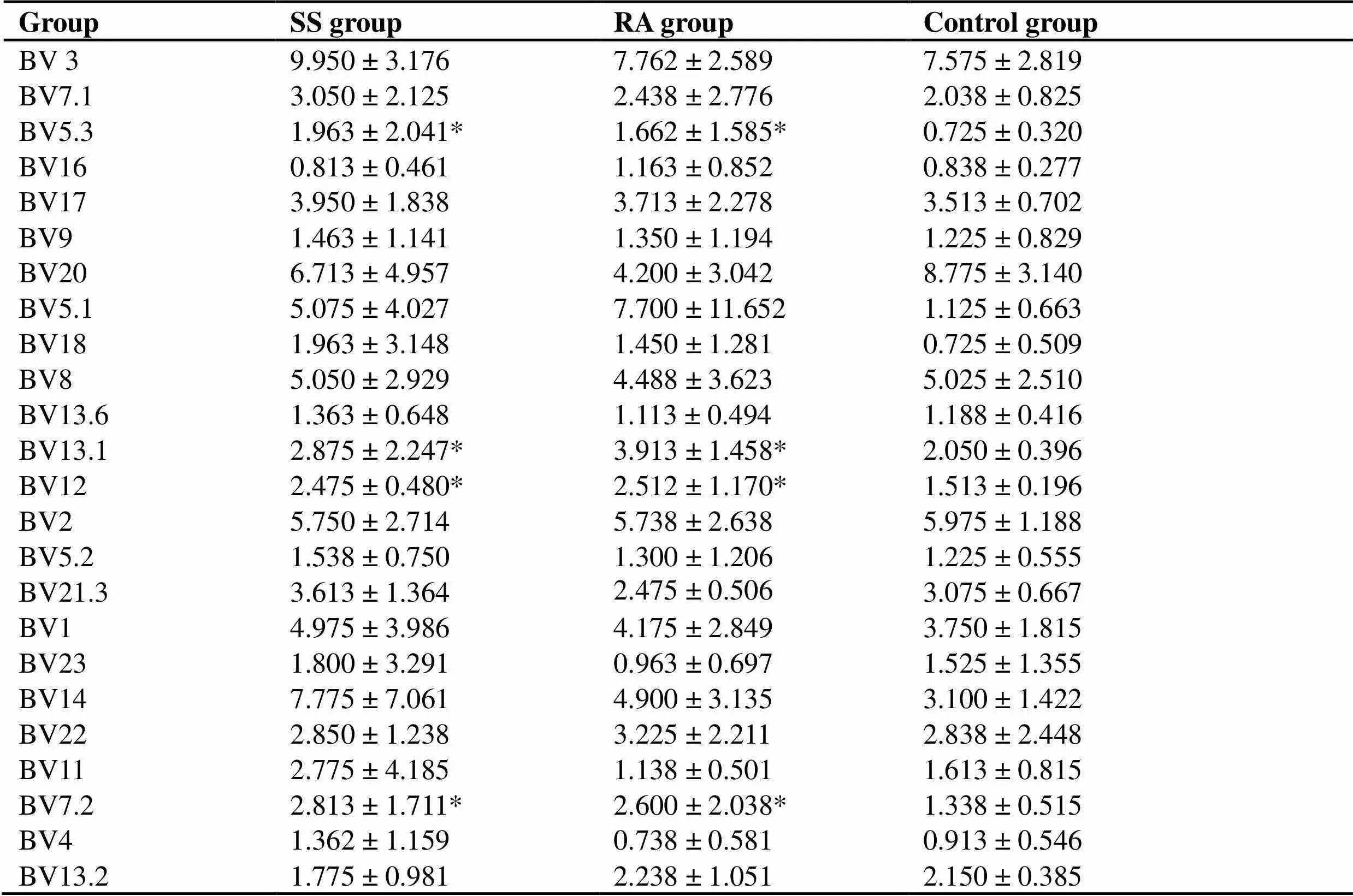
Table 4 Expression of TCR V beta (BV) subfamily in peripheral blood (%)
Note: Compare with control group, *< 0.05
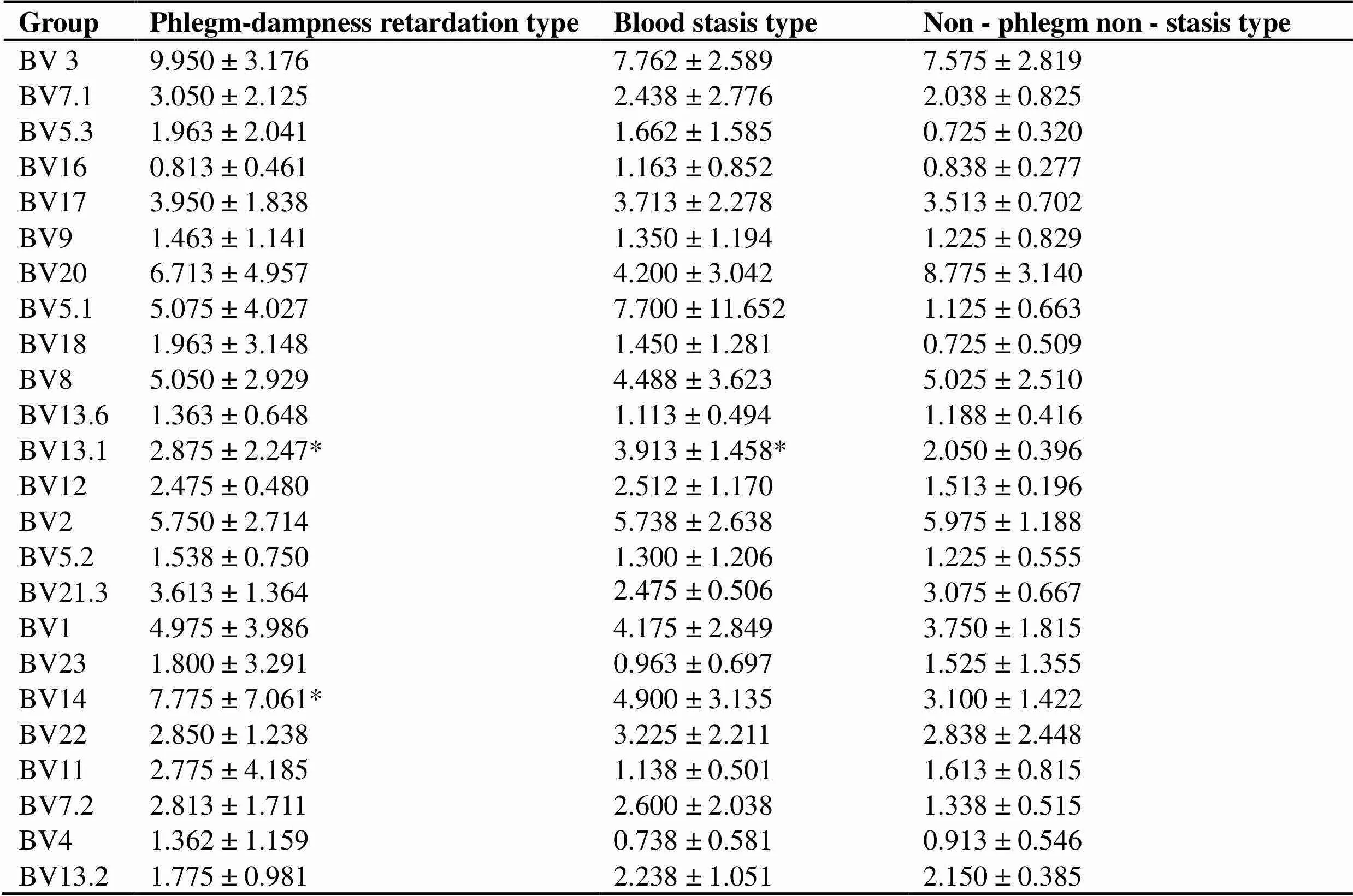
Table 5 Expression of TCR V beta (BV) subfamily in SS patients with different TCM syndromes (%)
Note: Compare with non - phlegm non - stasis type, *< 0.05
Discussion
SS patients are affected by toxic and pathogenic factors due to positive deficiency, resulting in phlegm and blood stasis, stasis and phlegm flowing and stopping accumulation of viscera, meridians and skin, forming various symptoms, which are manifested as a single syndrome or combined syndromes, such as blood stasis syndrome, phlegm-dampness syndrome, stasis and phlegm accumulation syndrome or non-phlegm and non-stasis syndrome. Syndrome is a pathological summary and an abstract summary of the essence of symptoms of the body at a certain stage of the disease. We take SS, an autoimmune disease, as the research object, and study the clonal characteristics of T lymphocytes at the molecular level to reflect some microscopic manifestations of syndromes, providing certain reference for microscopic standards of evidence. This experiment proves that the expression of TCR Vβ subfamily in ss patients with different TCM syndromes is different (< 0.05). it is possible that different activated autoreactive t cells mediated autoimmune reactions make patients tend to express different TCM syndromes.
Professor Zeng Shengping [8] thinks that according to the clinical manifestations of patients at different stages of the disease course, the disease can be divided into common syndrome types: phlegm-dampness blocking syndrome, blood stasis blocking syndrome, phlegm-blood stasis mutual accumulation syndrome,Tutors prefer to use large doses of yang-warming and qi-invigorating drugs represented by aconite root for supportive treatment, because phlegm-dampness and blood stasis are yin pathogens that need to be warmed and dispersed.
Professor Zeng Shengping discovered through long-term clinical observation and experimental research that Chinese and western medicine have the same understanding of SS in essence, but the theoretical language is slightly different. The toxic and side effects of western medicine are relatively large, so TCM should be the main treatment for this disease. In terms of TCM, some symptoms of SS can be classified into the corresponding categories of TCM diseases. However, all diseases with these symptoms can be classified into similar diseases, so the characteristic understanding between various diseases is relatively vague. The study of SS in modern medicine is more in-depth and has more advantages in distinguishing the characteristics of diseases. In short, the teacher's idea of integrating traditional Chinese and western medicine is to combine the theory of modern medicine and clinical research, and to "translate" it into the language of TCM by comparing images, and finally to treat diseases with TCM under the guidance of TCM theories such as holism, syndrome differentiation and treatment.
As mentioned above, recent studies have found that the onset of SS is related to the activation and dysfunction of T lymphocytes and their different subpopulations [10]. Most of the studies are aimed at CD4+T cells and CD8+T cells. There are still few reports on the analysis of TCR Vβ gene in peripheral blood T cells of SS patients by monoclonal antibodies and flow cytometry, and few studies show the surface markers of abnormal activation and apoptosis of T lymphocytes.
The results of this experiment show that the TCR Vβ (BV) gene in peripheral blood of SS case group does have unbalanced expression and has dominant expression subfamily, which is statistically different from that of the control group. This shows that the specific rearrangement of TCR Vβ gene is driven by different specific autoantigens in patients with autoimmune diseases. The experimental results show that compared with the control group, the number of CD4+T lymphocytes, CD8+T lymphocytes and CD4+/CD8+ratio in SS patients are significantly decreased. This is consistent with relevant literature [11-12]. Some studies have shown that CD4+T cells decrease and anti-CD4+antibodies may be produced in SS patients [13]; It may be that the expression variation of La/SS-B gene leads to the expression variation of MHC on the cell surface and produces anti-CD4+antibody [14]. Decreased CD4+T cells can lead to abnormal distribution of Thl and Th2 subsets, and abnormal production of inflammatory cytokines can lead to disease. CD8+T cells are divided into inhibitory CD8+T cells (CD8+TVV-) and anti-inhibitory CD8+lymphocytes (CD8+V+) [15]. We speculate that the increase of CD8+T cells, most of which are CD8+V+cells, can resist the negative immunoregulation of Ts cells, break immune tolerance and trigger autoimmune response. At the same time, CD8+cells can induce apoptosis of target tissue cells by secreting cytotoxins (perforin, granzyme) after proliferation, resulting in immune injury.
The results of this experiment show that compared with inactive patients, the ratio of CD4+/CD8+in active SS patients is increased (< 0.05), suggesting that the ratio of T lymphocytes in active SS patients is increased, which leads to an increase in autoantibody production, consistent with clinical observation.
CD69 [16-19] can be expressed rapidly after the early activation stage of different cells. At the same time, CD69, as a cell co-stimulation signal, further enhances the activation or proliferation and differentiation of cells, which makes it a marker molecule for cell activation in many studies. We detected CD69 on the surface of T cells to judge the activated self-reactive T lymphocyte population in patients with Sjogren's syndrome. Recent studies [20-21] show that T cells with high CD69 expression can detect an increase in CD95 expression and are prone to rapid spontaneous apoptosis.
CD95 (Fas) is an extremely important death receptor, which can mediate apoptosis of Fas-expressing cells by binding with FasL or anti-Fas antibodies. CD69 induced apoptosis can selectively act on activated cells, but not on non-activated cells [17]. CD95 can be expressed in both quiescent cells and activated cells, so apoptosis induced by CD95 has nothing to do with cell activation. Therefore, compared with CD95, using CD69 as a target target for inducing apoptosis has less side effects. Therefore, the combined detection of CD69 and CD95 can more accurately reflect the apoptosis of activated cells.
The experimental results showed that compared with the control group, the expression ratio of CD69 decreased and the expression ratio of CD95 increased in SS patients with statistical significance (< 0.05). The number of t cells expressing CD69 & CD95 at the same time increased (> 0.05). The reason may be that there are many inactive cases in the group, all of which have been treated with TCM for a long time, which just shows that the condition has been alleviated after the activation of self-reactive T cells induced by TCM. Zeng Shengping's mentorbelieves that apoptosis after activation is the main mechanism of negative feedback regulation of immune response, leading to clone deletion, namely, T cells are automatically transferred to the apoptosis procedure after full activation [2]. The higher the degree of activation, the closer the self-reactive T cell clone is to full activation, and the easier it is to be induced to apoptosis and deleted. Therefore, theoretically, the immune tolerance obtained by inducing apoptosis of self-reactive T cells after activation with TCM is more complete and stable than that obtained by inducing certain specific antigens abroad. The results of this experiment show that warming yang and removing dampness drugs mainly containing large dose of aconite root may indeed induce apoptosis after activation of self-reactive T lymphocytes, thus achieving the purpose of treating autoimmune diseases. Through the pharmacological study of Aconitum carmichaeli components, the polysaccharide components with high efficiency and low toxicity have surfaced and attracted the attention of scholars. Modern pharmacological research found that astragalus polysaccharide and lycium barbarum polysaccharide have the functions of regulating immunity and resisting tumor [22-24]. Aconitum carmichaeli polysaccharide, as an important natural macromolecular compound, has the functions of enhancing immune function, anti-tumor, anti-aging and so on, and has a wide range of effects on cellular immunity and humoral immunity [25]. In recent years, studies have found that TCM polysaccharide, as a highly active immunomodulator, can activate T and B lymphocytes, induce apoptosis in early stage, and treat autoimmune diseases such as rheumatism [26-27].
Professor Zeng Shengping has long-term research experience in Aconitum carmichaeli polysaccharide extract. Its "Aconite Lateralis Polysaccharide Injection and its preparation process" has been authorized by Chinese invention patent number ZL 96117809.4. cell culture experiments prove that Aconitum carmichaeli polysaccharide has the effect of inducing lymphocyte apoptosis after activation, which has accumulated certain experience for the preparation of series of TCM polysaccharide injections and further development of in vitro culture drug screening experiments on target pathogenic clones. In the future, further follow-up clinical trials will be carried out to expand the sample size of the subjects and increase relevant detection indexes that can confirm apoptosis after inducing lymphocyte activation.
1. Yasutomo K. Pathological lymphocyte activation by defective clearance of self-ligands in systemic lupus erythematosus. Rheumatology (Oxford) 2003, 42: 214-222.
2. Zheng SP, Ren LY, Zhao X,. A preliminary study on the induction of immune tolerance in the treatment of rheumatoid arthritis by traditional Chinese medicine. Chin Archives Tradit Chin Med 2007, 25: 1327-1329.
3. Vitali C, Bombardieri S, Jonsson R,. Classification criteria for Sjoren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002, 61: 554-558.
4. Zheng XY. Guiding principles for clinical research of new Chinese medicine. China Medical Science and Technology Press 2002, 115: 383-385.
5. Fang YQ. Quantitative study on macro syndrome differentiation of phlegm. Liaoning J Tradit Med 1995, 22: 490.
6. Van Woerkom JM, Kruize AA, Wenting Vanwijk MJ,Salivary gland and peripheral blood T helper l and 2 cell activity in Sjogren’ s syndrome compared with non Sjogren’s sicca syndrome. Ann Rheum Dis 2005, 64: 1474-1479.
7. Bertorellor R, Cordone MP, Contini P,. Increased levels of interleukin-10 in saliva of Sjogren’ s syndrome patients. Clin Exp Med 2004, 4: 148-151.
8. Wen B, Shi YS, Zeng SP,Professor Zeng Shengping's experience in treating sjogren's syndrome. Yunan J Tradit Chin Med Materia Medica 2011, 8: 6-8.
9. Zheng SP. The effect of warming Yang and benefiting qi on immune regulation in patients with cold deficiency autoimmune disease. Chin J Basic Med Tradit Chin Med 1999, 5: 48-49.
10. Mandl T, Bredberg A, Jacobeson LT,. CD4+T-lymphocytopenia-a frequent finding in anti-SSA antibody sempositive patients with primary Sjogren's syndrome. J Rheumatol 2004, 31: 726-728.
11. Li X, Qiu YJ, Han JP. Changes of T cell subsets in peripheral blood of patients with primary sjogren's syndrome. J Tianjin Med Univer 2008, 14: 51-54.
12. Margit Z, Peter S, Edit G,. Correlation of increased susceptibility to apoptosis of CD4+T cells with lymphocyte activation and activity of disease in patients with primary Sjogren's syndrome. Arthritis Rheum, 1999, 42: 1673.
13. Henriksson G, Manthorpe R, Bredberg A. Antibodies to CD4 in primary Sjogren's syndrome. Rheumatology 2000, 39: 142.
14. Troster H, Metzger TE, Semsei I,One gene, two transcripts: isolation of an alternative transcript encoding for the autoantigen La-SS-B from a cDNA library of a patient with primary Sjogren's syndrome. J Exp Med 1994, 180: 2059.
15. Liu MF, Wang CR, Fung LL,. Decreased CD4+CD25+T cells in peripheral blood of patients with systemic lupus erythematosus. Scand J Immuno1 2004, 59: 198.
16. Wang HY, James J Lee, Nancy AL. The effect of CD69 expression on the activation and apoptosis of eosinophilic cells in mice. Chin J Pathophysiol 2009, 26: 1-6.
17. Wu DD, Wang HY. The bi-directional immune regulation of CD69 in cell activation and apoptosis. Chin J Pathophysiol 2010, 26: 1859-1862.
18. Cho D, Campana D. Expansion and activation of natural killer cells for cancer immunotherapy. Korean J Lab Med 2009, 29: 89-96.
19. Vazquez BN, Laguna T, Carabana J,. CD69 gene is differentially regulated in T and B cells by evolutionarily conserved promoter-distal elements. J Immunol 2009, 183: 6513-6521.
20. Pajusto M, Ihalainen N, Pelkonen J, et a1. Human in vivo-activated CD45R0(+) CD4(+) T cells are susceptible to spontaneous apoptosis that can be inhibited by the chemokine CXCL12 and IL-2, -6, -7, and-15. Eur J Immunol 2004, 34: 2771-2780.
21. Esplugues E, Sancho D, Vega-Ramos J,. Enhanced antitumor immunity in mice deficient in CD69. J Exp Med 2003, 197: 1093-1106.
22. Deng W, Dou XB, Shi YQ,Experimental study on differentiation of monocytes from umbilical cord blood into dendritic cells induced by astragalus polysaccharide. Chin Archives Tradit Chin Med 2007, 25: 492-495.
23. Zhou ZW. Effects of lycium barbarum polysaccharide on proliferation and differentiation of hematopoietic stem cells and granulocytic progenitor cells in mouse bone marrow. Chin J Pharmacology Toxicology 1991, 5: 44-46.
24. Shao P. A new perspective on the regulation of polysaccharides on immune cells - effects on dendritic cells. Inter J Immunology 2006, 29: 237-241.
25. Li FS, Xu HH, Li MY,Study on extraction and immunoactivity of polysaccharide from aconite. Modern Preventive Med 2008, 35: 2290-2291.
26. Ma BX, Chen X, Deng JE. Research progress of polysaccharides in traditional Chinese medicine. Chin Hosp Pharm 2003, 23: 360.
27. Feng G, Luo LQ, Kong QZ,Study on cell apoptosis induced by Chinese traditional medicine polysaccharide in signal transduction. Cancer Res Prey Treat 2005, 32: 559.
15 June 2019,
6 July 2019.
Sheng-Ping Zeng, Affiliated Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu Gaoxin Huisheng Rheumatism Hospital, Chengdu, China. Email: 1761210020@qq.com
SS, Sjgren syndrome;
The authors declare that there is no conflict of interests regarding the publication of this paper.
10.12032/TMRIM201903015
Bi R, Zeng SP, Wen B,Study on immunological characteristics of T lymphocyte in peripheral blood from patients with Sjogren's syndrome. TMR Integrative Medicine 2019, 3: e19015.
Chang Liu
