Effects of Da-Cheng-Qi decoction on enteroparalysis and serum inflammatory cytokines in patients with severe acute pancreatitis
Xiao Wang*, Guo-Hong Yang, Chen-Xiao Wang, Chun-Ying Li, Min Guo, Ming-Hao Liu, Zhen-Jun Zeng,Jun Ma,Qin-Sheng Zhang
1Department of Gastroenterology, First Affiliated Hospital of Henan Traditional Chinese Medicine University,Zhengzhou, Henan, China.2Department of Gastroenterology, Second Affiliated Hospital of Zhengzhou University,Zhengzhou, Henan, China.3Department of Gastroenterology, Henan Provincial Hospital of Traditional Chinese Medicine,Zhengzhou,Henan,China.
Background
Severe acute pancreatitis (SAP) is a common acute abdominal disease with rapid progression and high mortality of 15-30%[1,2].Overall,20-40%of patients with acute pancreatitis (AP) die from multiple organ failure induced by systemic inflammatory response syndrome (SIRS) in the early stages [3].Enteroparalysis is one of its severe complications.Furthermore, enteroparalysis caused by SAP is an important cause of SIRS and multiple organ dysfunction syndrome [4].The pathogenesis of enteroparalysis in SAP is complex.The most studied mechanisms include inflammatory factors,gastrointestinal hormones, and pancreatitis-associated ascites.Among these, inflammatory factors play an important role in the development of enteroparalysis[5].
Chemokines are a family of small (8-10 kDa)inducible secreted cytokines with chemotactic and activating effects on leukocyte subsets, and they play key roles in the pathogenesis of SAP-associated enteroparalysis [5].Levels of proinflammatory cytokines such as serum tumor necrosis factor-α(TNF-α), interleukin (IL)-1β, IL-6, and IL-8 are significantly higher in patients with SAP than in patients with mild acute pancreatitis [6].C-C motif chemokine ligand 2 (CCL2), also known as monocyte chemotactic protein-1, is a potent chemotactic for monocytes that is produced constitutively or after stimulation of various cell types.CCL2 provides chemotactic cues for the recruitment of monocytes from the bloodstream to the tissues [7-10].It is an early proinflammatory mediator that plays an important role in SAP[11].
The effects of conventional treatments are unsatisfactory and there is no specific therapy available for SAP-associated enteroparalysis.Current Western medical treatment strategies include intravenous fluid resuscitation, prophylactic antibiotics, probiotics,endoscopic retrograde cholangiopancreatography(ERCP) in acute biliary pancreatitis [12] and gastrointestinal motility therapy.Enteroparalysis is a“bottleneck”in the current treatment of SAP.
Traditional Chinese medicine (TCM) has been used for the treatment of SAP for a number of years.Da-Cheng-Qi decoction (DCQD), a famous Chinese herbal formula, is usually used for the treatment of intestinal obstruction, acute cholecystitis, acute appendicitis and AP.The components of DCQD includeRadix et Rhizoma Rhei(Dahuang),Cortex Magnoliae Officinalis(Houpu),Fructus Aurantii Immaturus(Zhishi), andNatrii Sulfas(Mangxiao).Record about DCQD can be traced back to the 3rd Century A.D.According to the description inShanghan Lun, an ancient book of Chinese medicine published in Han Dynasty of China, DCQD had the effect of purging accumulation and clearing heat from the stomach and the intestines.Clinical study indicated that the DCQD could improve symptoms of postsurgical gastrointestinal dysfunction and was beneficial to functional gastrointestinal disorders patients [13, 14].Studies using a rat model of acute pancreatitis have shown that DCQD could alleviate pancreatic, intestinal, and lung injury by altering levels of IL-4, IL-6, IL-10 and TNF-α in AP rats [15].The study by Zhang YM,et al.showed DCQD could alleviate liver damage by altering the inflammatory response in rats with SAP [16].But the exact mechanisms of DCQD remains unclear.Thus,the aim of the present study was to investigate the effects of DCQD on enteroparalysis and levels of the serum inflammatory cytokines CCL2 and IL-8 in patients with SAP.
Materials and methods
Ethics statement
This study protocol (2015HL-043-01) was approved by the Ethics Committee of the First Affiliated Hospital of Henan Traditional Chinese Medicine University(Zhengzhou,Henan,China),and all patients provided written informed consent for the use of clinical specimens for medical research.
Inclusion criteria
Male and female patients (18 to 65 years old) who hospitalized within 24 h of the onset of SAP’s symptoms were enrolled.The diagnostic criteria of SAP follows revised Atlanta Classification[18].
Exclusion criteria
The following patients were excluded: patients with pancreatic infection or peripancreatic infection caused by another disease, patients sent directly to the intensive care unit for multiorgan failure, post-ERCP,or traumatic/operative pancreatitis, malignancy,pregnancy, immunodeficiency, and patients in a moribund state < 48 h before enrollment, regardless of cause.
Withdrawal criteria
The following patients were withdrawn: patients were unable to continuously receive TCM treatment; patient strongly requested to withdraw from the study for other reasons, patients died or received an operation because they were unresponsive to intensive care treatment within 72 h of admission.
Preparation of DCQD
Chinese medicinal herbs in DCQD were provided by the First Affiliated Hospital of Henan Traditional Chinese Medicine University (Zhengzhou, Henan,China).Spray-dried DCQD powder comprised 20 g of Dahuang (Radix et rhizoma rhei), 30 g of Zhishi(Fructus aurantii immaturus), 15 g of Houpu (Cortex magnoliae officinalis), and 10 g of Mangxiao (Natrii sulfas) (Chengdu Green Herbal Pharmaceutical Co.Ltd, China).Every herb was made into a powder.Before the experiment,DCQD powder was prepared as a solution(200 mL).
Study design
This study was conducted at the First Affiliated Hospital of Henan Traditional Chinese Medicine University (Zhengzhou, Henan, China).Patients enrolled in the study were randomly assigned to the treatment or control groups using a random number table generated by the software SPSS 17.0 (Chicago,IL, USA).All enrolled patients were administered the following standardized comprehensive medical treatment: intensive care, oxygen inhalation,gastrointestinal decompression, fluid resuscitation,nutritional support, as well as treatment with proton pump inhibitors and prophylactic antibiotics for 10 days.Patients in the treatment group received additional DCQD via gastric perfusion (50 mL/2 h)and retention enema (200 mL/3 h) for purgation for 10 days.
Indicators monitored
Patients were observed during their hospital stay.Follow-up evaluations included duration of abdominal pain and the time when abdominal distension disappeared and normal bowel sounds returned.Blood was collected from the elbow vein to measure levels of CCL2, IL-8, amylase, lipase, and C-reactive protein (CRP) using an MDI600 automatic biochemical analyzer (Beckman, USA).These parameters and acute physiology and chronic health evaluation (APACHE) II scores [19] were recorded on days 1 and 10.Serum CCL2 and IL-8 concentrations(CCL2 and IL-8 antibodies were purchased from Abcam, Cambridge, UK) were measured using xxxxxxx enzyme-linked immunosorbent assay (ELISA) kits(Raybiotech, USA) according to the manufacturer’s instructions.
Statistical analysis
Data are expressed as means±standard deviation(SD)and were analyzed using Student's t-test.Non-parametric test was performed for non-normally distributed samples.Statistical analyses were conducted using SPSS 17.0 software.P< 0.05 was considered to indicate statistically significant differences.
Results
Clinical characteristics
A total of 56 patients with SAP who hospitalized in First Affiliated Hospital of Henan Traditional Chinese Medicine University from May 1, 2016 to May 30,2018 were initially screened.3 patients died and 4 patients received operation.48 patients were enrolled(26 in the treatment group and 22 in the control group).No patient was withdrawn and no patient showed verified evidence of adverse effects.There were no statistical differences between the two groups with regard to sex, age, etiology, APACHE II score, and Balthazar computed tomography score in the initial stage of hospitalization(P>0.05,Table 1).
Effect of DCQD on enteroparalysis
No differences between the two groups with regard to abdominal pain and abdominal distension on day 1 and no bowel sounds were present in patients of either group.After 10 days of hospitalization, the duration of abdominal pain and distension and the time when bowel sounds returned to normal were significantly shorter in the treatment group than those in the control group(P=0.026,P=0.031,P=0.042,Table 2).
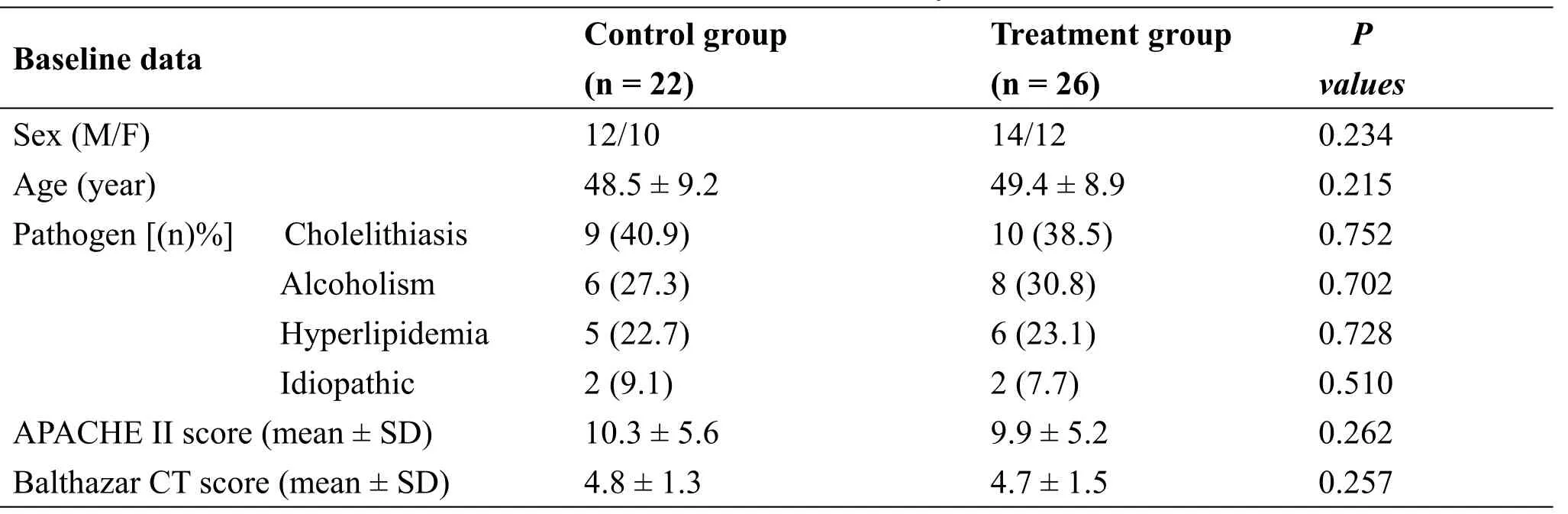
Table 1 Baseline data analysis
Effects of DCQD on APACHE II score and CRP level
No significant differences in APACHE II scores and serum CRP level between the treatment and the control groups were observed on day 1.However, their significantly decrease were present on day 10 (5.61 ±1.08 of treatment groupvs7.00±1.13 of control group,P< 0.001, Figure 1A; 66.04 ± 13.94 of treatment groupvs104.23 ± 21.61 of control group ,P< 0.001,Figure 1B).
Effects of DCQD on the levels of amylase and lipase
Though there were no significant differences in the levels of serum amylase and lipase between the treatment and control groups on day 1.On day 10, the levels of serum amylase and lipase in the treatment group were lower than those in the control group(80.46 ± 21.98vs137.41 ± 34.03,P< 0.001, Figure 2A; 193.27 ± 80.50vs335.23 ± 86.22,P< 0.001,Figure 2B).
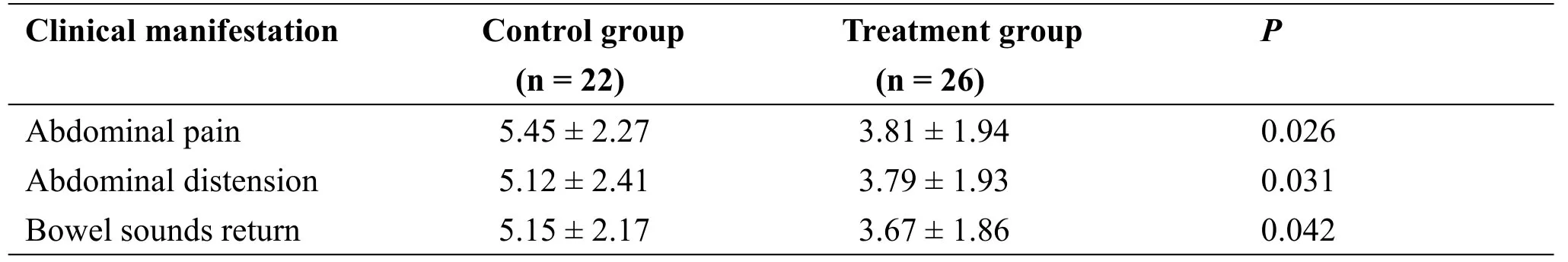
Table 2 The days of abdominal pain,abdominal distension and bowel sounds return to normal
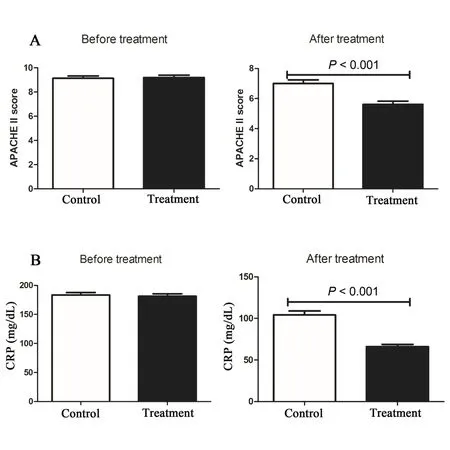
Figure 1 Effects of DCQD on APACHE II score and CRP level
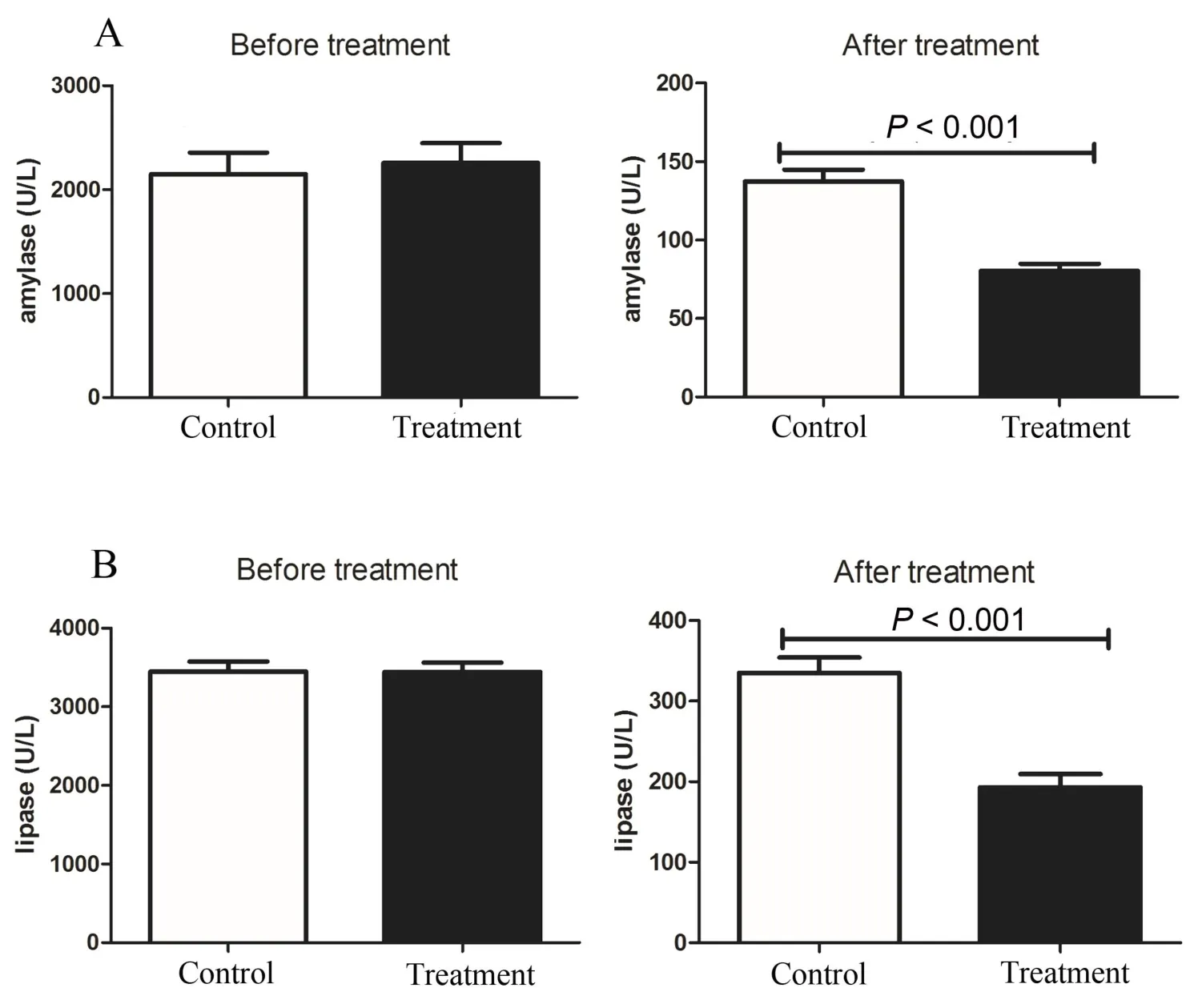
Figure 2 Effects of DCQD on the levels of amylase and lipase
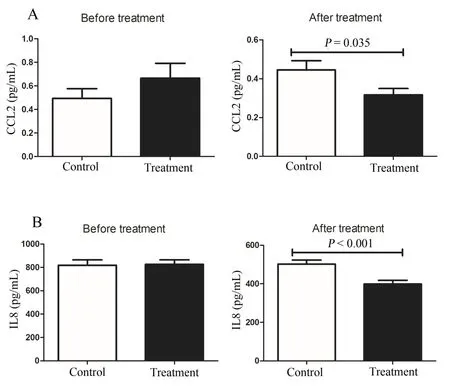
Figure 3 Effects of DCQD on the levels of CCL2 and IL-8
Effects of DCQD on the levels of CCL2 and IL-8
There were no significant differences in the levels of serum CCL2 and IL-8 between the treatment and control groups on day 1.However, on day 10, the levels of serum CCL2 and IL-8 in the treatment group were lower than those in the control group(0.33±0.15vs0.65 ± 0.52,P= 0.009, Figure 3A; 398.66 ± 97.95vs502.13±95.98,P<0.001,Figure 3B).
Discussion
SAP is a serious systemic disease with a mortality of approximately 15-30%, and with high morbidity of acute lung injury, acute kidney injury, and impairment of intestinal motility [3].Enteroparalysis is the predominant clinical symptom in patients with SAP.Abdominal pain,abdominal distension, and the lack of bowel sounds are its main clinical characteristics.DCQD is a commonly used herbal prescription in the treatment of early-stage SAP in China.It can effectively improve symptoms and reduce the incidence of complications and mortality of patients with SAP by protecting intestinal mucosal barrier and organ function.This clinical study also showed that the duration of abdominal pain and distension and the time when normal bowel sounds returned were shorter in the treatment group than those in the control group.Inflammatory chemokines are important mediators during SAP and are involved in the activation and migration of leukocytes into the tissues.In SAP, the local inflammatory process is amplified, and the inflammation spreads via the circulation throughout the body, resulting in a systemic inflammatory response[20,21].
This process is characterized by the release of proand anti-inflammatory cytokines and other inflammatory mediators, which recruit neutrophils,monocytes, and lymphocytes to the pancreas.CCL2 is a chemotactic molecule for monocytes/macrophages,B cells and T lymphocytes and belongs to the CC subfamily of chemokines.It is an early proinflammatory mediator that plays an important role in SAP.Our data showed that CCL2 production decreased after DCQD treatment.IL-8 is the most thoroughly characterized member of the chemokine family studied in AP.It is a powerful secondary chemoattractant of neutrophils in the inflammatory process [22].A previous study indicated that changes in serum CCL2 and IL-8 levels in patients with AP follow a pattern, thus allowing the determination of reference values for the diagnosis and evaluation of the severity of AP[23].
In this study, we also observed that serum IL-8 levels in the treatment group were lower than those in the control group.This indicates that DCQD may inhibit the production of proinflammatory chemokines,thereby preventing the pathological progress of SAP.
Furthermore, we noticed that DCQD treatment improved APACHE II scores, which is the traditional multifactorial scoring system used to predict the severity of AP.Currently, CRP is the most frequently used single biomarker to assess the severity of AP,as it is inexpensive, widely available, and easy to measure.We also noticed that DCQD treatment significantly reduced serum CRP level.Moreover, DCQD treatment decreased the levels of serum amylase and lipase.These data indicate that DCQD may effectively improve parameters used in the prognostic evaluation of SAP.
Conclusion
The occurrence and development of SAP are closely related to various inflammatory mediators and cytokines.The action of inflammatory factors that may damage the body may worsen the condition of SAP.Therefore, the key to SAP treatment is to reduce the inflammatory response, control the release of inflammatory transmitters,and alleviate multiple organ damage.This study demonstrated that DCQD treatment decreased the levels of CCL2, CRP,and IL-8 in patients with SAP, effectively relieved enteroparalysis, and shortened the duration of hospitalization.The specific mechanisms of DCQD involved in the treatment of SAP require further study.
 Traditional Medicine Research2019年4期
Traditional Medicine Research2019年4期
- Traditional Medicine Research的其它文章
- Artificial tiger bone powder for improving the quality of life in elderly patients with fracture
- Study on multi-target mechanism of Radix et Rhizoma Rhei(Dahuang)and Semen Persicae (Taoren) on adhesion intestinal obstruction based on network pharmacology
- Development and validation of the perioperative recovery scale for integrative medicine
- Acupuncture preconditioning protects against myocardial ischemia/reperfusion injury mediated apoptosis through miR-214/NCX1 activation:a hypothesis
- Is naturopathic medicine necessarily safe?
- Is IL1R1 required for celastrol’s leptin-sensitization and antiobesity effects?
