In-vitro pharmacological evaluation of Sulforaphane from Brassica oleracea
Sodum Nalini, Shaik Chand Basha, TS Mohamed Saleem
In-vitro pharmacological evaluation of Sulforaphane from Brassica oleracea
Sodum Nalini1, Shaik Chand Basha2, *, TS Mohamed Saleem3
1Annamacharya College of Pharmacy, New Boyanapalli, Rajampet, India.2Department of Pharmaceutical Chemistry, Annamacharya College of Pharmacy, New Boyanapalli, Rajampet, India.3Department of Pharmacology, Annamacharya College of Pharmacy, New Boyanapalli, Rajampet, India.
: Sulforaphane has numerous Pharmacological and therapeutic effects like anti-oxidant, anti-cancer, anti-diabetic, anti-arthritic, anti-ulcer, anti-viral. The present study was designed to evaluate the anti-arthritic activity of Sulforaphane by in-vitro screening methods.: The anti-arthritic activity of Sulforaphane was investigated by protein denaturation assay by using Bovine serum albumin (BSA) and egg albumin. Sulforaphane used in various concentration (10, 50, 100, 250, 500 µg/ml) against both the methods and Diclofenac sodium was used as reference standard.: The anti-arthiritic activity sulforaphane was directly proportional to inhibition of denaturation of albumin. Sulforaphane shows concentration dependent inhibition activity in both bovine serum albumin and egg albumin assay. The maximum inhibition was observed in the concentration of 500 µg/ml with 92.8% inhibition for BSA and 96.3% for egg albumin respectively.: Sulforaphane have significant anti-arthritic activity accessed by BSA denaturation and egg albumin denaturation assay. Based on present finding future direction also planned to conform the activity by using well established in-vivo methods.
Sulforaphane, Anti-arthritic activity, Protein denaturation methods
Sulforaphane shows significant anti-arthritic activity through in-vitro screening models and the activity was observed as concentration dependent against bovine serum albumin (BSA), egg albumin denaturation assay.
Introduction
Traditional medicinal plants are practiced worldwide for treatment of arthritis especially in developing countries where resources are meager [1]. Arthritis is a disease related to chronic joint pain and inflammation. Typically arthritis shoes heavy morbidity of pain, aching, stiffness and swelling in and around one or more joints characterize rheumatic conditions [2-3].
According to WHO 0.3-1% of the world population is affected from rheumatoid arthritis (RA) and among them females are three times more prone to the disease as compared to males. RA is a chronic, inflammatory and systemic autoimmune disease [4]. Presently for treatment of RA, strategies have changed from traditionally used NSAIDs or disease modifying antirheumatic drugs (DMARDs) to novel biological agents like TNF monoclonal antibody [5].
The most common adverse effects were gastrointestinal symptoms (abdominal pain, diarrhoea, dyspepsia and nausea), headache and upper respiratory infection, with an incidence of about 5% during the 12-week treatment period [6].
Although the treatment of RA is available but due to potential adverse effects or irreversible organ damage the new approaches of herbal therapies are developed for maintaining the balance between these potential risk and acknowledged benefits. Since ancient time India uses herbal medicines in the officially alternative systems of health and it is not an exaggeration to say that the use of herbal drugs is as old as mankind.
Sulforaphane (Figure 1) is a phytochemical which exists as sulforaphane Glucoraphanin (4-methylsulphinylbutyl glucosinolate) which has tends to exist in foods as its glucose moiety removed by Myrosinase (Thioglucosideglucohydrolase) an enzyme occurring in the broccoli family of plants. It is found in cruciferous vegetables such as broccoli, cauliflower, cabbage and kale. It is an antioxidant and stimulators of natural detoxifying enzymes. Sulforaphane may reduce the risk of breast, bladder and prostate cancer [7].
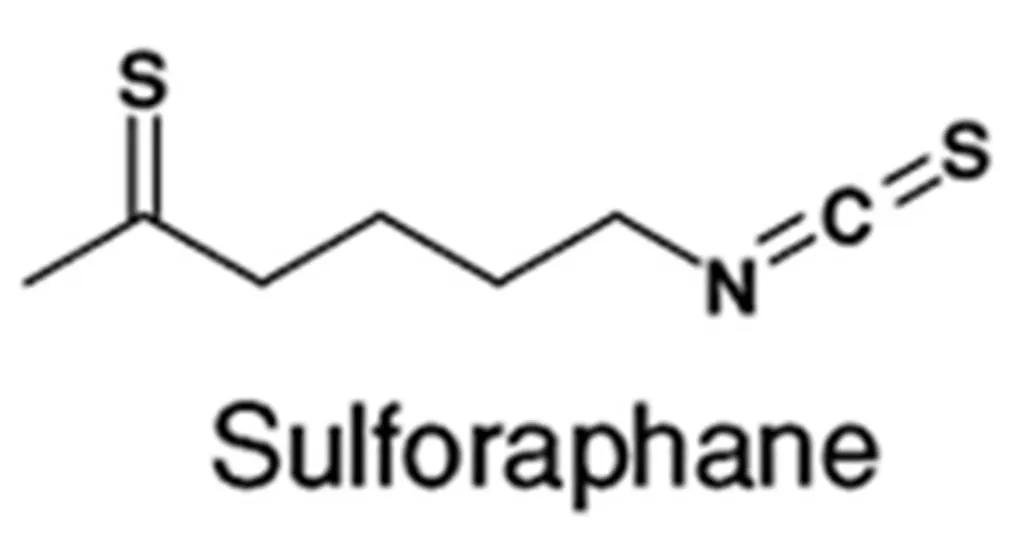
Figure 1: Chemical structure of Sulforaphane
The present study was designed to evaluate the anti-arthritic activity of sulforaphane by using in-vitro screening models.
Methods
Drugs and chemicals
Sulforaphane (from Broccoli Spout Extract standardized) was obtained as gift sample from Lebepur, Germany and bovine serum albumin was purchased from Sisco Research Laboratories Pvt. Ltd. (SRL) - India. All chemicals were of analytical grade purchased from Sigma-Aldrich, India.
In-vitro anti-arthritic activity
Anti-arthritic activity of sulforaphane was determined by using bovine serum albumin (BSA) denaturation and egg albumin denaturation assay [8, 9].
Bovine serum albumin denaturation assay
The reaction mixture contain 100 μl of various concentration of (10, 50, 100, 250, 500 µg/ml) sulforaphane and 100 μl of 5 % aqueous solution of BSA; pH was adjusted adding a small volume of glacial acetic acid. The mixtures were incubated at 37 °C for 20 min and then heated to 70 °C for 10 min. The mixture was permitted to cool for 10 min after which turbidity was measured at 660 nm. The blank comprised the sample and distilled water. Distilled water was used as the negative control. The positive control was diclofenac sodium (similar concentration as sulforaphane). The test was carried out in triplicate. Percentage inhibition was calculated using the formula:
Inhibition % = 100*(Abs Sample‐Blank/control‐1)
Egg albumin denaturation assay
The reaction mixture contain 0.2 ml of Egg Albumin (from fresh hen’s egg), 2.8 ml of Phosphate-buffered saline (PBS, PH-6.4) and 2 ml of varying concentrations (10, 50, 100, 250, 500 µg/ml) of Sulforaphane. A similar volume of double distilled water served as the control. Next, the mixtures were incubated at 37 °C in a BOD incubator for 15 minutes and then heated at 70 °C for 5 minutes. After cooling, their absorbance was measured at 660 nm by using the vehicle as a blank. Diclofenac Sodium in the concentrations of (10, 50, 100, 250, 500 µg/ml) was used as a reference drug and treated similarly for the determination of absorbance. The percentage inhibition of protein denaturation was calculated by using the following formula:
Inhibition %= 100*(Abs Sample‐Blank/control‐1)
Results
The anti-arthiritic activity sulforaphane was directly proportional to inhibition of denaturation of albumin. Sulforaphane shows concentration dependent inhibition activity in both bovine serum albumin and egg albumin assay. The maximum inhibition was observed in the concentration of 500 µg/ml with 92.8% inhibition (Figure 2) for BSA and 96.3% (Figure 3) for egg albumin respectively.
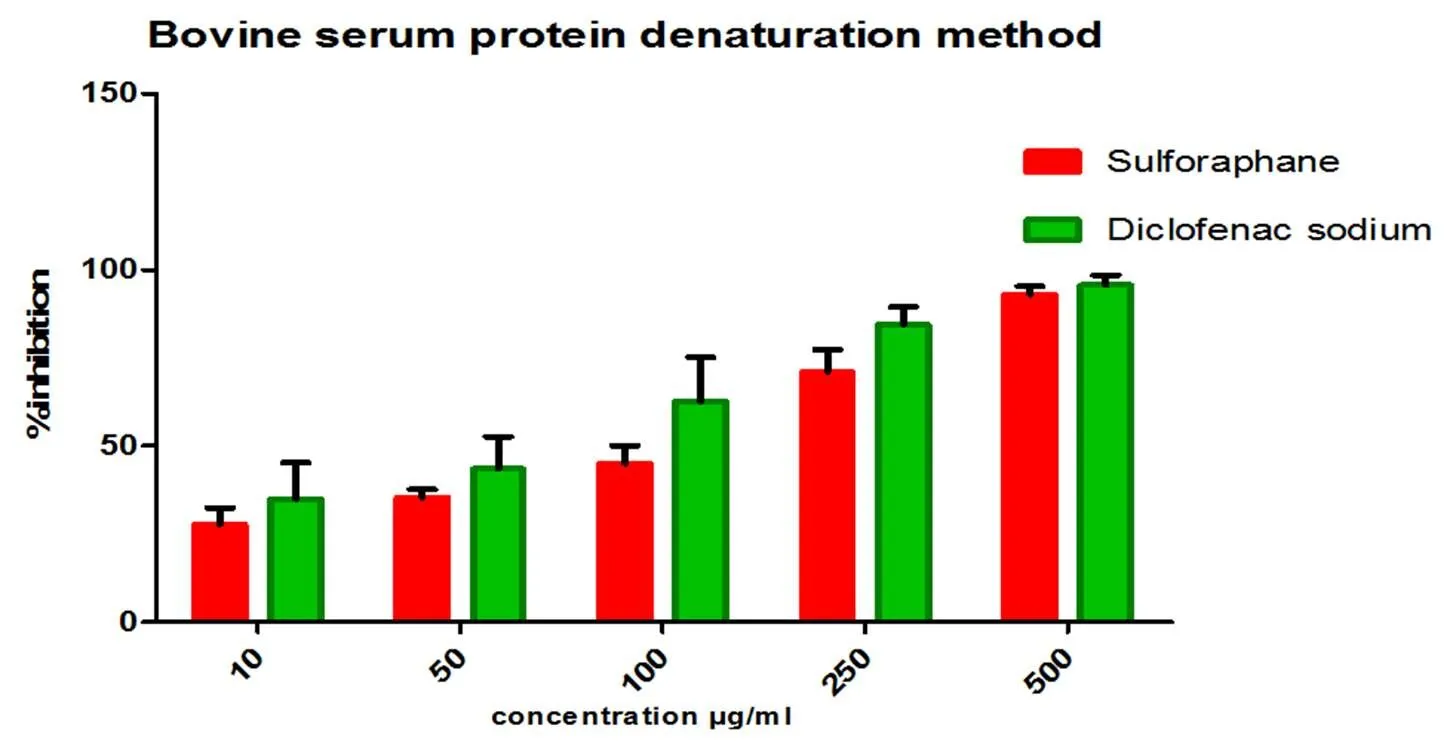
Figure 2 Anti-arthritic activity of Sulforaphane by bovine serum albumin method
Discussion
Arthritis is a public problem perceived in elderly people. Globally many people suffered by this devastating disease [10]. Disease modifying anti-rheumatic drugs (DMARDs) were used for the management of arthritis. These drugs were documented with many side effects which decline the regular and long term administration for disease management [11]. Hence, there is a unremitting search for substitute drugs from plants and other natural sources.
Medicinal plants are outstanding sources of antioxidants, anti-arthritic and anti-inflammatory agents [12, 13]. The presence of active constituents like phenols, flavonoids, tannins, flavonols, proanthocyanidins, nitrogenous compounds, vitamins and terpenoids were attributed with pharmacological property [14, 15]. Sulforaphane is an organic isothiocyanate (ITC) found in cruciferous plants such as broccoli with several medicinal properties [16]. In the present study we have demonstrated the anti-arthritic activity of suforaphane by in-vitro methods.
Denaturation of tissue protein is one among the well documented cause of inflammatory and rheumatoid diseases. Production of auto antigen insures rheumatic diseases could also because of denaturation of protein in vivo. Agent which will forestall protein denaturation thus may be worthy for anti-arthritic drug development. Some literature declared that protein denaturation and macro globulin formation cause the proteins to become antigenic, therefore initiating the immunologic response and producing organic chemistry changes in animal tissue that ultimately results in rheumatism [17].
In the present study sulforaphane shows potent anti-arthritic activity via inhibition of albumin denaturation in concentration dependent manner.
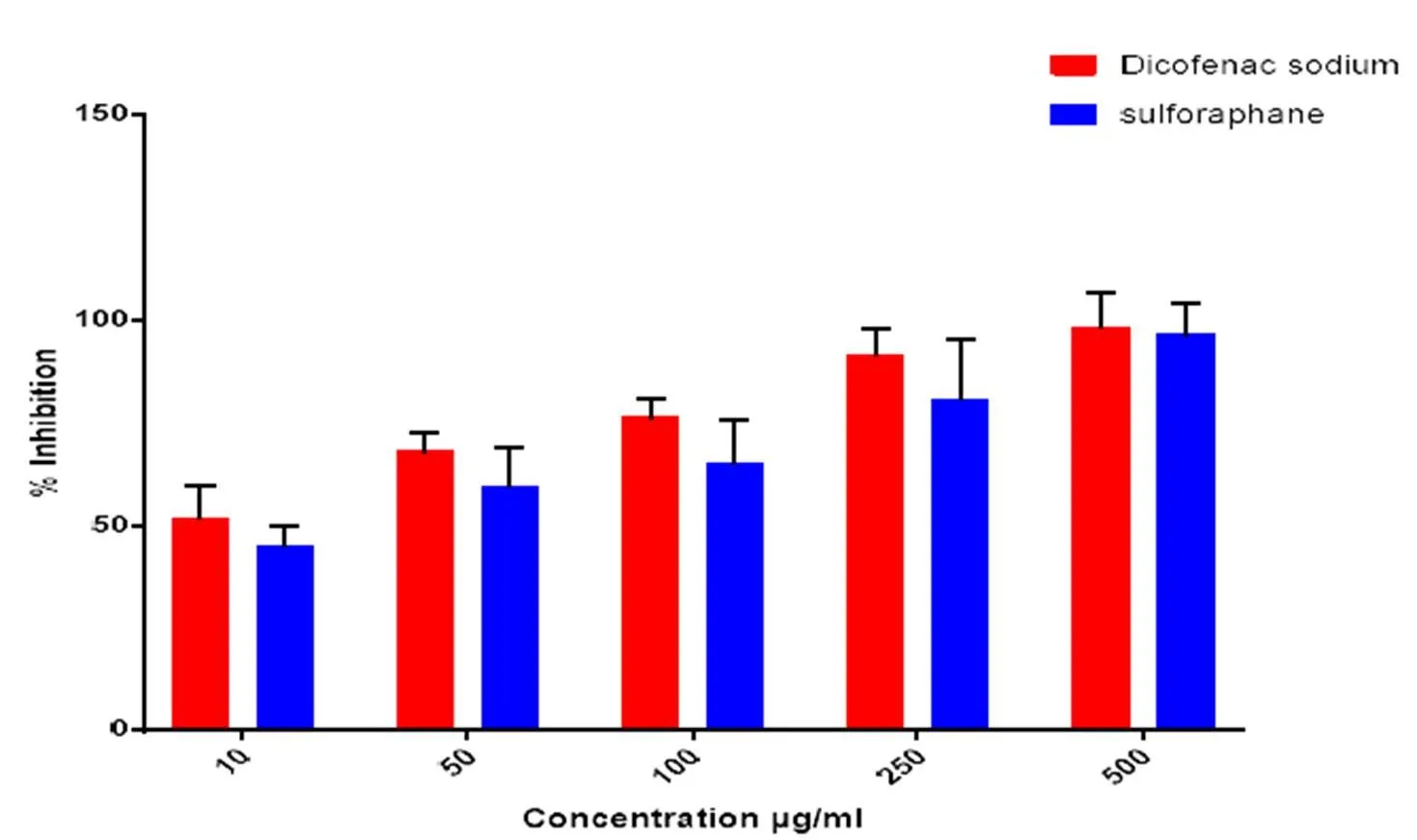
Figure 3 Anti-arthritic activity of Sulforaphane by egg albumin method
Conclusion
From the research findings we have concluded that sulforaphane have significant anti-arthritic activity accessed by BSA denaturation and egg albumin denaturation assay. Based on present finding future direction also planned to conform the activity by using well established in-vivo methods.
1. Choudhary M, Kumar V, Malhotra H,. Medicinal plants with potential anti-arthritic activity. J Intercultural Ethanopharmacology 2015, 4: 12-18.
2. Khanna D, Sethi G, Ahn KS,. Natural products as a gold mine for arthritis treatment. Current Opinion Pharmacology 2007, 7: 344-351.
3. Chunxia C, Peng Z, Huifang P,Extracts of Arisaema rhizomatum C.E.C. Fischer attenuate inflammatory response on collagen-induced arthritis in BALB/c mice. J Ethnopharmacology 2011, 133: 573-582.
4. Tripathy S Pradhan, Njana D. Anti-inflammatory and anti-arthritic potential of Ammania baccifera Linn. Int J Pharmaceutical Bio Sci 2010, 1: 1-7.
5. Firestein GS. Evolving concepts of rheumatoid arthritis. Nature 2003, 423: 356-361.
6. Lin J, Zhang W, Jones A,Efficacy of topical non-steroidal anti-inflammatory drugs in the treatment of osteoarthritis: meta-analysis of randomized controlled trials. BMJ 2004, 1: 1-6.
7. Chand Basha S, Rajesh Babu K, Madhu M,In-vitro anti diabetic activity of Sulphoraphane. PTB Reports 2017, 3: 47-49.
8. Rahman H, Eswaraiah C, Dutta M. In-vitro anti-inflammatory and anti-arthritic activity of oryza sativa var. Joha American-Eurasian J Agriculture Environmental Sci 2015, 15: 115-121.
9. Dutta SK, Basu SK, Sen KK. Binding of diclofenac sodium with bovine serum albumin at different temperatures, pH and ionic strengths. Indian J Exp Biol 2006, 44: 123-127.
10. Murugananthan G, Sudheer KG, Sathya CP,. Anti-arthritic and anti-inflammatory constituents from medicinal plants. J Appl Pharm Sci 2013, 3: 161-164.
11. Reddy VJS, Rao PGD, Lakshmi GR. A review on antiarthritic activity of some medicinal plaants. J Glob Trends Pharm Sci 2014, 5: 2061-2073.
12. Zubair M, Anwar F, Shahid SA. Effect of extraction solvents on phenolics and antioxidant activity of selected varieties of pakistani rice. J Agric Biol 2012, 14: 935-940.
13. Atawodi SE, Yakubu OE, Umar IA. Antioxidant and hepatoprotective effects of parinari curatellifolia root. Int J Agriculrure Biol 2013, 15: 523-528.
14. Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts torilis leptophylla L. BMC Complement Altern Med 2012, 12: 221.
15. Dzoyem JP, Kuete V, McGaw LJ,The 15-lipoxygenase inhibitory, antioxidant, antimycobacterial activity and cytotoxicity of fourteen ethnomedicinally used African spices and culinary herbs. J Ethnopharmacol. 2014, 156: 1-8.
16. Gray SG. The potential of epigenetic compounds in treating diabetes. Epigenetics in Human Disease, Academic Press, 2012.
17. Manvarl MN, Desai TR. In-vitro anti-inflammatory and anti-arthritic activities of fruits of vernonia anthelmintica willd. Asian J Pharmacy 2014, 4: 186-188.
30 April 2019,
15 June 2019.
Shaik Chand Basha M. Pharm, Department of Pharmaceutical Chemistry, Annamacharya College of Pharmacy, New Boyanapalli, Rajampet, India. E-mail: schandbasha20@gmail.com
The authors declare that there is no conflict of interests regarding the publication of this paper.
10.12032/TMRIM201903012
Sodum N, Shaik CB, TS Mohamed S. In-vitro pharmacological evaluation of Sulforaphane from Brassica oleracea. TMR Integrative Medicine 2019, 3: e19012.
Chang Liu
