Effect of gloriosa superba in combination with fluconazole on anti-Candida activity against biofilm development and mature biofilm growth patterns
Somwanshi Sachin M, Dhawale Shashikant C, Zore Gajanan B, Choudhary Harshad W
Effect of gloriosa superba in combination with fluconazole on anti-Candida activity against biofilm development and mature biofilm growth patterns
Somwanshi Sachin M1, *, Dhawale Shashikant C1, Zore Gajanan B2, Choudhary Harshad W1
1Department of Pharmacology, School of Pharmacy, Swami Ramanand Teerth Marathwada University, Vishnupuri, Nanded- 431606, (M.S.) India.2School of Life Sciences, Swami Ramanand Teerth Marathwada University, Vishnupuri, Nanded- 431606, (M.S.) India.
Use of G. Superba L. alone or in combination with available antifungal drugs would be a novel approach against drug resistant biofilms of C. albicans. In future it will be useful to avoid side effects associated with high dosages and long term usage of the conventional antifungal drugs during anti- biofilm therapy.
This study was to evaluate the efficacy of methanolic extract of G. superba L in combination with Fluconazole against biofilm development and mature biofilms of C. albicans. Synergism between MEGS and Fluconazole combination against biofilm formation was evident with FICI of 0.187. Combination of MEGS and Fluconazole did not have synergistic potential against mature biofilm growth, evidenced in FICI of 0.916. MIC of standard Fluconazole was found to be 0.5 and >0.5 in biofilm development and mature biofilm respectively.
G. superba L.; FICI; MTT; Germ tube assay
Introduction
Studies on the use of plant extracts for the control of diseases have shown the importance of natural Phytochemicals as possible sources of non-phytotoxic and easily biodegradable alternative fungicides or antibiotics [1]. A combinatorial approach in antifungal therapy offers several potential advantages like increased potency, reduced dosages of individual drugs, minimized toxicities, and prevention of the emergence of drug-resistant mutant strains [2].
Against this background the endangered medicinal plant Gloriosa superba L. to evaluate its potential inhibitory effect against C. albicans. Involvement of C. albicans biofilms in clinical infections is a serious problem for immunocompromised patients. Also it is reported to form biofilms on urinary catheters, intra-venous catheters, denture materials, central nervous system prostheses, artificial heart valves, joint prostheses, contact lenses, penile implants and intrauterine devices as well as host tissue surfaces [3, 4]. A notable feature of C. albicans biofilms is resistance to various antifungal including the widely prescribed drug Fluconazole. Additionally biofilms may act as reservoirs of infectious cells to cause re-infections. Toxic side effects limit the use of high concentrations of available antifungal drugs, as such new strategies to combat biofilm-associated C. albicans infections are necessities. Plants were found effective against multiple drug-resistant strains as well as biofilm growth of C. albicans [3, 4]. Attempts have been made to study the anti-biofilm activities of plant roots extract in combination with antifungal drugs. In this study, we propose to analyze the efficacy of Gloriosa superba L. extracts in combination with Fluconazole against biofilm development and mature biofilms of C. albicans.
Materials and methods
Collection, authentication and preparation of extracts of Gloriosa superba L. tubers
The tubers of G. superba L. were collected from Shree Baidyanath Ayurved Bhavan Pvt. Ltd, Nagpur, India. The tubers were sliced into round shaped pieces then ground into a powder using a blender. The powder was subjected to solvent extraction; 25 gm. of powder was extracted in 250 ml of solvent for 5 h. The extracts were filtered and concentrated. The concentrated extracts were weighed and kept in sterile bottles under refrigerated conditions until use. For the activity testing the stock solutions of extracts of tubers i.e. methanolic extract of G. superba L. (MEGS) were prepared by dissolving known quantity of the extracts in dimethyl sulfoxide (DMSO).
Culture media and culture conditions
C. albicans ATCC 90028 was obtained from the School of life sciences S.R.T.M. University, Nanded. The strain was maintained on Yeast-Peptone-Dextrose (YPD) agar slants at 4° C. A single colony from the yeast extract-peptone-dextrose (YPD) agar plates was inoculated in 50 ml of YPD broth (pH 6.5), in a 250 mL Erlenmeyer flask. The flasks were incubated at 30° C on an orbital shaker at 120 rpm for 24 h. Cells from the activated culture were harvested by centrifugation for 5 min at 2000 g speed. Collected cells were washed three times with phosphate buffer saline (PBS), pH 7.4.
For susceptibility testing various concentrations of extracts were prepared in RPMI-1640 (Rosewell Park Memorial Institute) medium by double dilution. Concentration of the solvent used i.e. Dimethyl Sulphoxide (DMSO) was never > 1 % Fluconazole was used as a standard antifungal drug. Marketed preparation of Fluconazole (Forcan, Cipla Pvt. Ltd., Mumbai, India) was purchased from local market. MTT [3-(4, 5-dimethyalthizole-2-yl)-2, 5-diphenyltetrazolium bromide) were purchased from Hi-media laboratories Ltd. Mumbai, India. The required organic solvents and other media components, chemicals were obtained from Hi-Media Laboratories Ltd., Mumbai, India.
Microtiter plate based morphological assay
Germ tube assay in which firstly YPD medium were prepared and in which 20% serum is added. After that 24h. activated culture is harvested and centrifuged then wash cell three times in sterile dist. water then cells keep side for starvation for 1hr. After the starvation cell density is calculated 1 * 105 in -1 or -2 dilution take slide and 20 µl cells and observe under microscope and calculated cell density for given medium. 100 µl of YPD medium was added in each micro titer 96 well plate and 200 µl YPD medium was added in 12th well row. Then drug is added in 12th no. well and then further dilution is carried out in each well. In 1st row is control and 2nd row is solvent. Then plate is incubated 370 c for 24 hr. in incubator. After 24 hr. plate were observed under microscope and then calculate the total number of cell and total number of germ tube and then calculate % germ tube. Microphotographs of these plates were carried out with the help of camera attached of microscope.7
Minimum inhibitory concentration (MIC)
Effect of MEGS on the growth of C. albicans was studied by using the standard broth micro dilution methodology, as per Clinical and Laboratory Standards Institute (CLSI) guidelines (CLSI, 2002). Extemporaneously various concentrations of the extracts (ranging from 0.0025 to 04 mg/ml) were prepared in RPMI-1640 medium, in 96 well plates. Wells without test extracts served as a control. Inoculums from washed C. albicans cells was added to each well so that to get 1×103 cells /mL. The plates were incubated at 35° C for 48 h. To analyze the growth, absorbance was read visually as well as using a micro plate reader (at 620 nm) (Multiskan EX, Thermo Electron Corp., USA). The lowest concentrations of the four extracts which caused 50 % reduction in the absorbance compared to that of control were considered as minimum inhibitory concentrations (MICs) for growth of C. albicans .4
Biofilm formation and drug susceptibility
C. albicans biofilms were developed on polystyrene surface of 96-well plates as per standard methodologies [5], a cell suspension of 1×107 cells/mL was prepared in PBS and 100 μL was inoculated in each well. In the adhesion phase, plates were incubated at 37° C for 90 minutes to allow attachment of cells on the surface. Non-adhered cells were removed by washing the wells with sterile PBS, two to three times. 200 μL of the RPMI-1640 medium was added to each well and the plates were incubated at 37° C for 48 h to allow biofilm formation.
To observe its effect on development of biofilms, RPMI-1640 medium along with concentrations 0.0025 to 4 mg/ml of the extracts were added to each well immediately after adhesion phase and incubated for 48 h at 37° C. Fluconazole was used as standard antifungal drug in the range 0.001 to 1.024 mg/ml. To analyze activity against mature C. albicans biofilms, the extracts were added to 24 h mature biofilms and further incubated for 48 h at 37° C. After incubation, wells were washed to remove any planktonic cells, and biofilms were observed using an inverted light microscope [6]. Biofilm growth was quantitated with MTT metabolic assay.
Chequerboard format for determination of FICI for the combination of drug and MEGS extract against biofilm development and mature biofilm growth.
Dilutions of Fluconazole and MEGS were prepared in a chequerboard format as per standard methodology [7]. Two dimensional array of serial concentrations of MEGS was used for preparation of combination dilutions. 100 µl of cell suspension was added to each well and the micro plates were incubated at 35 °C. After 48 h of incubation, absorbance was read using micro plate reader at 620 nm. MIC was determined as the concentrations of combination dilutions were showing 50 % reduction in the absorbance compared to that of control. FIC indices were calculated using formula as
ΣFIC = FICA + FICB. Where,
FICA = (MIC of Fluconazole in combination / MIC of Fluconazole alone)
FICB = (MIC of MEGS in combination / MIC of MEGS alone)
When the value of ΣFIC ≤ 0.5, it is the synergism, while with ΣFIC >4 it is antagonism. A ΣFIC result of >0.5 but ≤ 4 is considered as indifference. This was followed for planktonic as well as biofilm growth forms of C. albicans.
Microscopic analysis
Biofilms were observed under an inverted light microscope (Metzer, India). Photographs were taken by Labomed microphotography system (Labomed, Korntal, Germany) at × 200 magnification and Microscope Primovert iLED with Epi-Fluorescence 470 nm, transmitted-light bright field while universal phase contrast with objectives 4x, 10x Ph1, LD 20x Ph1, LD 200 magnification.
Results
Inhibition of induced germ tube formation by MEGS, Micro titer plate based morphological assay showed that MEGS inhibited serum induced hyphae in C. albicans at very low concentration, 71 % reduction in biofilm growth compared to that of control biofilms. MTT assay revealed MEGS were highly effective, causing more than 79 % inhibition at 0.0025 mg/ml, while 1 and 2 mg/ml shows >95 % inhibition and 4 mg/ml show 100% germ tube inhibition. It reveals that MEGS exhibited a little prevention of growth after 24 h of incubation, while have not very efficient inhibition of planktonic growth of C. albicans. The highest concentration analyzed in this study was 2 and 4 mg/ml, reduced the growth by 50 and 68 % compared to that of control. In the MTT assay revealed that development of biofilm concentration of 2 and 4 mg/ml caused significant growth decrease of 67 and 70%, while with of mature biofilm 43 and 55%. Microscopic observations confirmed the decrease in biofilm density with increasing concentration of the MEGS.
Synergism between MEGS and Fluconazole against development of biofilm was evident with FICI of 0.187. Mature biofilm were comparatively less sensitive to the activity of MEGS alone and combined with Fluconazole. Combination of MEGS and Fluconazole did not have synergistic potential against mature biofilm growth, evidenced with FICI of 0.916.
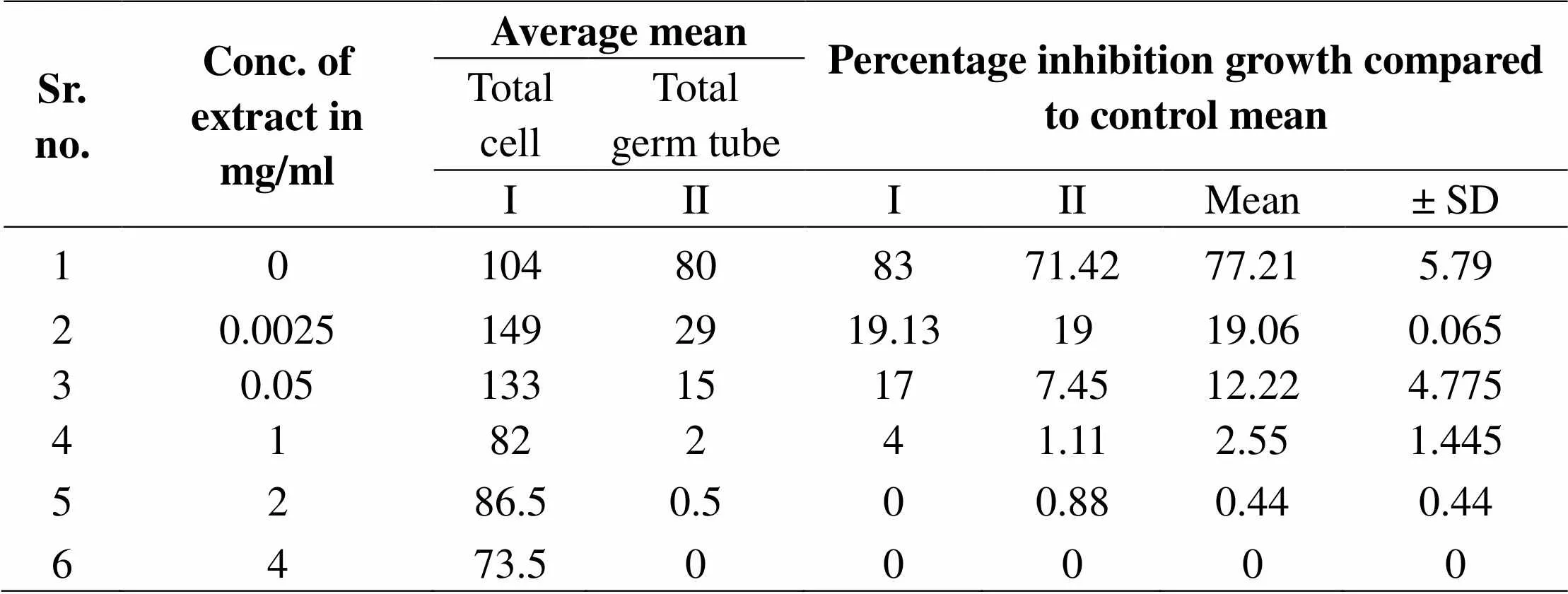
Table 1: Effect of MEGS on germ tube growth of C. albicans
Figure 1: Germ tube cell growth of C. albicans ATCC 90028, in presence of various concentrations of MEEGS. Germ tube cell growth was analyzed as absorbance of cell density at 595 nm. Where absorbance of colored end product was measured at 450 nm and compared with that of control biofilms;
Microphotographs
MEGS-Germ tube Assay

Figure 2 Microphotographs showing inhibition of C. albicans Germ tube formation in presence of MEGS A) Control without MEGS; B) Treatment with 1mg.mL-1 of MEGS; C) Germ tube growth in presence 2mg.mL-1 D) Concentration 4mg/ml of MEGS. The complete inhibition of biofilm and presence of only a layer of yeast form cells in fig. C and fig. D and lowering in density of Germ tube growth in fig. B, compared to that of dense network of biofilm in control, fig. A
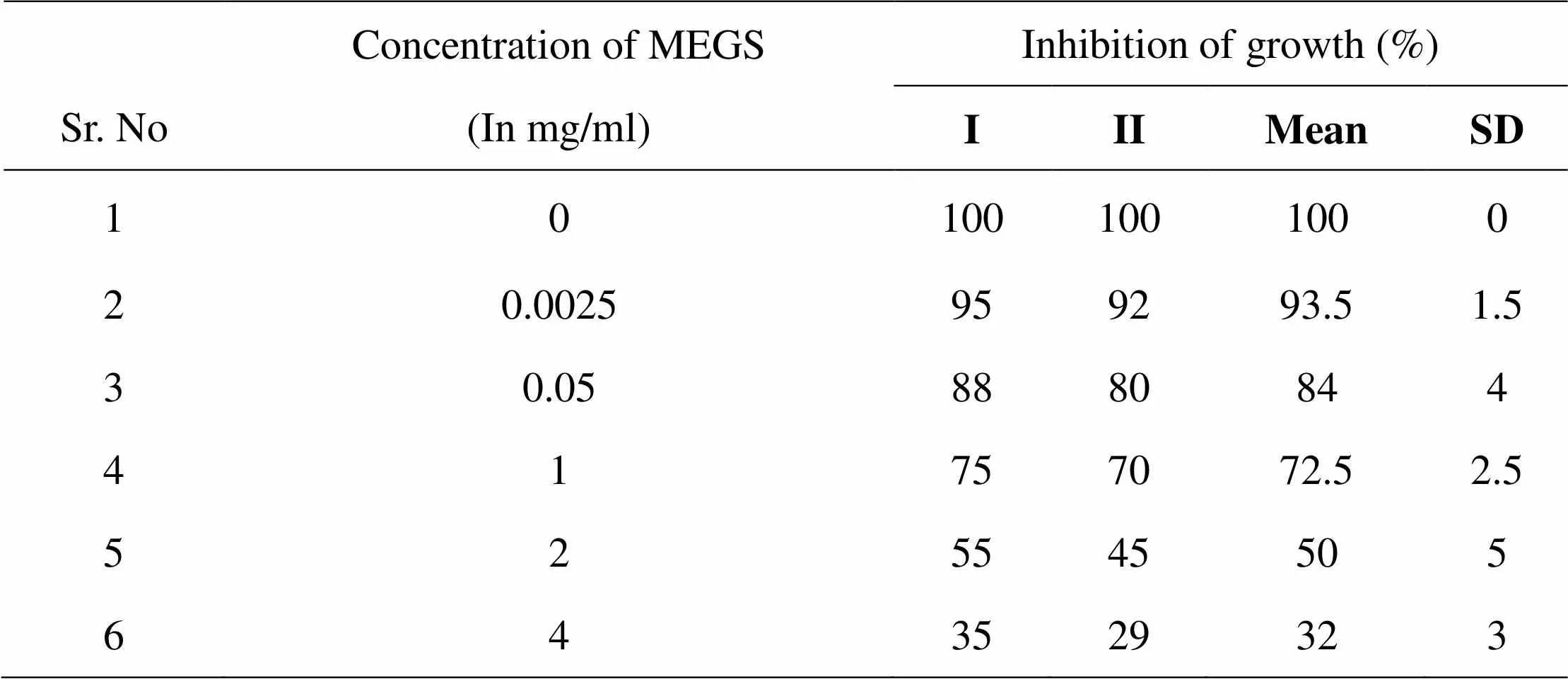
Table 02: MIC of MEGS on planktonic growth
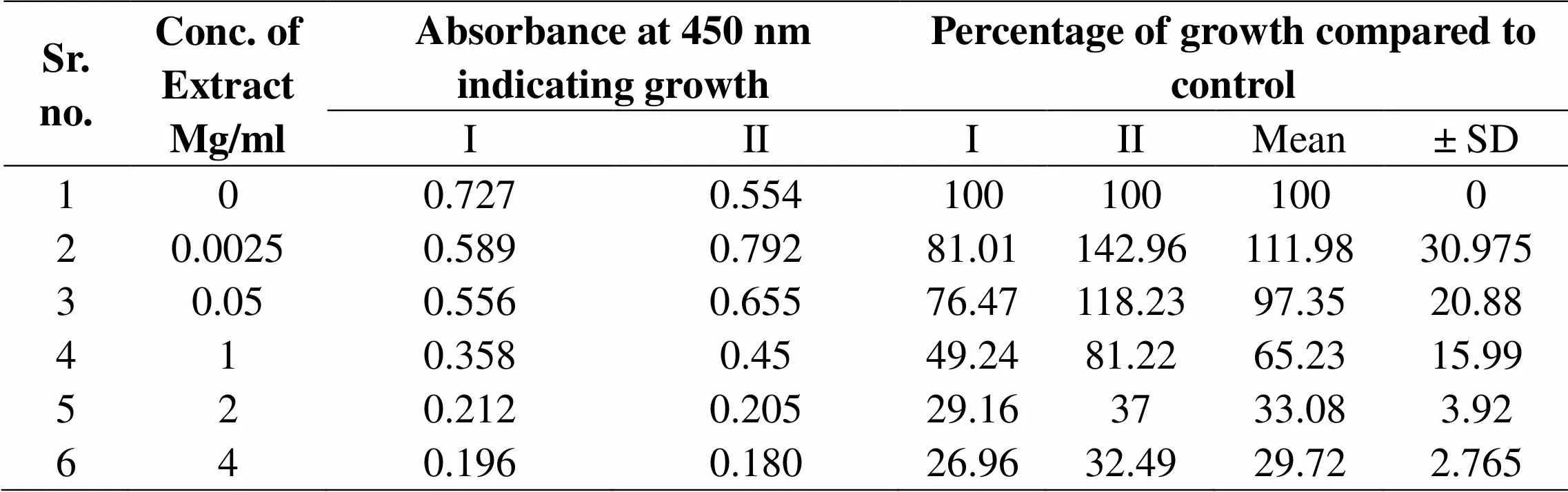
Table 03: Effect of MEGS on Biofilm development of C. albicans
Figure 3 Development of biofilm growth of C. albicans ATCC 90028, in presence of various concentrations of MEEGS. Development of biofilm growth as analyzed as absorbance of cell density at 595 nm. Biofilm developments were quantified using MTT assay, where absorbance of colored end product was measured at 450 nm and compared with that of control biofilms
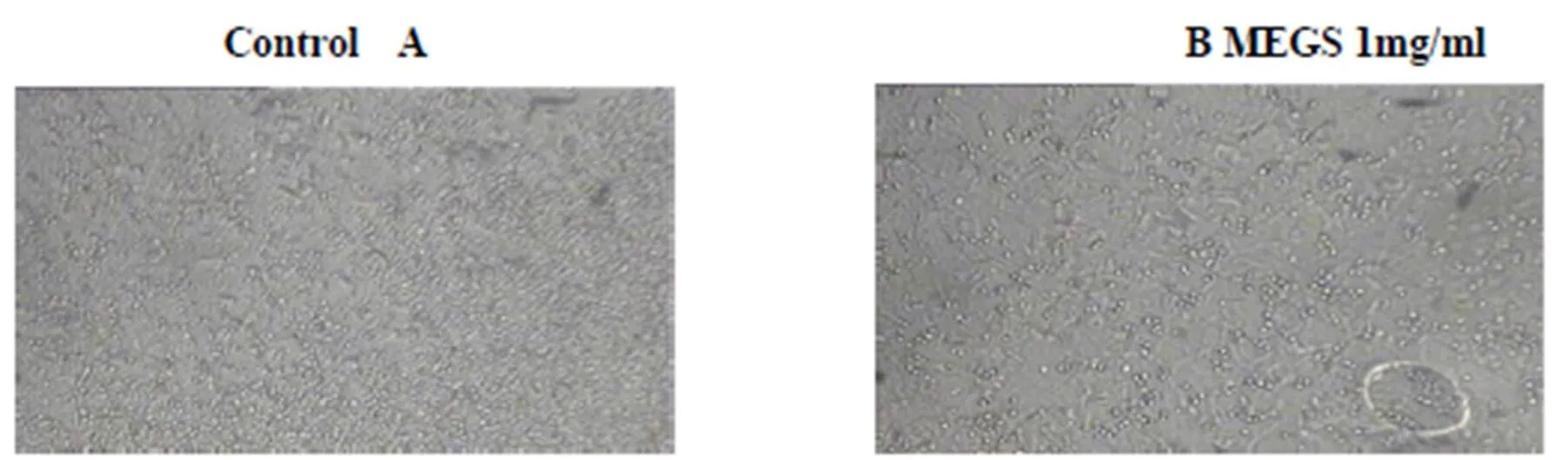
Figure 4 Microphotographs showing inhibition of C. albicans Biofilm development in presence of MEGS. A) Control without MEGS; B) Treatment with 1mg.mL-1 of MEGS; C) Biofilm growth in presence 2mg.mL-1 D) Concentration 4mg/ml of MEGS. The complete inhibition of biofilm and presence of only a layer of yeast form cells in fig. D and lowering in density in fig. C, compared to that of dense network of biofilm in control, fig. A
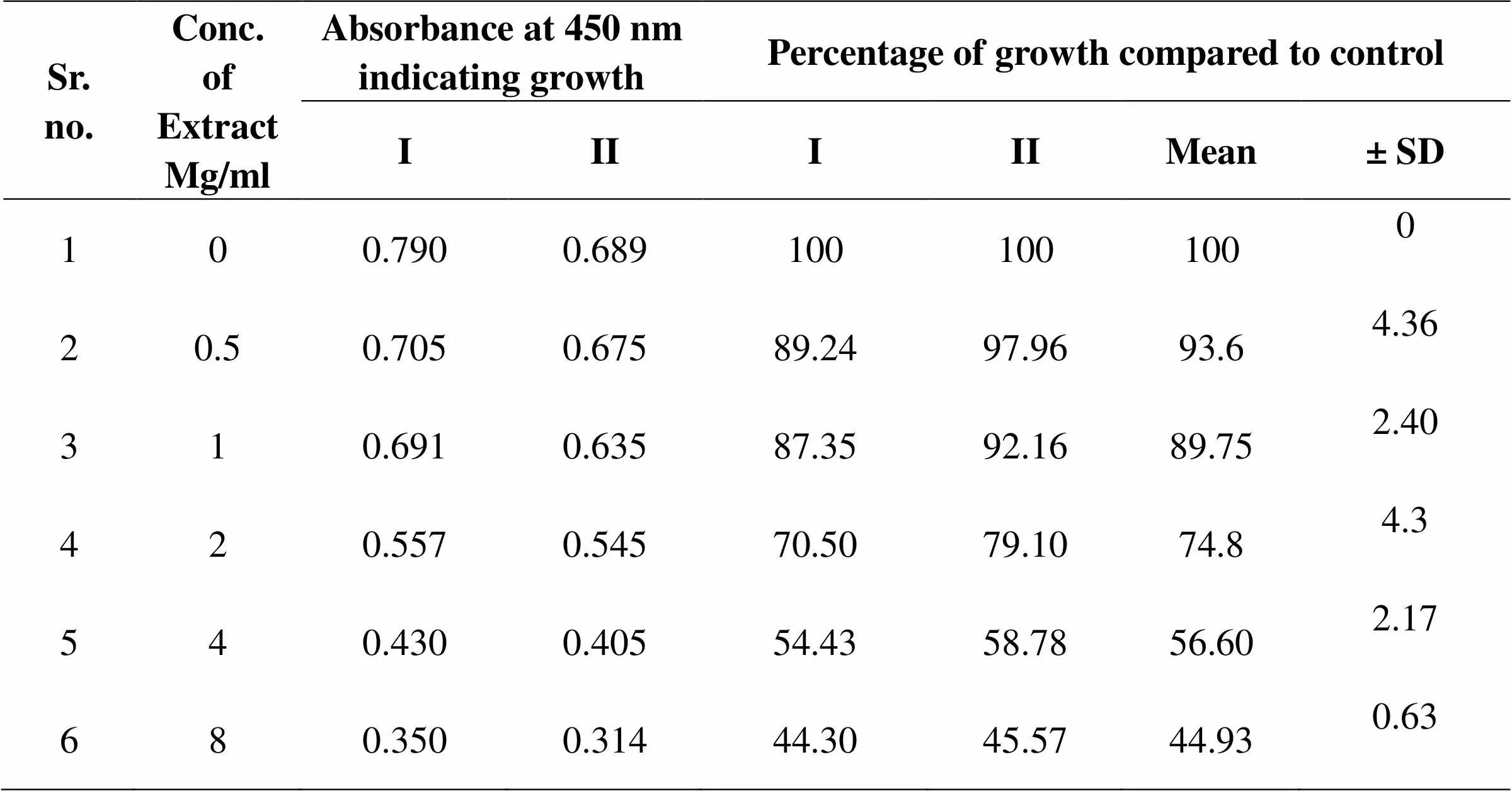
Table 04: Effect of MEGS on Mature Biofilm of C. albicans
Figure 5 Mature of biofilm growth of C. albicans ATCC 90028, in presence of various concentrations of MEEGS. Mature biofilm growth as analyzed as absorbance of cell density at 595 nm. Mature biofilm were quantified using MTT assay, where absorbance of colored end product was measured at 450 nm and compared with that of control biofilms.
Effect of MEGS on Mature Biofilm of

Figure 6 Microphotographs showing inhibition of C. albicans Mature Biofilm in presence of MEGS. A) Control without MEGS; B) Treatment with 1mg/ml of MEGS; C) Biofilm growth in presence 2mg/ml. D) Concentration 4mg/ml of MEGS. The complete inhibition of biofilm and presence of only a layer of yeast form cells in fig. D and lowering in density of growth in fig. C, compared to that of dense network of biofilm in control fig. A
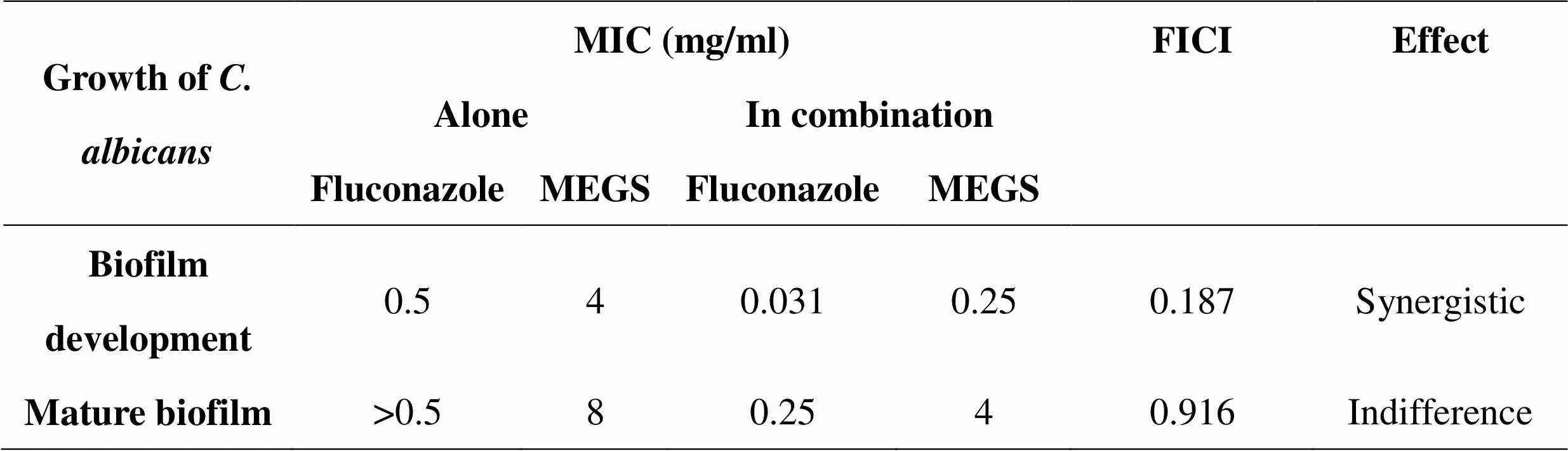
Table 05: Effect of MEGS on Development and mature Biofilm Candida albicans in combination with Fluconazole
Figure 7 Microphotographs showing inhibition of C. albicans biofilm development in combination with Fluconazole. A) Control without MEGS; B) MEGS alone 4mg/ml conc. C) Fluconazole alone >0.5 mg/ml concentration D) Complete inhibition of biofilm presence of Combination (0.25Fluco. + 4 mg/ml MEGS) and the lowering in density of biofilm growth in fig. B, compared to that of dense network of biofilm in control fig. A
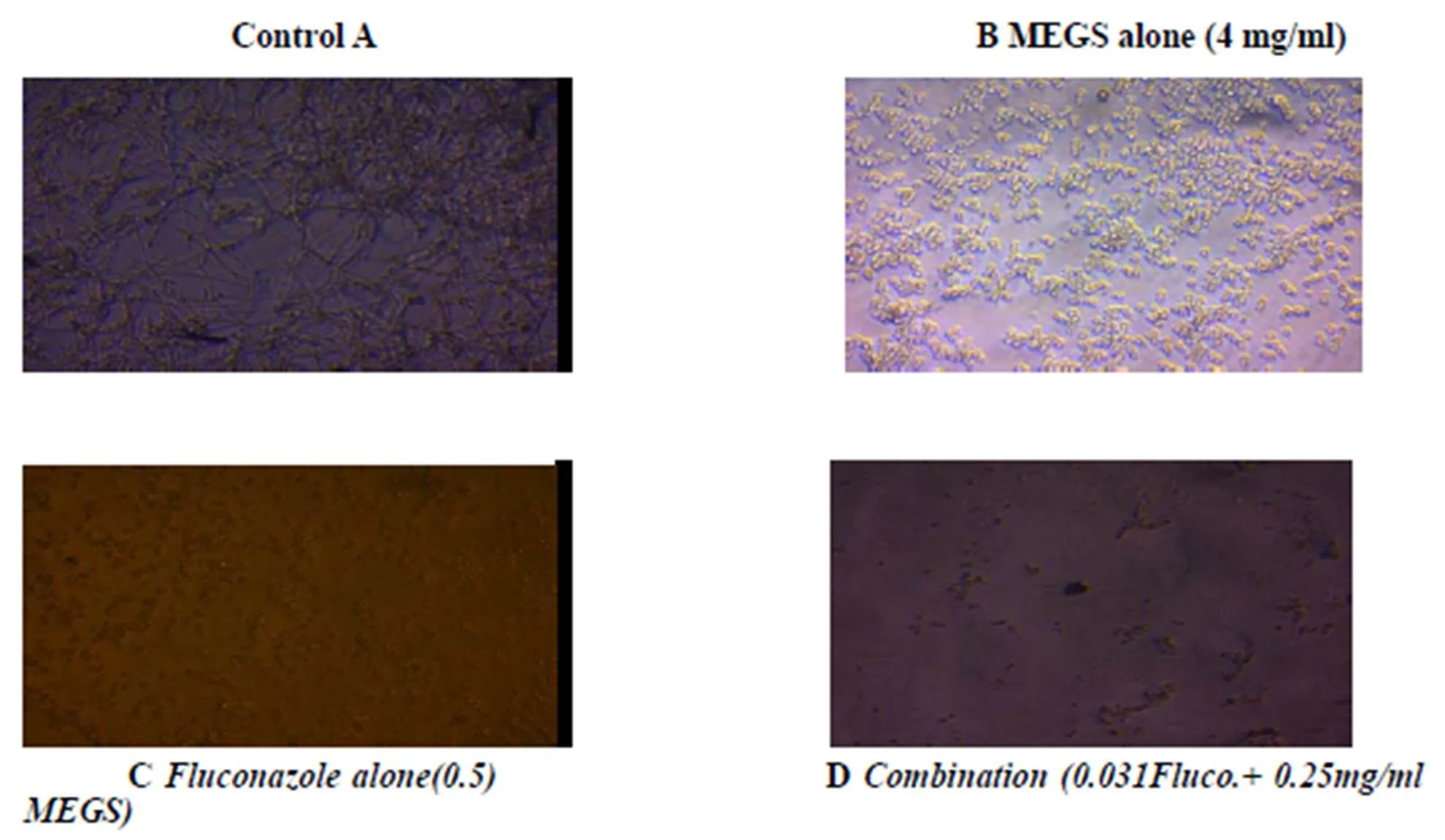
Figure 8: Microphotographs showing inhibition of C. albicans Mature biofilm formation in combination with Fluconazole. A) Control without MEGS; B) MEGS alone 8mg/ml conc. C) Fluconazole alone >0.5 mg/ml concentration D) Complete inhibition of biofilm presence of Combination (0.25Fluco.+ 4 mg/ml MEGS) and the lowering in density of biofilm growth in fig. B, compared to that of dense network of biofilm in control fig. A
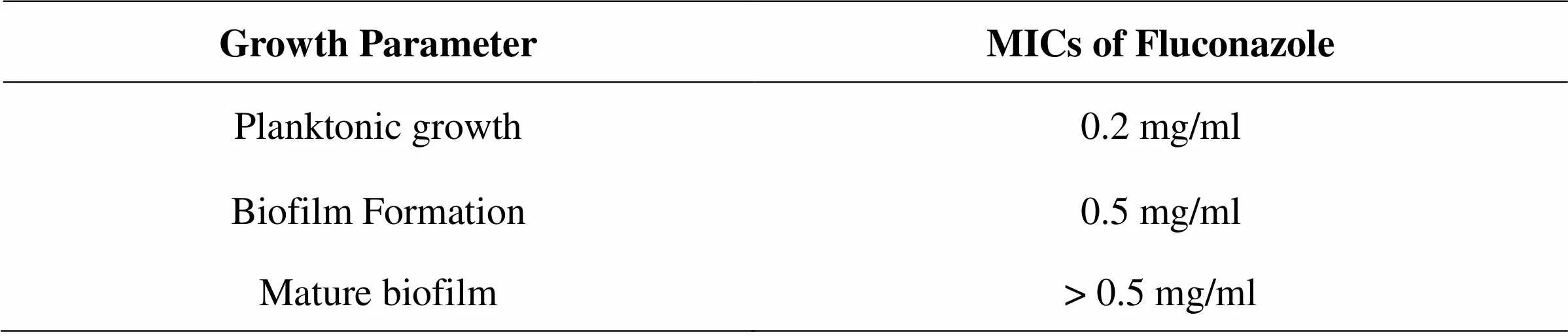
Table 06: Effect of Fluconazole on C. albicans
Discussion
Candida albicans form biofilms on host cells followed by maturation of biofilms, these biofilms of Candida albicans are resistant to most of the currently available antifungal drugs. In the present work extracts of G. superba were evaluated against planktonic, adhesion, developing biofilms and mature biofilms of Candida albicans [7]. The stated work analyzed the potential of Gloriosa superba roots extract in combination with Fluconazole against biofilm development and maturation of C. albicans biofilm. Biofilm inhibitory activities observed were at concentrations less than that of the standard drug, Fluconazole. Results depicted the MEGS and Fluconazole combinations were synergistic effect against biofilm formation. While 0.25 mg/ml concentrations of MEGS dramatically lowered the Fluconazole concentrations required to prevent biofilm formation. It shows the possibility of MEGS to be used as chemo sensitizers to enhance the activity of various standard antifungal. Developments of biofilms were inhibited only at 4 mg/ml concentrations of the MEGS. MEGS-Fluconazole combination against the development of biofilms revealed potential activity at 0.031mg/ml sensitizing the biofilm cells to eradicate it at 0.25 mg/ml concentrations of Fluconazole. Outcome of this in vitro study gives insight into the use of MEGS-Fluconazole combinations for treatment of biofilm associated C. albicans infections. Reduction in the effective dosages of Fluconazole to physiologically relevant concentrations after combination with MEGS would avoid toxicity associated with high concentrations and hence may be of in vivo future research interest.
Conclusion
Use of G. Superba L. alone or in combination with available antifungal drugs would be a novel approach against drug resistant biofilms of C. albicans. In future it will be useful to avoid side effects associated with high dosages and long term usage of the conventional antifungal drugs during anti- biofilm therapy.
1. Senthilkumar, M. Phytochemical Screening and Antibacterial Activity of Gloriosa superba Linn. International Journal of Pharmacognosy and Phytochemical Res 2013, 5, 31-36.
2. Marchetti O, Moreillon P, Entenza JM,Fungicidal synergism of Fluconazole and cyclosporine in Candida albicans is not dependent on multidrug efflux transporters encoded by the CDR1, CDR2, CaMDR1, and FLU1 genes. Antimicrobial. Agents Chemotherapy 2017, 47: 1565-1570.
3. Chandra, J, Kuhn, DM, Mukherjee, PK,2001. Biofilm formation by fungal pathogen Candida albicans development, architecture and drug resistance. J Bacteriol 2015, 183: 5385-5394.
4. Pfaller, MA, Messer SA, Coffmann S. Comparison of visual and spectrophotometric methods of MIC endpoint determinations by using broth micro dilution methods to test five antifungal agents, including the new triazole. J Clin Microbial 2015, 33: 1094- 1097.
5. Raut JS, Chauhan NM, Shinde RB,Inhibition of planktonic and biofilm growth of Candida albicans reveals novel antifungal activity of caffeine. J Med Plants Res 1998, 7: 777-782.
6. Mary A, William AF, Timothy F. Fungal biofilms and drug resistance, emerging infectious diseases 2004, 10: 14-19
7. Gajanan BZ, Archana D, Thakre, SS. Mohan Karuppayil∗Terpenoids inhibit Candida albicans growth by affecting membrane integrity and arrest of cell cycle. Phytomedicine 2011, 18: 1181-1190.
:Somwanshi Sachin M, Department of Pharmacology, School of Pharmacy, Swami Ramanand Teerth Marathwada University, Vishnupuri, Nanded-431606, (M.S.) India. Email: sachinsomwanshi007@gmail.com
Chang Liu
The authors declare that there is no conflict of interests regarding the publication of this paper.
10.12032/TMRIM201903011
Somwanshi Sachin M, Dhawale Shashikant C, Zore Gajanan B,. Effect of Gloriosa Superba in Combination with Fluconazole on Anti-Candida Activity against biofilm Development and Mature biofilm Growth Patterns. TMR Integrative Medicine 2019, 3: e19011.
