Efficacy of entacapone and pramipexole in treating non-motor symptoms of Parkinson's disease: a prospective randomized controlled trial
Zheng Bei ,Guo-Qiang Wen,Yi ChenNing Bei
1 Department of Neurology,Geriatric Hospital of Hainan,Haikou,Hainan Province,China
2 Department of Neurology,Hainan General Hospital,Haikou,Hainan Province,China
Abstract
Key words: Parkinson's disease; entacapone; pramipexole; autonomic neurological symptoms; psychiatric symptoms; paresthesia; non-motor symptoms; prospective self-controlled trials
INTRODUCTION
Background
Parkinson's disease (PD) is a common neurological disease that affects middle-aged and older adult patients.It mainly manifests as static tremor,postural reflex disorder,myotonia,and motor retardation (Buetow et al.,2016; Ikeda et al.,2017;Rodríguez-Violante et al.,2017).In recent years,PD patients have also been found to present non-motor symptoms,such as anxiety,pain,sleep disorders,dysuria or constipation,chest distress,and abdominal pain (Campolo et al.,2016; Pfeiffer,2016; Nagayama et al.,2017; Schaeffer and Berg,2017;Schapira et al.,2017).At present,there is no gold standard pharmaceutical drug for the treatment of non-motor symptoms of PD.Many studies have explored the treatment of non-motor symptoms of PD (Table1).
Entacapone is a selective and reversible inhibitor of the enzyme catechol-O-methyltransferase.Entacapone can inhibit the metabolism of catechol-O-methyltransferase and levodopa in peripheral tissues,thereby increasing plasma levodopa levels and stimulating the production of dopamine to improve PD symptoms (Kuoppamäki et al.,2015; Li et al.,2017; Senek et al.,2017).Pramipexole,a synthetic aminobenzothiazole derivative,can restore the dopamine signal required for normal basal ganglion function in PD patients by directly stimulating dysfunctional dopamine receptors in the corpus striatum(Kvernmo et al.,2006; Schapira et al.,2013; Olanow et al.,2017; Shen et al.,2017; Wang et al.,2017).Pramipexole has been widely used in the treatment of PD in clinical settings and has been found to have strong therapeutic efficacy (Meriç et al.,2014; Silindir and Ozer,2014).
Main objective
In this prospective randomized controlled trial,we will compare entacapone and pramipexole in the treatment of non-motor symptoms of PD.Objective data regarding such differences will be beneficial in the development of treatment protocol for the non-motor symptoms of PD.
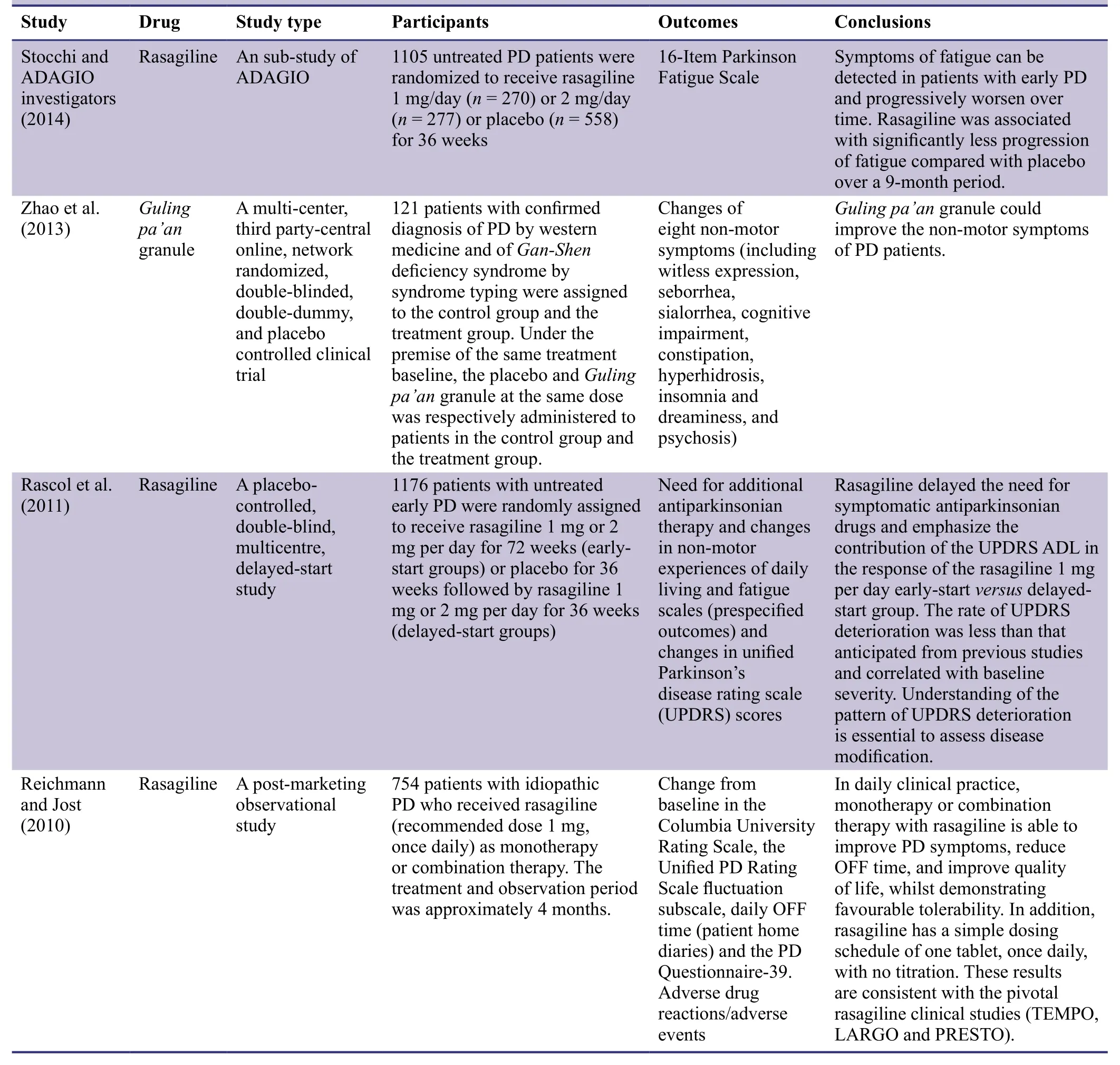
Table1: Clinical trials of the effects of different drugs on non-motor symptoms of Parkinson's disease (PD)
PARTICIPANTS/METHODS
Design
This will be a prospective randomized controlled clinical trial.
Setting
Geriatric Hospital of Hainan,Haikou,Hainan Province,China.
Clinician qualifications
The Geriatric Hospital of Hainan is the only geriatric hospital in Hainan Province.It is a third-tier hospital that specializes in rehabilitation,and is considered to be a base for osteoporosis research by the Chinese Medical Association.It is also a medical cooperative hospital of Beijing Geriatric Hospital,China,a teaching hospital of Hainan Medical University,China,and Hainan Provincial Health School,China,a medical association cooperative unit of the Second Affiliated Hospital of Hainan Medical University,China,and a general training base for medical and nursing personnel.It is the chairman unit of the National Union of Geriatric Hospitals and the vice-chairman unit of the Hainan Health Management Association,China.
The members of the research team have all received doctoral or master's degrees,have been awarded the title of associate chief physician (or higher),and have at least 10 years of clinical experience.
Participants Recruitment
Recruitment notices will be posted on the bulletin board of outpatient clinic and admission office of Geriatric Hospital of Hainan.Interested patients will contact the principal investigatorviatelephone,email or WeChat.
Inclusion criteria
· Accordance with British Brain Bank Diagnostic Criteria for idiopathic Parkinson's Disease (Hughes et al.,1992)
· Provision of accurate records of daily activities
· Age 40 to 75 years,either sex
· Provision of written informed consent
Exclusion criteria
· History of drug abuse
· Schizophrenia or severe cognitive impairment
· Epilepsy
· Severe metabolic dysfunction such as diabetes mellitus· Malignant tumor
· Participation in other clinical trials
Interventions
Patients in the entacapone group will receive 200 mg entacapone (Novartis; import drug registration certificate number:H20160680) 10 times per day for 3 weeks.
Patients in the pramipexole group will receive 1 mg pramipexole (Boehringer-Ingelheim; import drug registration certificate number: 20140918) 3 times per day for 3 weeks.
Outcome measures Primary outcome measure
Degree of improvement of non-motor symptoms 3 weeks after treatment
Non-motor symptoms comprise autonomic neurological symptoms such as postural hypotension,urinary urgency,urinary frequency,sexual dysfunction,dry mouth and salivation,sweating,and cold limbs.Additional non-motor symptoms include constipation,psychiatric symptoms such as cognitive dysfunction,hallucination,depression,and anxiety,as well as sensory abnormalities such as spasm,pain,and restless leg syndrome (Rana et al.,2015).
Secondary outcome measures
· Serum soluble interleukin-2 receptor levels: We will conduct an enzyme-linked immunosorbent assay before and 3 weeks after treatment (Weiland,1978).We purchased a soluble interleukin-2 receptor detection kit from Senxiong BioTech,Shanghai,China.The operation and parameter settings will be in accordance with the kit instructions.
· Serum homocysteine levels: We will conduct an enzymelinked immunosorbent assay before and 3 weeks after treatment (Weiland,1978).We purchased a homocysteine detection kit from Jining Biotechnology,Shanghai,China.The operation and parameter settings will be in accordance with the kit instructions.
· Adverse events: The following adverse events will be assessed: dyskinesia,nausea,abnormal urine color,diarrhea,aggravation of PD symptoms,dizziness,abdominal pain,insomnia,dry mouth,fatigue,hallucination,constipation,dystonia,sweating,hyperkinesia,headache,leg spasm,confusion,nightmare,fall,postural hypotension,vertigo,and tremor.
The participants will be asked to truthfully and accurately complete the adverse event record form (Additional file 1)during the trial to record the time,severity,duration,measures,and outcomes of adverse events.
The research team will assess possible associations between adverse events and the trial drugs.Adverse events will be reported to the ethics committee and medical administration department of Hainan Geriatric Hospital within one working day.Researchers will complete the report form pertaining to serious adverse events and send it to the person in charge of serious adverse events.If serious adverse events occur during the trial,the trial will be terminated,followed by unblinding.The principal investigators will ensure that medical emergencies are properly handled by experts during the trial.In case of emergency medical treatment,patients will be asked to contact the main researcher to start the emergency plan,which will involve immediate termination of the test and administration of the corresponding medical treatment.
The schedule for the primary and secondary outcome measures is listed in Table2.
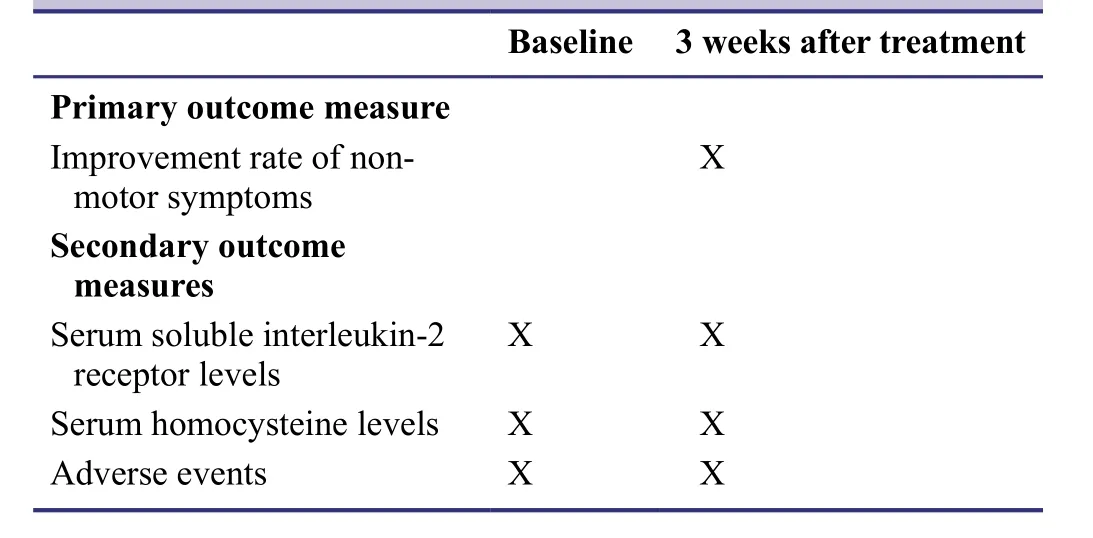
Table2: Timing of primary and secondary outcome measures
Sample size
According to previous results (Zhao et al.,2018),it is hypothesized that the efficacy in the entacapone and pramipexole groups will be 85% and 70%,respectively.Assumingα= 0.05(two-sided) and (1 -β) = 0.90,a final effective sample size ofn= 161 per group will be calculated using the PASS 11.0 software (PASS,Kaysville,UT,USA).Assuming a patient loss rate of 20%,we will require 194 patients per group.
Randomization
We will use the Visual Binning function in SPSS 21.0 software(IBM,Armonk,NY,USA) to generate two groups of random numbers.The patients will be grouped according to the order of enrollment.The data distribution form will be sealed and signed,and kept strictly confidential.
Blinding method
From randomization to database locking,patients,clinicians,evaluators and statisticians will be blind to group allocation.
Ethical approval
This study will be performed in strict accordance with theDeclaration of Helsinkiformulated by the World Medical Association and Standards for Clinical Trial Research in China.This study was approved by the Ethics Committee of Geriatric Hospital of Hainan,China on August 30,2013(approval number: S2013-038-01; Additional file 2).If it is necessary to modify the protocol and the informed consent form after the start of the trial,it will be examined and approved by the ethics committee.The writing and editing of the article will be performed in accordance with the CONsolidated Standards Of Reporting Trials (CONSORT)(Additional file 3).This study was registered with the Chinese Clinical Trial Registry on April 15,2019 (registration number: ChiCTR1900022534).Protocol version is 1.0.
Informed consent
Before recruitment,the clinicians from the study team will explain the study details to the patients and the patients will be asked to sign an informed consent form (Additional file 4).
Statistical analysis
All data will be statistically analyzed using SPSS 21.0 software.Measurement data will be expressed as the mean ±SD.Count data will be expressed as the number of cases and percentages.The incidence of non-motor symptoms at various time points will be compared using the Kruskal-WallisHtest.Serum soluble interleukin-2 receptor and homocysteine levels will be compared using pairedt-tests (normally distributed data) or the Wilcoxon signed rank test (non-normally distributed data).Symptom incidence will be compared between the groups using the Kruskal-WallisHtest.Soluble interleukin-2 receptor and homocysteine levels will be compared between groups using two-samplet-tests (normally distributed data) or the Mann-WhitneyUtest (non-normally distributed data).A value ofP< 0.05 will be considered statistically significant.The per-protocol data set will be used for analysis.
RESULTS
Patient flow chart
A flow chart outlining the experimental intervention is shown in Figure1.A total of 194 eligible patients per group will be included in the final analysis.
Patient recruitment
According to patient admissions at the Department of Neurology,Geriatric Hospital of Hainan,we anticipate that 388 patients will be included in the study by December 31,2020.
Baseline data collection
Prior to recruitment,patient information including sex,age,onset time,cognitive status,past medical history,non-motor symptoms,serum levels of soluble interleukin-2 receptor,and homocysteine will be collected.
Anticipated outcomes
We will collect data regarding the non-motor symptoms,serum levels of soluble interleukin-2 receptors and homocysteine,and adverse events before treatment and during the follow up assessment.
Anticipated possible adverse events
Possible adverse reactions during the trial include dyskinesia,nausea,abnormal urine color,diarrhea,aggravation of PD symptoms,dizziness,abdominal pain,insomnia,dry mouth,fatigue,hallucination,constipation,dystonia,sweating,hyperkinesia,headache,leg spasm,confusion,nightmare,fall,postural hypotension,vertigo and tremor.Any adverse reactions will be reported to the Ethics Committee and Medical Administration Department at the Geriatric Hospital of Hainan within 24 hours.
Small-sample-size pilot study results Baseline information for 100 patients
A pilot study was performed at the Department of Neurology at the Geriatric Hospital of Hainan from January 2014 to May 2017.The pilot study involved 52 men and 48 women aged 51-72 years (mean 66.5 ± 8.3 years).
Improvement of autonomic neurological symptoms and psychiatric symptoms in 100 patients
The results of the pilot study indicated that the incidence of autonomic neurological symptoms such as postural hypotension,urinary urgency,urinary frequency,sexual dysfunction,dry mouth,and salivation,as well as psychiatric symptoms such as cognitive impairment,hallucination,depression,and anxiety were significantly reduced after combined treatment with entacapone and pramipexole (P< 0.05; Table3).
Sensory abnormalities in 100 patients
The pilot study indicated that the incidence of sensory abnormalities such as spasm,pain,olfactory abnormality,and restless leg syndrome was significantly reduced after com-bined treatment with entacapone and pramipexole (P< 0.05;Table4).
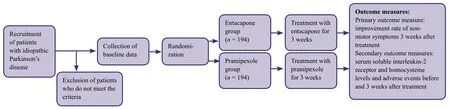
Figure1: Trial flow chart.
Serum levels of soluble interleukin-2 receptor and homocysteine in 100 patients
The pilot study indicated that serum levels of soluble interleukin-2 receptor and homocysteine were significantly diminished after combined treatment with entacapone and pramipexole(P< 0.05; Table5).
DISCUSSION
Study limitations
This trial is a self-controlled study without a control group and with only one follow-up time point.
Generalizability
Non-motor symptoms such as psychiatric symptoms,pain,and autonomic neurological symptoms have a great impact on quality of life in PD patients (Campolo et al.,2016; Pfeiffer,2016;Nagayama et al.,2017; Schaeffer and Berg,2017; Schapira et al.,2017).This clinical trial will focus on non-motor PD symptoms.Although pramipexole has been widely used in the treatment of PD and has shown good therapeutic efficacy(Kvernmo et al.,2006),pramipexole and entacapone have not been compared in terms of efficacy.We plan to simultaneously explore the mechanism of treatment by analyzing the serum levels of soluble interleukin-2 receptor and homocysteine.
Explanation
The incidence of PD is increasing each year.Previous studies have focused particularly on the treatment of PD-related dyskinesia (Lemay et al.,2004; Zhang et al.,2013; Samoudi et al.,2015; Stożek et al.,2016; Kurt et al.,2018),and have directed less attention to non-dyskinesia.Indeed,the treatment of non-motor PD symptoms is still being explored.Previous small-sample-size tests found that the combination of pramipexole and entacapone had clear positive effects on autonomic neurological symptoms such as postural hypotension,urinary urgency,urinary frequency,sexual dysfunction,dry mouth and salivation,psychiatric symptoms such as cognitive dysfunction,hallucination,depression,and anxiety,and sensory abnormalities such as spasm,pain,and restless leg syndrome.Moreover,serum levels of soluble interleukin-2 receptor and homocysteine were also remarkably decreased.
Our study has the potential to produce new information about differences in the efficacy of entacaponeversuspramipexole in the treatment of non-motor symptoms of PD.This information would facilitate future clinical treatment of non-motor symptoms of PD and thus improve patient quality of life.
DATA AUTHENTICITY MANAGEMENT
Data collection
The project leader will complete a case report form for each participant who provides written informed consent.To ensure the authenticity of the data in this trial,all the researchers participating in the trial will be trained on how to complete case report forms.Epi-Data 3.0 software will be used to establish the corresponding entry procedure based on the items in the casereport form.Data will be input using the double entry method based on on-site electronic acquisition and real-time data storage.After data entry,real-time quality control and online error correction will be performed by investigators responsible for data entry using a two-pass verification method.Transparency in the research process will be ensured.The research progress and the existing problems will be managed at any time and rationally solved under the permission of protocol principle.The person in charge will confirm the accuracy and completeness of the case report form.
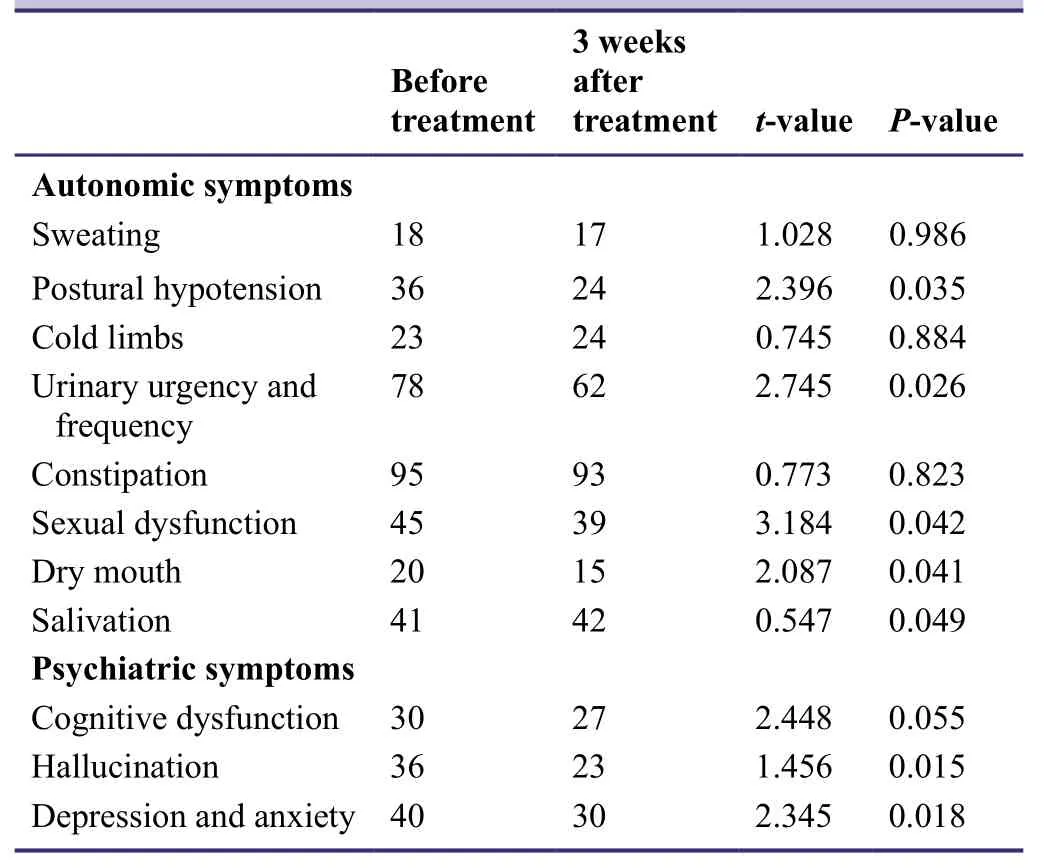
Table3: Effects of entacapone combined with pramipexole on autonomic neurological symptoms and psychiatric symptoms in patients with idiopathic Parkinson's disease
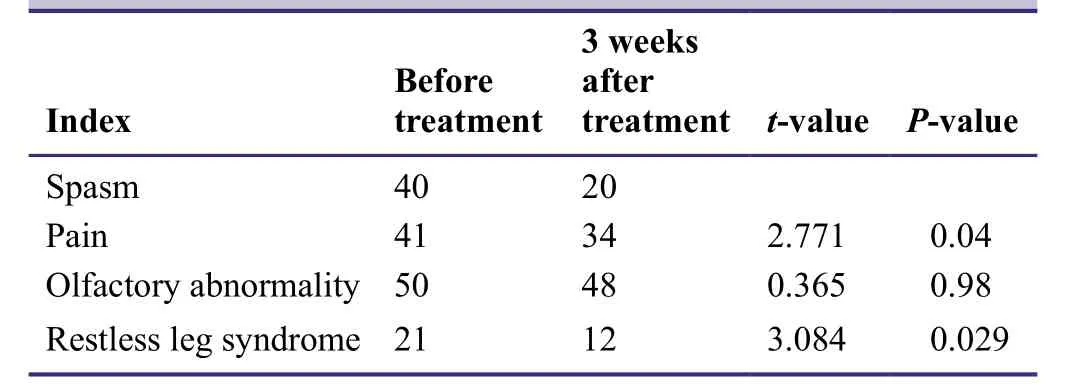
Table4: Effects of entacapone combined with pramipexole on sensory abnormalities in patients with idiopathic Parkinson's disease
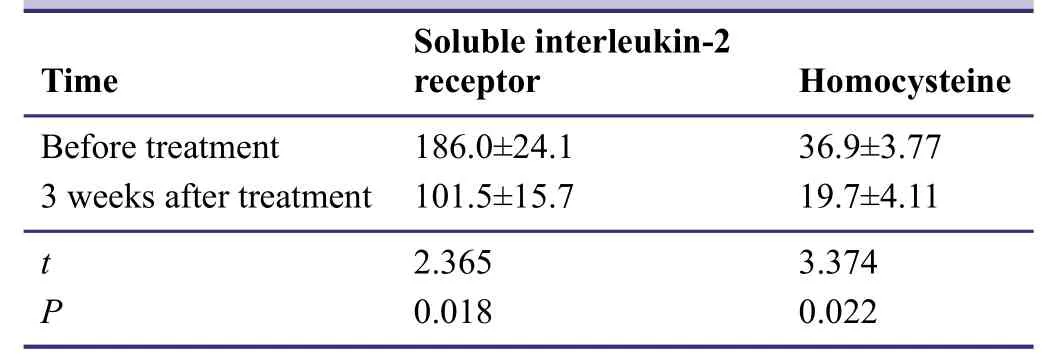
Table5: Effects of entacapone combined with pramipexole on serum soluble interleukin-2 receptor (pM)and homocysteine (μM) levels in patients with idiopathic Parkinson's disease
Data management
The project leader or his/her designated staff will authorize the principal investigator or researcher to access the electronic case report system.Only investigators will have access to the data.
Quality control
An Independent Data Monitoring Committee will include experts in medicine (physicians with relevant professional background),epidemiology,clinical imaging,clinical trial management,statistics,and ethics.Researchers who participate in the trial will carefully implement the standard operating procedures for clinical trials before,during,and after the study.The research group will provide a set transportation subsidy for patients,and medical expenses will be exempted during the treatment and follow-up periods.The trial will provide participants with liability insurance for clinical trials to cover the cost of treatment and financial compensation for participants who suffer from test-related injury or death.
Audits
Clinical inspectors will monitor the quality of the case reports and laboratory records for all participants every six months and will report to the Medical Ethics Committee regarding the trial progress.Trial registration will be updated simultaneously.
Confidentiality
All clinical and other relevant participant data will be recorded in the case report form,which will be kept confidential by a responsible person.Researchers may not disclose information contained in case reports without the written approval of the project leader.
Result release
Results will be disseminated through presentations at scientific meetings and/or by publication in a peer-reviewed journal.
TRIAL STATUS
Registration time:April 15,2019
Recruitment time:June 1,2019-December 31,2020
Study completed:December 31,2021
Trial status:Active,recruiting.
Additional files
Additional file 1: Adverse event record form (Chinese).
Additional file 2: Hospital Ethics Approval (Chinese).
Additional file 3: CONSORT checklist.
Additional file 4: Informed Consent Form (Chinese).
Author contributions
Study design,subject recruitment and data analysis: ZB,GQW,YC,NB.All authors approved the final version of this manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Financial support
None.
Institutional review board statement
This study was approved by the Ethics Committee of Geriatric Hospital of Hainan,China on August 30,2013 (approval number: S2013-038-01).This study will be performed in strict accordance with theDeclaration of Helsinkiformulated by the World Medical Association.This study was registered with the Chinese Clinical Trial Registry on April 15,2019 (registration number: ChiCTR1900022534).Protocol version is 1.0.
Declaration of patient consent
The authors certify that they will obtain all appropriate consent forms from the patients or their legal guardians.In the forms,the patients or their legal guardians will give their consent for patients' images and other clinical information to be reported in the journal.The patients or their legal guardians understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement
This study follows the Consolidated Standards of Reporting Trials(CONSORT) Checklist.
Biostatistics statement
The statistical methods of this study were reviewed by the biostatistician of Hainan General Hospital/Geriatric Hospital of Hainan,China.
Copyright license agreement
The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement
Individual participant data that underlie the results reported in this article,after deidentification (text,tables,figures,and appendices).Data will be available immediately following publication,with no end date.Results will be disseminated through presentations at scientific meetings and/or by publication in a peer-reviewed journal.Anonymized trial data will be available indefinitely at www.figshare.com.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open access journal,and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
 Asia Pacific Journal of Clinical Trials:Nervous System Diseases2019年2期
Asia Pacific Journal of Clinical Trials:Nervous System Diseases2019年2期
- Asia Pacific Journal of Clinical Trials:Nervous System Diseases的其它文章
- Efficacy and safety of traditional Chinese medicine combined with western medicine for early-phase treatment of acute ischemic stroke based on the primary syndrome elements: protocol for a randomized controlled trial
- Effect of transcranial direct current stimulation on the level of consciousness in patients with traumatic coma:study protocol for a self-controlled trial
- Influence of bladder management on long-term quality of life in patients with neurogenic lower urinary tract dysfunction
