Crohn’s disease of esophagus,stomach and duodenum
Andréa Maia Pimentel,Raquel Rocha,Genoile Oliveira Santana
Abstract
Key words: Crohn’s disease; Upper gastrointestinal tract; Upper digestive endoscopy;Esophagus; Stomach; Duodenum; Chronic gastritis; Focal gastritis; Epithelioid granuloma
INTRODUCTION
Crohn’s disease (CD) is a heterogeneous disorder with a multifactorial etiology,including genetic factors,environmental insults and intestinal microbiota,characterized by chronic,segmental and transmural inflammation that affects the gastrointestinal tract and may involve any segment of the oral cavity up to the anus[1,2].This disease was first described in 1932 by Crohn,Ginzburg and Oppenheimer,initially as a disease involving only the terminal ileum,but two years after its initial publication,Crohnet al[3]and Daviset al[4]described that other segments of the gastrointestinal tract could also be affected.Changes in the upper gastrointestinal tract (UGT) were described by Gottlieb and Alpert in 1937,and since then,recognition of gastroduodenal CD has been increasing[5].With the evolution of the digestive endoscopy in recent decades,it has become possible to improve the detection of lesions involving the esophagus,stomach and duodenum.Previously,this evaluation was performed only through radiological studies or surgical specimens.Despite the great advances in the knowledge of CD,the experience with the involvement of the UGT remains limited,even though it is a predictor of recurrence and progression to complications[6].
According to the Vienna classification,involvement of the UGT (L4) is characterized by the involvement of any segment proximal to the lower of the ileum[7].The Montreal classification[8],which was created after the revision of the Vienna classification,the location in the UGT (L4) was considered a modifier of the disease and may or may not be associated with other locations (L1-L3).Patients with proximal CD (L4) often present with evidence of colonic disease or distal small intestine.The involvement of the upper tract in CD represents a risk for complications and surgery[4,9-11].It’s worth pointing out that the contribution of esophagogastroduodenalvsjejunal involvement among patients with proximal disease has not been evaluated[9].
A cross-sectional study has demonstrated that proximal CD (L4) affects younger,nonsmoking patients,is less likely to involve the colon (L2),is more frequently present with concomitant ileal involvement and stenosing behavior,and has a higher probability of having one or more abdominal surgeries.When L4 (with jejunal involvement) and L4 esophagogastroduodenoscopy (esophageal and gastroduodenal involvement) are compared,the former are twice as likely to present ileal disease,stenosing behavior and one or more abdominal surgeries.CD with involvement of the esophagus,stomach and duodenum is diagnosed at an earlier age and has a lower number of abdominal surgeries.In view of these differences,the authors suggest a review of the Montreal classification in order to reclassify the L4 location in L4a(upper tract proximal to the Treitz ligament) and L4b (below the Treitz ligament until proximal to the distal third of the ileum) in adult patients,as is done in the pediatric population[9,12].
The prevalence of lesions in the UGT in symptomatic patients varies from 0.5% to 5%,but more recent observational studies show a higher frequency of endoscopic and histological alterations when upper digestive endoscopy (UDE) is performed routinely as part of the evaluation of the disease,regardless of the presence of gastrointestinal symptoms[13-16].Studies performed in the pediatric population demonstrate that the involvement of the UGT has a higher prevalence,since it is recommended to perform UDE with biopsies of the UGT routinely upon the initial presentation of inflammatory bowel disease (IBD) in this patient group[17-21](Table 1).In a prospective cohort of 1015 adult patients with CD,upper tract involvement occurred in 11.7%,and 60% of them also had concomitant involvement of the ileocolonic region[22].Despite the high prevalence of macroscopic and microscopic findings with the most frequent endoscopy of the UGT,many of them are not specific and present uncertain relevance in clinical practice[23].

Table 1 Studies that evaluated the involvement of the upper gastrointestinal tract in patients with Crohn's disease

CD:Crohn disease; UC:Ulcerative colitis; GI:gastrointestinal; UGI:upper gastrointestinal; IBD:Inflammatory bowel disease.
The European consensus on the diagnosis and management of CD [European Crohn’s and Colitis Organization,(ECCO)] published in 2017 recommends that,irrespective of colonoscopy findings,additional research should be performed to define the location and extent of CD in the small intestine.However,performing routine EDA in adult patients with asymptomatic CD remains controversial[11].In patients with unclassified IBD (10% to 15%),HDE may contribute to the differentiation between CD and ulcerative colitis (UC)[24].
Despite the inclusion of UGT as a disease modifier,there is no objective definition of the endoscopic and histological criteria needed to adequately characterize the involvement of these segments in CD[16].Nugentet al[25],in their series of patients with duodenal CD,defined the following criteria for the diagnosis:the presence of noncaseous granuloma in the duodenum with or without involvement of the lower intestinal tract in the absence of any granulomatous disease or the presence of CD associated with duodenal inflammation consistent with CD.Although this definition does not consider other histological aspects,it can also be applied to the esophagus,stomach and jejunum[26].The ECCO guideline recommends that the diagnosis be confirmed by integrating clinical,endoscopic,histological,radiological and/or biochemical findings[11].
ESOPHAGEAL CROHN’S DISEASE
Esophageal CD is rare and was described in 1950 by Frankly and Taylor[27,28].The prevalence of esophageal CD ranges from 3.3% to 6.8% in the adult population and from 7.6% to 17.6% in pediatric patients,but these data may be underestimated in adults due to the failure to perform HDE in asymptomatic patients[29].Deckeret al[30]reported 20 cases of esophageal involvement (0.2%) among 9900 CD patients,evaluated between 1976 and 1998,of whom 19 (95%) also had active bowel disease.The diagnosis was based on the endoscopic,histological,radiological and clinical impressions of the gastroenterologist.The mean age at diagnosis was 31 years,and the mean interval between the diagnosis of CD and involving the esophagus was 1 year[30].A retrospective observational study conducted at the Mayo Clinic between 1998 and 2012 identified 12367 patients with CD,and 57 of them were submitted to HDE to evaluate UGT symptoms,of which 24 presented esophageal involvement.The mean age at diagnosis was 16 years,and the mean time between diagnosis of CD and esophageal involvement was 3 years.The authors demonstrated that most of the patients were diagnosed with extraesophageal CD before the diagnosis of esophageal involvement.Only 3 cases were limited to the esophagus,and two of them developed extraesophageal CD in the follow-up[29].
DIAGNOSIS
Clinical condition
Patients with esophageal involvement may present with symptoms such as heartburn,regurgitation and chest pain,similar to gastroesophageal reflux disease (GERD),and in more advanced cases,dysphagia,odynophagia,vomiting and weight loss.Less frequent symptoms such as hyperoxia and growth retardation may be present[28-30].In general,other segments of the gastrointestinal tract are affected,but some cases of isolated esophageal involvement are reported in the literature[31-33].The main complications associated with the disease are stenosis with or without obstruction,fistulas and perforation.Rarely,patients present fistulization to the bronchus,pleura,lungs,stomach and mediastinum,as well as abscess formation to adjacent organs[34-37].De Feliceet al[29]demonstrated that 86% of patients with esophageal CD showed symptoms,the main ones being dysphagia (54%),odynophagia (33%) and epigastric pain (33%).Oral ulcers were present concomitant to esophageal involvement in 33%and gastroduodenal involvement in 21%.Similar findings were reported by Deckeret al[30].
Radiology
The radiological study of the esophagus may show mucosal irregularities,ulcers,and polyps,but it is currently more commonly used to evaluate stenoses and fistulas.The most commonly described radiological findings are aphthous ulcers that appear as small particles of barium retained with a halo of translucent mucosal edema[28].Esophageal echoendoscopy may contribute to cases of difficult diagnosis,since it demonstrates the transmural character of the inflammatory process,characteristic of CD[28].
Digestive endoscopy
The endoscopic findings are not specific,being described as friability,erosions,aphthoid ulcers,superficial or deep ulcerations (Figure 1),nodularity and stiffness of the mucosa,cobblestone appearance,inflammation and,less frequently,masses,stenoses and fistulas[28,38,39].Some reports and a series of cases demonstrate that ulcers may have a characteristic appearance called punched-out ulcers,which are larger and deeper than aphthoid ulcers and resemble ulcers caused by herpesviruses and cytomegalovirus[40-42].
Aphthoid ulcers,followed by “punched-out” ulcers and linear erosions,were the main endoscopic findings identified by D’Haenset al[41].The most affected sites are the middle third and the distal third,and there may be,to a lesser degree,the diffuse involvement of the organ and the proximal third[29,30].Deckeret al[30]reported involvement of distal esophagus,middle and distal third and whole esophagus in 80%,15% and 5% of the patients,respectively.The main findings were ulcers in 85%of patients,erythema or erosions in 40%,stenoses in 20%,fistulas in 5% and pseudopolyps in 5%[30].De Feliceet al[29]also demonstrated that the most common sites of involvement were the middle third (29%),distal (29%),medium and distal (17%),diffuse (21%) and proximal (4%).Surface ulcerations were present in 58%,erythema and/or erosions in 50%,deep ulcerations in 13%,pseudopolyps in 4%,stenoses in 17% and fistulas in 8%[29].
Histology
Performing biopsies is critical to warding off other conditions,especially in the absence of involvement of other segments in the CD.It is important to ensure adequate fragments for the analysis,through larger and deeper biopsies,since superficial biopsies contribute little to the diagnosis because it is a transmural disease[37].The most frequent histological findings are chronic inflammatory infiltrate with a predominance of lymphocytes in the lamina propria and presence of ulcers[30].Noncaseous granulomas are rare and observed in fewer than 25% of cases[28,30,38].The granulomas are focal and are located in the deep submucosal and muscle,which could affect their identification[43].D’Haenset al[41],in a series of cases,demonstrated esophageal granulomas in 57% of patients[41].However,this prevalence was different in the studies by Deckeret al[30]and De Feliceet al[29],who found 0% and 21.7%,respectively[29,30].
Differential diagnosis
It is important to pay attention to other conditions that may involve the esophagus,such as viral esophagitis (cytomegalovirus,herpesvirus,human immunodeficiency virus),sarcoidosis,tuberculosis,reflux esophagitis,pill esophagitis,eosinophilic esophagitis,disseminated fungal disease,syphilis,Behçet’s disease,carcinoma,lymphoma,metastasis of melanoma or lung cancer,and epidermolysis bullosa[28,44,45].
TREATMENT
There are no guidelines for the treatment of esophageal CD due to the absence of randomized controlled trials conducted with this goal.Several options are reported in the literature.In general,they are based on experience with the treatment of ileocolonic disease,such as systemic corticosteroids,aminosalicylates,immunomodulators or biological therapy.The more intensive therapy is reserved for moderate to severe disease or refractory to corticosteroids[28].Progressive esophageal dilation with Savary-Gillard or balloon candles is indicated for cases with stenosis with persistent dysphagia[42].Placement of stents may represent an alternative treatment with good efficacy for benign stenoses.Esophagectomy is also an option considered in the disease complicated by fistulas,extensive stenoses,or abscesses and in refractory cases,for which medical therapy was not effective[28].

Figure 1 Esophageal ulcers in a patient with Crohn’s disease.
D’Haenset al[41]demonstrated that esophageal lesions in CD have,in most cases,a good response to clinical treatment but require courses of corticosteroids.They reported three patterns of disease behavior after initial treatment:those responding rapidly to corticosteroid therapy,achieving clinical and endoscopic response without relapse (57.2% of cases); those with persistent lesions despite the therapy (21.4%); and others with initial response and subsequent relapse (21.4%).They point out that“punched-out” ulcers recurred more frequently than aphthoid ulcers[41].Deckeret al[30]demonstrated that,among the 20 patients diagnosed with esophageal CD,90%received corticosteroid therapy,60% aminosalicylates,25% H2 blockers and 25%proton pump inhibitors (PPIs).Esophageal dilation was required in six patients and surgery in three.The authors comment that the use of PPIs resulted in symptomatic improvement,but these drugs did not act in the control of the inflammatory response and should not be used as monotherapy,as is the case for aminosalicylates,since they are activated in the proximal intestine.They observed that half of the patients improved spontaneously or with the use of the first-line therapy,but one of the limitations was the non-accomplishment of HDE to evaluate the healing of the mucosa after the treatment.The remaining patients were treated with immunosuppressants,with a favorable response,and one of them with anti-tumor necrosis factor (anti-TNF) antibody.The authors suggest the use of acid suppressive therapy and corticosteroids in mild disease and immunomodulatory therapy in disease refractory or dependent on steroids[30].De Feliceet al[29]demonstrated that patients who presented inflammatory behavior responded to prednisone,topical budesonide and anti-TNF agents (infliximab and adalimumab).Stenting disease was treated successfully with immunomodulators and anti-TNF agents associated with dilation with or without steroid injection.Intensive treatment of fistulizing esophageal disease with immunomodulators,anti-TNF antibodies,or tacrolimus was not universally effective.Eight patients were treated with anti-TNF therapy,and all had a clinical response with dose adjustment,combination with immunosuppressive therapy or switching to another anti-TNF agent.Esophagectomy was not required[29].Some case reports reinforce the usefulness of biological therapy for the treatment of esophageal CD complicated or refractory to conventional therapy,both with infliximab and with adalimumab,avoiding esophagectomy[46-48].
GASTRODUODENAL CROHN’S DISEASE
Gastroduodenal CD has a prevalence of 0.5% to 4%.The isolated involvement of the stomach and duodenum occurs in less than 0.07% of all cases of CD[13,49-52].A higher frequency of findings is reported in more recent studies,though with different diagnostic criteria.CD involving the duodenum is rare.A case series performed by Nugentet al[25]demonstrated 36 cases of duodenal involvement,which represented 2% of the total population of new CD patients over a 20-year period.The proximal duodenal involvement was accompanied by distal involvement of the stomach in most cases,with a smaller number of patients with isolated involvement of the duodenum or in contiguity with the proximal jejunum[25].
DIAGNOSIS
Clinical condition
In general,the patients are asymptomatic.Clinically symptomatic disease often arises at the same time or after the onset of bowel symptoms[25].The clinical picture includes abdominal pain,which can be localized in the epigastrium,nausea,vomiting,postprandial fullness,anorexia,and fever.The presence of symptoms such as abdominal distension,postprandial vomiting,weight loss,and early satiety may indicate more severe disease,usually due to stenosis obstruction[53].Gastroduodenal involvement usually manifests itself through insidious,gastritis-like symptoms,but there are reports of atypical conditions,with rapid progression to obstructive forms[52,54].Less frequent presentations are upper gastrointestinal bleeding,delayed puberty,and chronic iron-deficiency anemia[49,55].Perforation,a frequent complication in peptic ulcer disease,is rare in gastroduodenal CD[56].Gastroduodenal fistulas are uncommon and usually result from inflammatory activity in adjacent organs such as the transverse colon and ileocolonic anastomosis[26,57].Duodenopancreatic fistula with refractory abdominal pain is rare[58].Duodenal CD may be a cause of acute or chronic pancreatitis due to the inflammatory process and fibrosis in the region of the vater ampulla,with duodenopancreatic reflux or fistulas between the duodenum and the pancreatic duct[26,59,60].
Radiology
With the advancement of the digestive endoscopy,the radiological examinations have become less often used for the diagnostic evaluation of the UGT.The most frequent radiological findings are thickening of the folds,nodularity or cobblestone appearance,ulcerations,asymmetrical irregularities,pyloroduodenal deformity,stenoses,fissures and pseudodiverticula[61,62].The antrum and the duodenal bulb may be rigid and have a reduced peristalsis.The descending duodenum stenosis can be identified through the radiological finding called “string sing”[26].
Gastroduodenal involvement with progressive pyloric stenosis can produce two radiological features:the “pseudo-Billroth I” characterized by the tubular appearance of the antrum,pylorus and duodenum,and the ram’s horn sign,characterized by distensibility of the proximal stomach and rigid and stenotic aspect of the antrum and duodenum[63].The radiological study of the upper tract detects only one third of the gastrocolic fistulas,with barium enema being the examination of choice[56,64].Gastric fistulas often originate from the transverse colon and duodenal fistulae are derived from the colon or anastomosis colonic ileus.Primary gastroduodenal fistulas are rare and generally enterocutaneous[56].The three major radiological patterns of disease in the duodenum,in the case series described by Nugentet al[25],were the contiguous involvement of the proximal antrum and duodenum,followed by the isolated involvement of the descending duodenum and the distal duodenum[25].
Endoscopic aspects
Endoscopic findings are similar to those described in other segments of the gastrointestinal tract,such as edema,enanthem,longitudinal or irregular erosions,ulcers that may be superficial or deep or aphthoid,linear or serpentine (Figure 2);nodularity of the mucosa and thickening or narrowing of the antrum,with reduction of distensibility; “cobblestone” appearance,duodenal stenosis,“notching” of the duodenal folds (Figure 3),protruding lesions in the bulb and second duodenal portion and a “bamboo-joint-like (BJL)” appearance[4,38,49,65,66].The protruding lesions may have a longitudinal arrangement described as “rosary-like protuberant lesions”.Erosions may be circular or longitudinally aligned,usually located in the antrum and difficult to differentiate from other types of gastritis not associated with CD,so that the sensitivity of this finding is low[38].
The places most frequently affected by gastroduodenal CD are the antrum,pylorus and proximal duodenum,while the proximal stomach is usually preserved[64,66].A retrospective cohort study of 138 CD patients submitted to HDE for investigation of high gastroduodenal symptoms or as part of the diagnostic investigation demonstrated that the ratio of specific CD lesions were higher in upper-middle (47.8%),lower (24.6%) and bulb (31.9%)[15].The BJL appearance was first described by Yokotaet al[67]in 1997 and is characterized by linear fissures or grooves that cross the longitudinal folds of the small curvature of the cardia and upper gastric body.Although this finding can be identified by white light,it can be better observed through indigo carmine chromoscopy.A retrospective study by Kuriyamaet al[66]in Japan suggests that the BJL appearance may be a specific finding of CD (sensitivity:44% and specificity:95%).The detection rate was 44% in patients with CD,whereas in patients with UC and GERD,it was 5% and 0%,respectively.Fujiyaet al[68]demonstrated in a case-control study that included 81 CD patients,81 non-IBD patients and 66 UC patients that in those with CD the incidence of BJL in the stomach was higher (P< 0.001),suggesting that this finding may be characteristic of gastroduodenal CD.The accuracy,sensitivity and specificity of this endoscopic finding were 67.9%,38.3% and 97.5%,respectively [odds ratio (OR) 24.49; 95%confidence interval (CI) 5.61-106.85].The authors described that the notch-like appearance and erosions and/or ulcerations had an incidence of 9.9% and 32.1%respectively[68].This endoscopic aspect is also described in patients with the disease in remission[69].
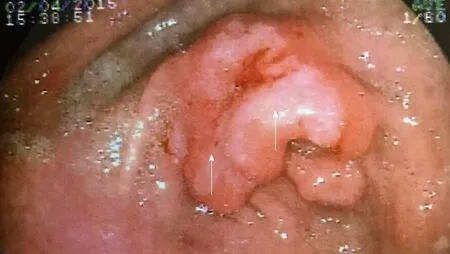
Figure 2 Pre-pyloric gastric ulcer in a patient with Crohn’s disease.
Histology
The main histological findings described in CD of the stomach and duodenum are nonspecific acute and chronic inflammation,focal inflammatory changes,lymphoid aggregates,mucosal-muscular fibrosis,chronicHelicobacter pylori(H.pylori) negative gastritis,focal gastritis,epithelioid granulomas,and duodenitis with or without granulomas.The biopsies should be done in endoscopically normal areas and in altered areas,since the disease presents a focal distribution[64].Removal of several fragments is recommended.
Noncaseous granuloma,although a marker of the disease,has a very variable prevalence in the literature,occurring in 0% to 83% of the cases,since some studies only include histological analysis of biopsies and others of surgical specimens or both[4,52,70].Epithelioid granuloma is characterized by the capacity of secreting such cytokines as IL1,TNF-alpha and TGF-beta[71].Several studies have shown that the presence of epithelioid granuloma may be associated with a worse prognosis,however the results regarding the frequency and clinical significance are conflicting[72-76].In the UGT,granulomas are located more frequently in the stomach than in the duodenum and more frequently in the antrum than in the stomach body[65]and can be detected in both normal-appearing and altered mucosa[4,26].
The prevalence of granuloma detection may vary according to the type and location of the lesions,being identified in less than 25% of the esophageal lesions,7%-87.7% of the gastric lesions,14.3%-45.5% of the lesions with appearance in BJL and 0-49% of duodenal lesions[38].Similar results are reported in the pediatric population[17].One study showed that granulomas occur more in the stomach (9.4%),with a prevalence of 3.4% in the duodenum and 4.9% in both stomach and duodenum.The incidence of granulomas was higher in patients with enterocolonic disease and shorter duration of disease[77].A retrospective cohort study showed that noncaseous granulomas were detected in 10.9% of the patients,mainly in the lower stomach (25%) and duodenal bulb (11.4%).However,this study did not evaluate other histological changes,such as focal gastritis,and not all patients were evaluated for the presence ofH.pylori[15].The variation between these results may be related to the biopsied site,the number of biopsies,the number of cuts performed,the use of serial sections and the pathologist's proficiency[38,78].
A study by Yaoet al[79]evaluated noninflamed gastroduodenal mucosa through histological and immunohistochemical analysis and identified that microaggregates of macrophages and epithelioid granulomas were present only in CD patients and not patients with UC,so that this finding helped differentiate between the two diseases.Microaggregates of macrophages were more frequent than epithelioid granulomas in this population (54.5% × 18.2%,respectively).

Figure 3 Duodenal involvement in a patient with Crohn’s disease and Helicobacter pylori negative.
Focal gastritis is characterized by infiltrating inflammatory cells in a foveolus or small groups of foveolae or gastric glands and has been described in several studies as a finding suggesting gastric CD (Figure 3).Magalhães-Costaet al[80]demonstrated that focal active gastritis and the presence of macrophage microaggregates were associated with CD,onceH.pyloriinfection was excluded.A prospective study evaluating the histology of gastric mucosa in 75 patients with CD of the small intestine or colon demonstrated that focal active gastritis was found in 76% ofH.pylori-negative patients,with a positive predictive value of 97.5%.It was located more frequently in the antrum than in the body.The immunohistochemical study aided in the differentiation between the type of inflammatory infiltrate in active focal gastritis of CD compared to the control group.An important feature was the presence of accumulations of CD68+and CD68R+histiocytes and peripheral or periglandular CD3+lymphocytes,findings not seen in the control group[81].A case-control study conducted by Parenteet al[82]showed that among 94 patients with CD who wereH.pylorinegative,40 (43%) had focal gastritis,compared to 5 of the 52 patients with UA(12%) and 11 of the 57 control patients (19%).Among the patients with focalH.pylori-negative gastritis,only 4 presented granulomas.The main immunohistochemical finding of focal gastritis in CD patients was the accumulation of CD8+and CD4+lymphocytes and histiocytes,with a predominance of CD68+cells,a similar finding in UC,whereas in controls without IBD,there was a predominance of T and B lymphocytes,histiocytes and CD8+lymphocytes.Other authors have also demonstrated that,although active focal gastritis is more common in CD,it may also be present in UC[16,83].The main histological findings described in the BJL appearance are the presence of fissure-like erosions,edema,eosinophils and lymphoid follicles[68].Hirokawaet al[84]demonstrated similar histological,including cleft-like erosions and/or mucosal clefts in 7 (50%) of the 14 CD patients and 1 (20%) of the 5 non-CD patients.Metaplasia,atrophy of fundic glands and abscesses of crypts were not identified.
ChronicH.pylori-negative gastritis is a common finding and has been reported at a high frequency in patients with CD[13,85-87],mainly in young children and adults,while in the general population its prevalence is 2% according to Gentaet al[88].Halmeet al[13]demonstrated an association between the finding of gastric involvement and greater severity of intestinal disease.A case-control study demonstrated that active chronic gastritisH.pylori-negative (OR 11.7,95%CI 7.5-18),focal gastritis (OR 40.8,95%CI 15.5-114.9),and duodenitis (OR 28,2; 95%CI 17.1-46.5) were more frequent in CD than in patients with UC and healthy controls,especially in younger patients[89].Korean pilot study showed that active,chronicH.pylori-negative gastritis was present in 40%of patients,andH.pylorigastritis positive in 25%[85].
The lower prevalence ofH.pyloriinfection in CD patients is a controversial issue[50,64,80].This possible lower frequency may be justified by the prolonged use of medicines such as sulfasalazine,frequent use of antibiotics,socioeconomic distribution or unknown immune and infectious mechanisms[13,87].The interaction with the immune system of the host,acting on the dendritic cells,leading to an upregulation of the inflammatory T cells and,in turn,the reduction of the production of pro-inflammatory cytokines,downregulation of the Th1/Th17 cascade and increased levels of some cytokines are some of the mechanisms proposed to justify the protective role ofH.pyloriinfection[90,91].
A meta-analysis by Lutheret al[90]suggests a beneficial protective effect ofH.pyloriinfection against the development of IBD.H.pyloriinfection was evidenced in 27.1%of patients with IBD and 40.9% of controls [Risk ratio (RR) 0.64,95%CI 0.54-0.75].In the subgroup analysis,a greater effect was observed in the group of subjects with CD(RR:0.60,95%CI 0.49-0.79) compared to UC (RR 0.75,95%CI 0.62-0.90).However,significant heterogeneity of the included studies was observed (I2=5.8%),which limits the interpretation of this association.Another meta-analysis,performed in an Asian population that included 1299 patients with IBD and 1817 controls,showed that 24.9% and 48.3% hadH.pyloriinfection,respectively.The RR ofH.pyloriinfection in patients with IBD when compared to the control group was 0.47 (95%CI 0.43-0.54,P<0.001),without significant heterogeneity (I2=21%).In the group of CD patients,21.3%of patients withH.pyloriinfection were observed,compared to 47.7% in the control group and (RR 0.43; 95%CI 0.37-0.50;P< 0.001),and there was nonsignificant heterogeneity (I2=43%)[92].Despite these results,high-quality controlled studies are needed to confirm these findings.Immunohistochemical study of the duodenum in CD patients demonstrates CD68-positive mononuclear cells that did not change after eradication ofH.pylori[93].
Isolated gastroduodenal CD represents a diagnostic challenge,since the histological analysis of biopsies may only demonstrate nonspecific inflammatory findings,and in some cases,only the study of the surgical specimens confirms the involvement of the UGT[54].The series of cases reported by Nugentet al[25]did not demonstrate granulomas in biopsies taken by endoscopy.In contrast,they were found in the surgical specimen of some patients operated on later,reinforcing that the finding of nonspecific changes as well as normal mucosa does not rule out CD,since the findings are focal[25].It is important to emphasize that the presence of gastric lesions does not always reflect the activity of the disease[38].
Differential diagnosis
The main differential diagnoses are peptic ulcer disease,Menetrier’s disease,Zollinger-Ellison’s syndrome,gastrinoma,tuberculosis,lymphoma,sarcoidosis,gastric syphilis,collagen diseases,plastic lymphoma,and amyloidosis[49,52,64].Intestinal tuberculosis should always be remembered in the differential diagnosis,especially in underdeveloped or developing countries where the incidence and prevalence of the disease are high.The main location in the intestinal tract is the ileocecal region,followed by the jejunum and colon.Gastric tuberculosis is rare (0.4%-2%) and is usually associated with pulmonary tuberculosis or immunodeficient states.Clinical symptoms are nonspecific and must be confirmed endoscopically.The main lesions described are ulcers located along the small curvature and pylorus,hypertrophic lesions and pyloric stenosis,with distortion of the anthropological region.Duodenal involvement is also rare,occurring in 2%-2.5% of all cases of gastrointestinal tuberculosis,the most common location being the third duodenal portion.The pattern of involvement may be intrinsic or extrinsic,ulcerative,hypertrophic or ulcerohypertrophic,with formation of fistulas or stenoses[45].
TREATMENT
The literature lacks controlled studies evaluating the effects of drugs available for the treatment of CD in the UGT,so treatment is based on the concomitant activity of the distal disease and clinical experience.PPIs relieve symptoms but have no effect on the chronic inflammatory process and therefore should not be used as an isolated therapy[50].Initial treatment of active gastroduodenal CD includes the use of systemic corticosteroids associated with PPIs.Immunomodulators such as 6-mercaptopurine and azathioprine are used to maintain corticoid remission in dependent or symptomatic patients who remain symptomatic despite corticosteroids[51,94].Aminosalicylates,even those with proximal release,have no benefit and can cause worsening of symptoms[50,64].
The use of infliximab for gastroduodenal CD has been reported in the literature for the treatment of complicated disease,such as in cases of refractory duodenal ulcers,duodenal stenosis and pancreatic duodenum fistulas,with good results[95-99].The ACCENT I study included 43 subjects with gastroduodenal CD out of a total of 573 participants (8%),of whom 2 (56%) responded to therapy at week 2[100].Adalimumab is another treatment option with a satisfactory response,in the most severe cases and with complications,according to some case reports[101,102].Annunziataet al[14]demonstrated in a prospective study that among 19 patients with gastroduodenal CD,72.7% of infliximab- or adalimumab-treated patients achieved mucosal healing at endoscopy 12 wk after the start of treatment compared to 12.5% of patients treated with conventional therapy,although this study was not designed with the objective of evaluating treatment efficacy.
Short pyloric or duodenal stenoses are treated through balloon dilatation,with a low risk of complications such as perforations (1%-2%).In general,there is a high rate of recurrence of obstructive symptoms,and repeated dilatations are required to treat them properly and avoid surgery[50,103].A retrospective study by Guoet al[104]evaluated the safety and efficacy of endoscopic balloon dilations for the treatment of stenoses in 24 patients with gastroduodenal DC associated with obstructive symptoms,less than 4 cm,not associated with fistulas or abscesses.They demonstrated that the procedure was safe but had unfavorable long-term effectiveness.The main predictor of longterm response was the absence of a new intervention in the first month after initial dilatation.Systematic review by Hassanet al[105]evaluated the efficacy and safety of endoscopic dilations.A total of 353 stenoses were dilated in 347 patients,the majority(66%) in anastomoses of the colonic ileum and only 3% in the UGT.Endoscopic dilation avoided future surgical interventions and was a safe method with a low complication rate (2%).Stenoplasty can be used as an option in the treatment of gastroduodenal obstruction,but few studies have compared their results with bypass surgery[94].This procedure may have serious complications such as ruptured anastomosis,persistent obstructive symptoms and restenosis at the site of previous stenoplasty[57].
The first reports of upper limb involvement cases in the prebiological therapy era and before the introduction of digestive endoscopy as a diagnostic method demonstrated that surgical treatment was the main option in the management of more advanced disease and with complications[106].The main surgical indications are fistulas,stenosis refractory to dilation,ulcers not responsive to medical therapy and persistent high digestive hemorrhage,with excellent results.The most commonly used techniques are bypass with gastrojejunostomy,gastroduodenostomy and duodenojejunostomy.Gastrojejunostomy with highly selective vagotomy is the surgery of choice for reducing the number of complications[64].
Vagotomy may reduce the incidence of anastomotic ulcers,but it increases the chance of diarrhea in these patients and has a controversial indication.Laparoscopic gastrojejunostomy is an option for the treatment of obstructive gastroduodenal disease,with lower morbidity,shorter recovery time and shorter hospitalization[107].In a retrospective study of 54 patients with gastroduodenal CD treated between 1958 and 1997,Yamamotoet al[57]demonstrated that surgical treatment was performed in 33 patients (61%),with gastroduodenal obstruction (30 patients) being the most frequent indication,followed by duodenocutaneous fistula in 2 patients and massive bleeding in 1 patient.Bypass surgery was the procedure of choice,but it had a high incidence of postoperative complications,such as anastomotic leakage (13%),enterocutaneous fistula (6%),intraabdominal abscess (13%),anastomotic obstruction(25%),stoma (13%),small bowel obstruction (6%),and persistent obstructive symptoms (19%),and of the need for reinterventions.
CONCLUSION
The prevalence,complications,therapeutic and prognostic implications of involvement of the esophagus,stomach and duodenum in CD has not been thoroughly elucidated to date.UDE and histological study are useful diagnostic methods for the adequate identification and characterization of the lesions that can affect these segments,but these results should be interpreted with caution,staying aware of other conditions that can mimic this disease.Despite the inclusion of upper tract involvement as a disease modifier in the Montreal classification,routine upper gastrointestinal endoscopy is not indicated for assessing the extent of the disease in adult patients without UGT symptoms,as the clinical impact of this conduct is uncertain.Prospective studies may help to define the real contribution of upper gastrointestinal endoscopy in CD patients and to define the endoscopic and histological criteria necessary to better characterize the involvement of the UGT in these patients.
 World Journal of Gastrointestinal Pharmacology and Therapeutics2019年2期
World Journal of Gastrointestinal Pharmacology and Therapeutics2019年2期
- World Journal of Gastrointestinal Pharmacology and Therapeutics的其它文章
- Nutritional status as a predictor of hospitalization in inflammatory bowel disease:A review
