硅化钴的简单固相合成与磁学性能
朱炳龙 陆铃鲸 戴伟城 张赐阳 方礼炜 王 迪 王佳健
(江苏理工学院化学与环境工程学院,常州 213001)
0 Introduction
In the past few decades,transition metal silicides have been received much attention for their unique range of properties such as high melting point,corrosion resistance,low density,low electrical resistivity and high resistance to oxidation[1-3].Thus,transition metal silicides have a wide range ofapplication including high temperature heating materials, interface diffusion barriersand large scale integrated (ULSI)circuit technology.Among them,cobalt monosilicide(CoSi)is a promising candidate for thermoelectric devices at medium temperatures (between 200 and 500℃)for its electrical conductivity (5.0×105S·m-1at 300 ℃)and relatively good Seebeck coefficient(-87 μV·K-1)[4-6].In addition,CoSi materials exhibit excellent activity in hydrogenation of naphthalene[7].
Up to now,several synthetic methods of cobalt monosilicide materials have been reported. CoSi nanowires and nanocables have been synthesized through chemical vapor deposition processes[8-11].CoSi nanowires on a silica support can also be obtained by metal organic chemical vapor deposition of Co(SiCl3)(CO)4[12].Microwave activated combustion synthesis is an effective method for the preparation of cobalt silicide intermetallic compounds[13].CoSi nanomaterials can be obtained by mechanical milling bulk CoSi for only four hours or by mechanical alloying of pure elements for twelve hours[14].Vertically aligned cobalt silicide nanowire arrays have been synthesized via a solid-state reaction[15].Zhang et al.have synthesized cobalt silicides using metal vapor vacuum arc ion source implantation[16].Nanostructured CoSi has been obtained by spark plasma sintering[17].
In contrast to the traditional synthetic methods,solid state reactions carried out in a stainless-steel autoclave provide a convenient and environmentally friendly pathway for synthesis of non-oxide compound nanocrystals such as carbides[18-20],nitrides[21-26],silicides[2-3],borides and other non-oxide nanomaterials under mild conditions[27-31].Here,we prepared CoSi simple phase by a simple solid-state route.CoSi particles with high crystallinity and yield have been prepared by metallic Mg,Co3O4and Si in a stainless-steel autoclave at 750℃.The reaction process can be represented as following:
4Mg+Co3O4+3Si=3CoSi+4MgO
1 Experimental
1.1 Synthesis
All reagents used in our experiments were analytical pure grade,purchased from Shanghai Chemical Co.(China).In a typical synthesis procedure,Si powder(0.28 g),Co3O4(0.80 g),and metallic Mg(2.40 g)were mixed and put into a 20 mL stainless-steel autoclave.Then the autoclave was sealed and heated from room temperature to 750℃ at a rate of 10℃·min-1and kept for 10 h.After the autoclave was naturally cooled to room temperature,the raw product was washed with ethanol,distilled water and hydrochloric acid(0.50 mol·L-1)to remove the impurities.The precipitate was washed with distilled water and ethanol for 3 times.The final sample was dried in vacuum at 50℃over night for further characterization.
1.2 Characterization
Powder X-ray diffraction measurements were carried out with a Philips X′pert X-ray diffractometer(Cu Kα λ=0.154 178 nm,voltage of 40 kV,current of 40 mA,scan range of 10°~80°)at room temperature.The scanning electron microscopy (SEM)images were taken by using a field-emission scanning electron microscope(FESEM,JEOL-JSM-6700F,voltage of 5 kV).The transmission electron microscopy(TEM)images and highresolution transmission electron microscopy(HRTEM)were taken on a JEOL-2010 transmission electron microscope with an accelerating voltage of 200 kV.Thermogravimetric analysis (TGA)profile was collected with a Shimadzu-50 thermoanalyzer apparatus under flowing air from room to 1 000℃.
2 Results and discussion
2.1 Crystalline phase and structure analysis
XRD was employed to investigate the crystalline phases and structures.A typical XRD pattern of the obtained sample was shown in Fig.1.All peaks in the XRD pattern could be indexed as cubic CoSi with lattice constant a=0.445 5 nm,which was very close to reported data (PDF No.50-1337).No diffraction peaks of any otherphaseswere detected in the XRD pattern,indicating the high purity of main product.The strong and sharp diffraction peaks suggested that the asprepared CoSi was well crystallized.
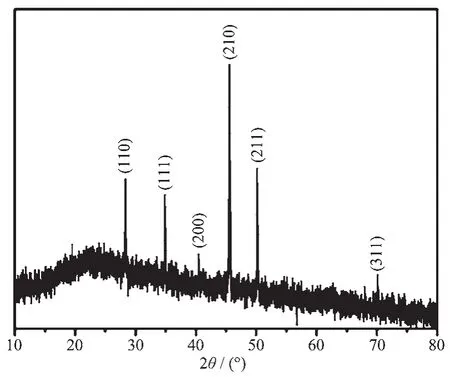
Fig.1 XRD pattern of the obtained CoSi sample
2.2 Morphologie and structure analysis
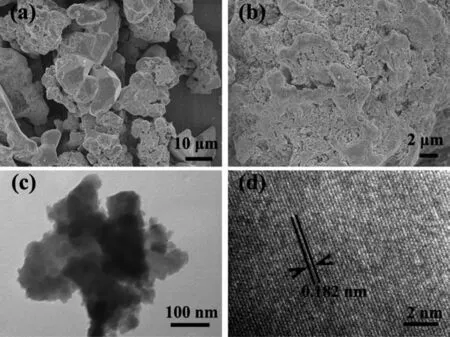
Fig.2 (a,b)SEM images,(c)TEM image and(d)HRTEM image of the CoSi sample
The morphologies and structures of the obtained CoSi sample were investigated by SEM,TEM and HRTEM.Fig.2(a,b)indicated that the obtained CoSi sample was composed of microparticles and aggregation of nanoparticles.A typical TEM image of the obtained CoSi sample (Fig.2c)showed that the size of the nanoparticles was about 20 nm.The lattice spacing was about 0.182 nm in Fig.2d,which was in agreement with the spacing d(211)of cubic phase CoSi.
2.3 Oxidation resistance analysis
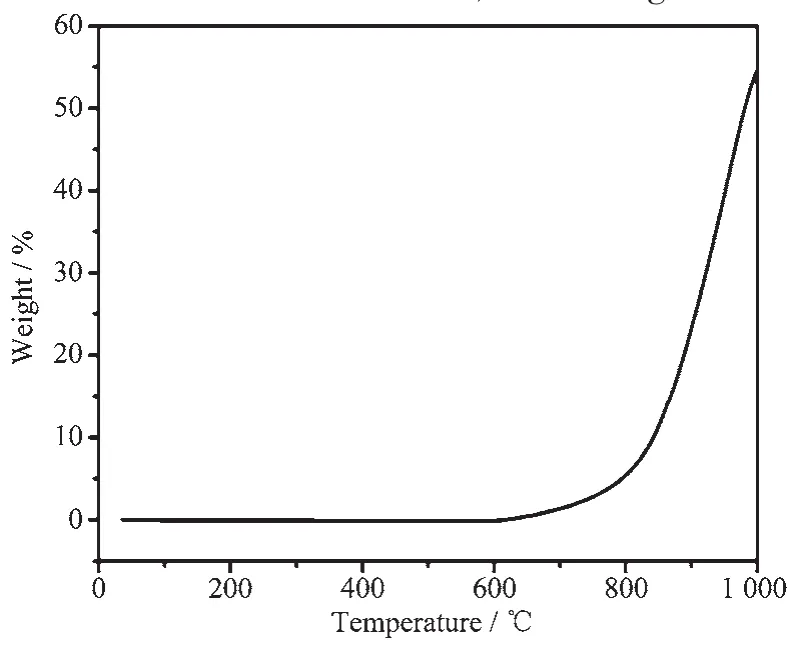
Fig.3 TGA curve of the obtained CoSi sample
Asto the materials,the oxidation resistance determined their application conditions.Therefore,it is important to investigate the oxidation resistance of the obtained CoSi sample.Fig.3 showed the thermogravimetric analysis(TGA)of the the obtained CoSi sample.The TGA curve in Fig.3 showed that the weight of the product did not change under 600℃.When the temperature was over 600℃,the weight increased significantly,which indicated the CoSi sample was oxidized by oxygen to form oxides.According to the theoretical calculation,the weight increment was about 55.17%by converting the CoSi into CoO and SiO2completely.The weightincrementofthe sample(54.06%)was below the theoretical calculating value,which ispossibly attributable to imperfect oxidation.Therefore,the CoSi sample obtained in this route had anti-oxidation under 600℃.As the literature reported[32],cobalt monosilicide (bulk material)is stable under 750℃,since the bulk material has a lower surface energy.Because of the smaller size(~20 nm in diameter)of the as-prepared CoSi nanocrystals,the surface energy of CoSi nanocrystals was very higher in contrast with the bulk material.It could be oxidized by oxygen at relatively lower temperature than that of bulk materials.
2.4 Magnetic behavior
The magnetic behavior of the materials is important for their practical applications.The magnetic hysteresis curve for the obtained CoSi sample at room temperature is shown in Fig.4.The hysteresis loop of the CoSi sample exhibited ferromagnetic behavior.It could be seen that the magnetization,remanent magnetization and magnetic coercivity of the obtained CoSi sample were 4.18 emu·g-1,0.71 emu·g-1and 30.77 Oe.
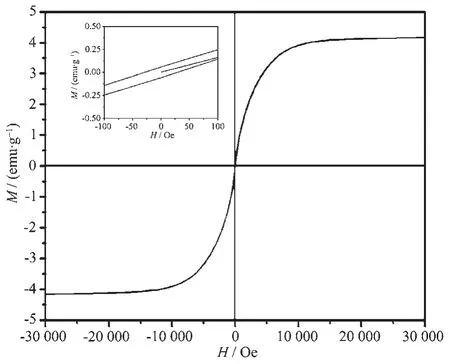
Fig.4 Magnetic properties of the obtained CoSi sample at room temperature
2.5 Formation mechanism
In our experiment,the overall reaction may proceed via a reduction route,which can be described as follows:
4Mg+Co3O4→4MgO+3Co
Co+Si→CoSi
All the total reaction process can be represented as follows:
4Mg+Co3O4+3Si→4MgO+3CoSi
It was found that the reaction temperature had significant influence on the formation of CoSi in our experiments.CoSi could not be detected at the reaction temperature of 700℃in the product.Therefore,the minimun reaction temperature for preparing CoSi was about 750℃.When metallic magnesium was replaced by metallic sodium,CoSi could not be obtained through the similar process.And CoSi could not also be obtained through the similar process when Co3O4was replaced by CoO or Co2O3.
3 Conclusions
Cobalt monosilicide was synthesized by a solidstate route at a relatively low temperature.The CoSi sample exhibited ferromagnetic behavior,and had good thermal stability and oxidation resistance below 600℃in air.This simple route may provide new insights into the synthesis of other transition metal silicides.

