Protective action of glutamine in rats with severe acute liver failure
Elizângela G Schemitt,Renata M Hartmann,Josieli R Colares,Francielli Licks,Jéferson O Salvi,Cláudio A Marroni,Norma P Marroni
Elizângela G Schemitt,Renata M Hartmann,Josieli R Colares,Francielli Licks,Jéferson O Salvi,Cláudio A Marroni,Norma P Marroni,Laboratory of Experimental Hepatology and Gastroenterology,Hospital de Clínicas de Porto Alegre,Porto Alegre 90040060,Brazil
Elizângela G Schemitt,Renata M Hartmann,Josieli R Colares,Francielli Licks,Jéferson O Salvi,Norma P Marroni,Laboratory of Oxidative Stress and Antioxidants,Universidade Luterana do Brasil,Canoas 92425900,Brazil
Abstract
Key words:Thioacetamide;Cytokines;Oxidative stress;Inflammation;Liver failure;Chemical and drug induced liver injury;Glutamine
INTRODUCTION
Severe acute liver failure (SALF) is a complex and rare syndrome characterized by rapid deterioration of liver function,usually in patients without underlying liver disease[1,2].Management includes intensive and comprehensive supportive care,but the only effective treatment to date is liver transplantation[3].
The xenobiotic thioacetamide (TAA) has been used to induce hepatic damage in animals,and constitutes an effective experimental model to study the mechanisms involved in the pathophysiology of SALF and investigate potential therapies thereof.
Several studies have implicated oxidative stress and inflammation as critical events involved in experimental SALF[4-9].
Reactive oxygen species (ROS) are generated during the process of oxygen metabolism and play several important physiological roles,including signal transduction,defense against microbial pathogens,and gene expression to support cell growth or cell death signaling pathways[5,10].Reactive nitrogen species (RNS),in turn,are derived from nitric oxide (NO),which is producedviainducible nitric oxide synthase (iNOS) activity[5,6].Because of their unique chemical characteristics,ROS and RNS can trigger lipid peroxidation and cause DNA strand breaks and protein oxidation,resulting in cellular injury.Oxidative and nitrosative stress represents an imbalance in the production and elimination of these reactive species and a decrease in the production of antioxidants[5,11,12].
The antioxidant defense system is essential for cell protection.Antioxidant enzymes such as superoxide dismutase (SOD),catalase (CAT),and glutathione peroxidase(GPx),as well as non-enzymatic electron receptors such as glutathione (GSH),are affected by oxidative stress conditions[12,13].The nuclear factor erythroid 2-related factor 2 (Nrf2) is an important regulator of redox balance.Under physiological conditions,Nrf2 is stores in the cytoplasm in inactivated form,bound to Kelch-like ECH-associated protein 1 (Keap1).Under stress conditions,Nrf2 dissociates Keap1 and is translocated to the nucleus,where it promotes expression of cytoprotective target genes,such as NADPH quinone oxidoreductase1 (NQO1),antioxidant enzymes,and phase-II detoxification enzymes such as glutathione S-transferase(GST)[5,14-16].
Inflammation is a key component of several liver diseases.The inflammatory process leads to parenchymal damage,which progresses to fibrosis,liver cancer,and,occasionally,SALF[17].Metabolites of hepatotoxic drugs bind to a toll-like receptor 4(TLR4) complex that activates nuclear factor kappa B (NFκB),triggering the production of pro-inflammatory cytokines,such as tumor necrosis factor-α (TNF-α),interleukin (IL)-1β,and IL-6,and modulation of anti-inflammatory cytokines such as IL-10[12,14,17,18].Furthermore,it can stimulate production of the enzymes cyclooxygenase-2 (COX-2) and iNOS,both of which act as inflammatory mediators[19].
Many isolated compounds have been investigated for their potential to eliminate oxidative stress and mitigate the inflammatory process in liver diseases.Within this context,glutamine (Gln) has been investigated due to its important role in a wide range of metabolic pathways.It is indispensable for nucleotide,glucose,and protein synthesis.It is a precursor of GSH and plays a key role in the immune defense of the gut mucosal barrier,participating in immunoglobulin formation[20,21].Several studies in humans have shown that Gln modulates the expression of genes related to the activation of pathways involved in several disease states[22-25].Furthermore,studies conducted in different experimental models have demonstrated beneficial effects of Gln on the rat liver,bowel,and stomach[21,26-30].
Within this context,the present study aimed to evaluate the effect of Gln as an antioxidant and its role in the inflammatory process in an experimental model of IHAG induced by TAA.
MATERIALS AND METHODS
Ethical considerations
This study was conducted at the Animal Experimentation Unit and the Laboratory of Experimental Hepatology and Gastroenterology,Hospital de Clínicas de Porto Alegre,after approval from the Institutional Animal Care and Use Committee(opinion no.CEUA 15-0175).
Animal handling followed the ethical principles for animal experimentation mandated by current Brazilian legislation (Law no.11794/2008),the standards of the Brazilian Council for the Control of Animal Experimentation (CONCEA),the State Code for Animal Protection,and established local procedures for the care and use of animals in experimental research.
Experimental procedures
Twenty-eight male Wistar rats (mean weight 300 g) were used in this study.During the experiment,the animals were housed in boxes (47 cm × 34 cm × 18 cm) lined with wood shavings,in a 12-h light/dark cycle,and a controlled temperature of 18 to 22°C.Water and feed were given ad libitum.The animals were randomized in four groups (n= 7 each):control (CO),control plus glutamine (CO + G),TAA,and TAA plus glutamine (TAA + G).
IHAG was induced by intraperitoneal administration of two doses of TAA,400 mg/kg in normal saline solution (0.9% NaCl),with an 8-hour interval between doses[8].The glutamine-treated groups received intraperitoneal Gln (Sigma Chemical®,St.Louis,MO,United States),25 mg/kg in 1 mL 0.9% NaCl.The first dose was administered 30 min after the last dose of TAA;the second and third doses of Gln were administered 24 and 36 h,respectively,after the start of the experiment.
At 48 h,the animals were weighed and anesthetized by intraperitoneal injection of a mixture of ketamine hydrochloride (95 mg/kg) and 2% xylazine hydrochloride (8 mg/kg).Blood was then collected from the retro-orbital plexus with a glass capillary tube and placed into a heparin-containing test tube to prevent coagulation.
After blood sampling,animals were euthanized by anesthetic overdose (three times the therapeutic dose,as per CONCEA guidelines)[31].Upon confirmation of death,the abdominal region was shaved and disinfected,a midline ventral laparotomy was performed,and the liver was removed in sections for storage and subsequent analysis.One liver fragment was submerged in 10% formaldehyde solution for 24 h for histological examination,while another fragment was frozen -80 °C for other analyses.
Histological examination of hepatic tissue
Tissue samples were fixed in 10% formalin and embedded in paraffin.The resulting paraffin blocks were then placed in a Leitz®1512 microtome and cut into sections 3 μm thick.The slides were then dipped in hematoxylin and eosin (HE) for 5 min each and placed in a running water bath.For dehydration,the slides were run through a graded ethanol series,followed by two xylol baths.Finally,coverslips were mounted with Canada balsam.Slides were examined under a light microscope coupled to a digital camera.Images were captured using Image-Plus software (Media Cybernetics®,Bethesda,United States) at a magnification of 200×.Hallmarks of acute liver injury were scored on a scale of 0 to 3,where 0 = normal hepatic tissue;1 = mild damage with scant inflammatory infiltration;2 = moderate damage and infiltration;and 3 = severe damage with infiltration and loss of hepatic architecture.
Spectrophotometric analysis of biochemical parameters
Hepatic integrity was assessed by measurement of the liver enzymes aspartate aminotransferase (AST),alanine aminotransferase (ALT),and alkaline phosphatase(ALP) in plasma,using the commercially available Liquiform Labtest® kit (a kinetic spectrophotometric assay).Protein content in liver homogenate was determined by Bradford's method[32].Lipid peroxidation was investigated by the thiobarbituric acidreactive substances (TBARS) method[33].Levels of GSH were evaluated[34],as well as activity of the antioxidant enzymes catalase (CAT)[35]and glutathione peroxidase(GPx)[36]and of the phase-II detoxification enzyme glutathione S-transferase (GST)[37].Production of nitric oxide metabolites (NO2/NO3) was measured indirectly by the Griess reaction[38].
Western blot analysis of protein expression
Cytoplasmic and nuclear extracts were prepared from liver homogenates[39].The supernatant fraction was collected,aliquoted,and stored at -80 °C.Protein content was determined by Bradford's method[32].Lysed proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes[40,41].The membranes were then blocked with 5% skim milk in Tris buffer containing 0.05% Tween (TTBS) for 1 hour at room temperature and probed overnight at 4 °C with anti-Nrf2 (57 kDa),anti-Keap1 (69 kDa),anti-NQO1 (31 kDa),anti-SOD (32 kDa),anti-TLR4 (95 to 120 kDa),anti-NFκB(65 kDa),anti-COX-2 (21 kDa),and anti-iNOS (120 kDa) antibodies (Santa Cruz Biotechnology,Santa Cruz,CA,United Staes),diluted 1:200 to 1:1000 with TTBS in dehydrated milk at 5%.Primary antibodies were detected with HRP-conjugated antirat IgG,anti-rabbit IgG,or anti-goat IgG secondary antibodies (Santa Cruz Biotechnology,Santa Cruz,CA,United States).Protein detection was performed with a commercially available electrochemiluminescence kit (Amersham Pharmacia Biotech,Little Chalfont,Bucks,England)[42].Density of the specific bands was quantified with imaging densitometry software (Scion Image,Scion Corporation,Frederick,MA,United States).
Multiplex analysis
Levels of IL-1,IL-6,IL-10,and TNF-α were measured in hepatic tissue homogenates using a MILLIPLEX™ MAP Rat Cytokine bead-based multiplex assay kit (RCYTO-80K,Millipore,Billerica,MA,United States).The MILLIPLEX™ MAP method is based on Luminex®xMAP™ technology.All procedures were performed in accordance with manufacturer recommendations.Tissue specimens were diluted 1:5 in sample diluent and incubated in duplicate overnight with TNF-α,IL-1,IL-6,and IL-10 capture beads.The beads were subsequently washed and incubated for 2 h with biotin-conjugated detection antibody,and then for 30 min with streptavidin-phycoerythrin.Bead fluorescence was read in a Luminex 100 IS Multiplex BioAssay analyzer.Cytokine concentrations were determined from these readings using four standard curves.Results were expressed as pg/mL.
Statistical analysis
Quantitative data were expressed as mean ± SE.Groups were compared by one-way analysis of variance (ANOVA).Differences in means were located by the Student-Newman-Keuls procedure.Data were analyzed in program GraphPad InsTat 3.1,and significance was accepted atP< 0.05.The statistical methods of this study were reviewed by Ceres Andréia Vieira Oliveira,from the Regional Statistical Council of Rio Grande do Sul and Santa Catarina,Brazil.
RESULTS
Histopathological analysis
On histological examination of H&E-stained slides at 200× magnification (Figure1),disruption of the hepatic parenchyma was observed in the TAA group,with inflammatory infiltration,massive necrosis,and ballooning;none of these features occurred in the CO or CO + G groups.Photomicrographs of liver tissue from rats in the TAA + G group show regeneration of the hepatic parenchyma,with a decrease in inflammatory infiltration and a substantial reduction in ballooning and spotty necrosis.
Assessment of hepatic cellular integrity
Liver function tests (Table1) showed increased levels of liver enzymes in TAA group animals,which decreased significantly when Gln was administered (P< 0.05).
Assessment of oxidative stress
Assessment of lipid peroxidation by the TBARS method (Figure2) revealed a significant increase in the TAA group in relation to the CO and CO + G groups (P<0.001).Again,significant reductions were observed in the TAA + G group (to which Gln was administered) as compared with the TAA group (P< 0.001).
Nuclear expression of Nrf2 (Figure3A) was decreased in the TAA group as compared with the CO and CO + G groups (P< 0.01),and increased in the TAA + G group as compared with the TAA group (P< 0.05).Conversely,expression of the inhibitory cytoplasmic protein Keap1 (Figure3B) was increased in the TAA group as compared to both control groups (P< 0.001) and significantly reduced in the TAA + G group when compared to the TAA group (P<0.01).
Expression of both NQO1 (Figure4A) and SOD (Figure4B) was decreased in the TAA group compared to the CO and CO + G groups (P<0.001 andP< 0.05,respectively) and increased in the TAA + G group as compared to the TAA group(P<0.01).
GSH levels (Figure5) were statistically lower in the TAA group than in both control groups (P< 0.01),but increased significantly in the TAA + G group (P< 0.01).
Table2 shows the findings of analysis of activity of the antioxidant enzymes CAT and GPx and the detoxification enzyme GST.GPx enzyme activity was increased in the TAA group as compared with the CO and CO + G groups (P< 0.001),and decreased in the Gln-treated group as compared with the TAA group (P< 0.01).CAT activity exhibited the inverse behavior;it was lower in the TAA group than in the control groups (P< 0.01) and higher in the TAA + G group than in the TAA group (P< 0.01).GST activity increased in the TAA group (P< 0.001) and decreased in the Glntreated group (P< 0.001),which is consistent with its detoxifying role.
Assessment of the inflammatory process
Cytoplasmic expression of TLR4 (Figure6A) and nuclear expression of NFκB (Figure6B) were significantly increased in the TAA group as compared with control groups(P< 0.001),and significantly decreased in the TAA + G groupvsthe TAA group (P<0.001).
Levels of the proinflammatory cytokines IL-1β,IL-6,and TNFα (Table3) were higher in the TAA group than in both control groups,CO and CO + G (P< 0.001).These levels decreased significantly in the TAA + G groupvsthe TAA group (P<0.01).In contrast,levels of the anti-inflammatory cytokine IL-10 were decreased in the TAA group in relation to the CO and CO + G groups (P< 0.001) and increased in the TAA + G groupvsthe TAA group (P< 0.001).
Expression of both COX-2 (Figure7A) and iNOS (Figure7B) was increased in the TAA group compared to the CO and CO + G groups (P< 0.001) and decreased in the TAA + G group as compared to the TAA group (P< 0.01).
Assessment of NO levels through their metabolites (nitrites and nitrates) (Figure8)revealed a significant increase in the TAA groupvsthe CO and CO + G groups (P<0.001) and a significant decrease in the TAA + G group as compared with the TAA group (P< 0.05).
DISCUSSION
SALF is a syndrome that causes a marked decline in hepatocyte function,leading to multiple organ failure and leads to an extremely high mortality rate due to several etiologies:drug-induced liver injury (DILI),xenobiotics,viral hepatitis,diseases metabolic,vascular and autoimmune.In many situations the cause remains unknown.The therapeutic armamentarium available to prevent or treat SALF is still very limited[18].Studies using TAA as an inducer of tissue damage have shown that this xenobiotic leads to different degrees of liver injury depending on the dose and timing of administration.This study aimed to evaluate the damage caused by acute administration of TAA in rats and assess the effects of Gln on the Nrf2-mediated antioxidant pathway and on chemical mediators of inflammation.
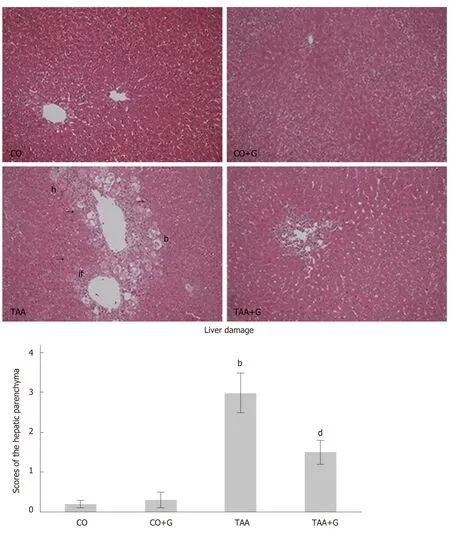
Figure1 Effect of glutamine on liver injury in animals exposed to an experimental model of severe acute liver failure.Representative photomicrographs;original magnification,200 ×.Hematoxylin and eosin (HE) stain.In the Thioacetamide (TAA) group,there is visible disruption of the hepatic parenchymal architecture,with inflammatory infiltration,hemorrhage,ballooning,and massive necrosis.The glutamine-treated group (TAA + G) exhibits a reduction of these parameters and restructuring of the hepatic parenchyma.There were no visible tissue changes in the CO and CO + G groups.CO:Control;G:Glutamine;TAA:Thioacetamide.If:Inflammatory infiltrate;h:Hemorrhage;b:Ballooning;n:Necrosis.Values expressed as mean ± SE.bP < 0.001,TAA group vs groups CO and CO + G;dP < 0.01,TAA + G group vs the TAA group.
We observed extensive destruction of the hepatic parenchyma with necrosis,spotty hemorrhaging,leukocyte infiltration,and ballooning in the group of animals administered TAA.These findings are consistent with previous studies that reported hepatic tissue damage in experimental models using TAA[43-45].In the present study,Gln was able to mitigate changes in the parameters of interest,thus restoring the hepatic parenchyma.Sellmannet al.showed that Gln contributed to a reduction of inflammatory infiltration in the livers of mice with non-alcoholic steatohepatitis induced by a liquid Western-style diet (WSD)[28].Hartmannet al.found that Gln reduced hemorrhagic spots,necrosis,and inflammatory infiltration in the liver of rats subjected to intestinal ischemia-reperfusion injury[21].
Similar results were found on analysis of hepatic cell integrity.Gln reduced serumlevels of AST,ALT,and ALP,while significantly increased levels of these enzymes were found in animals in the TAA group,suggesting massive hepatic impairment after exposure to the xenobiotic.In other studies,Gln was also able to decrease serum levels of these enzymes,thus corroborating our findings[7,14,46].
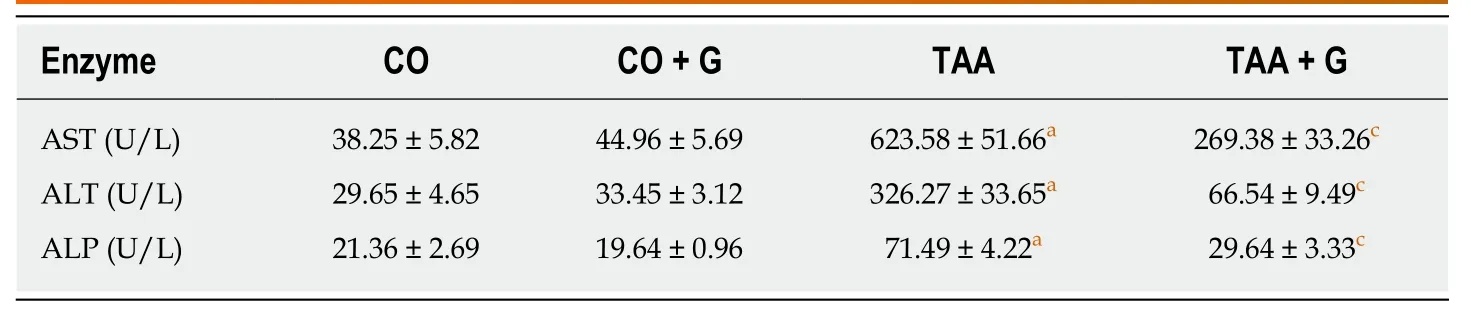
Table1 Effect of glutamine on hepatic integrity levels in rats with severe acute liver failure
We also observed an increase in TBARS levels in the livers of animals in the TAA group,which is indicative of increased lipid peroxidation.Other authors found similar increases in TBARS in studies that used TAA to induce liver damage[6,7,43].Again,Gln administration was able to reduce these levels in our animals,corroborating the findings of several other studies that have demonstrated its protective effect in different experimental models[21,26,47].
Cellular stress mediators activate Nrf2,which is translocated to the nucleus and binds to DNA through a region known as the antioxidant response element (ARE).This promotes expression of cytoprotective target genes,including antioxidant and detoxification enzymes[5].In the present study,Gln promoted increased nuclear expression of Nrf2,whereas cytoplasmic expression of its inhibitory protein Keap1 was downregulated.Gln also promoted increased expression of the NQO1 protein and the antioxidant enzyme SOD.Similar results were reported in a study that evaluated expression of the Nrf2/Keap1 antioxidant pathway,NQO1,and SOD in the liver of rats subjected to intestinal ischemia and reperfusion[14].
GSH is considered an important marker of the antioxidant defenses of the cell.High doses of TAA lead to liver damage because it is biotransformed into a rapidly reacting metabolite that causes an imbalance of the glutathione redox cycle[44].In this study,Gln was able to increase GSH levels in liver tissue,demonstrating its protective role.Similar results were observed by Cruzatet al.,who studied the antioxidant effects of Gln in endotoxemic animals[47].
The first line of antioxidant defense is represented by the antioxidative enzymes,such as CAT and SOD.In this study,CAT activity was decreased in the TAA group in relation to both control groups.This reduction may reflect the redox imbalance induced by stress resulting from xenobiotic administration.Conversely,CAT activity was increased in the group which received Gln treatment.Other studies using TAA-induced liver damage models have demonstrated increased CAT activity in animals treated with berberine and α-lipoic acid[19,46].
GPx enzyme activity was increased in the TAA group and decreased in the Glntreated group.The proper balance of antioxidative enzyme activity plays an important role in cellular protection against oxidative stress.GPx acts in an attempt to reduce molecules with oxidative potential,especially hydrogen peroxide and hydroperoxides,which may explain its increased activity in the group of animals that received TAA.Similar results regarding GPx activity were reported in other studies that used the experimental model of TAA-induced SALF and evaluated the antioxidant action of vitamin E and melatonin[6,7].
GST is an important cellular detoxification enzyme that works in tandem with GSH to remove toxic metabolites.In this study,GST was increased in the TAA group,and Gln treatment was able to reduce its activity.Other authors have reported similar increases in GST in other hepatotoxicity models[7,48].The protective action of Gln in this experimental model may be due to the fact that it is a precursor for GSH synthesis.
The link between oxidative stress and inflammation has been recognized in many disease states,including those which affect the liver.ROS stimulate signaling molecules such as TLR4 to activate inflammatory mediators,including NFκB,which upregulates the production of inflammatory cytokines (such as IL-1β,IL-6,and TNFα)and other mediators of inflammation (such as iNOS and COX-2)[46].

Figure2 Assessment of lipid peroxidation in the liver of rats with severe acute liver failure.Values expressed as mean ± standard error.aP < 0.05 TAA group vs groups CO and CO + G;dP < 0.01 TAA + G group vs the TAA group.CO:Control;G:Glutamine;TAA:Thioacetamide.
In this study,expression of TLR4 and NFκB in the TAA group was increased in relation to both control groups.Similar results were reported in another study that evaluated TLR4 signaling pathways in a model of TAA-induced hepatic fibrosis and liver carcinogenesis[49]and in a study that evaluated NFκB activity in an LPS-induced model of SALF[18].Gln was able to reduce TLR4 and NFκB expression,thus demonstrating its effectiveness in blunting the inflammatory process.A decrease in inflammatory modulators was also observed in other experimental studies that used allopurinol and Gln as treatments[14,50,51].
In the present study,levels of the proinflammatory cytokines IL-1β,IL-6,and TNFα increased while those of the anti-inflammatory cytokine IL-10 declined in animals that received TAA as a hepatotoxicant.Gln reversed the inflammatory process,as demonstrated by a decrease in levels of IL-1β,IL-6,and TNFα and an increase in levels of IL-10 in liver tissue after its administration.A previous study using the TAA model of liver injury also demonstrated an increase in levels of proinflammatory cytokines in animals that developed TAA-induced liver damage[46].Another study reported a similar anti-inflammatory action of Gln in the liver of animals subjected to experimental endotoxemia[47].
Activation of the TLR4/NFκB pathway promotes COX-2 production.In this study,COX-2 expression was increased in the group that received TAA and decreased in the group that received TAA followed by Gln as treatment.In another study of TAA-induced hepatotoxicity,the authors observed a similar increase in COX-2 expression,followed by decreased expression after treatment with allopurinol[50].
As a hepatotoxicant,TAA may induce production of iNOS and,consequently,NO synthesis,which further increases inflammation in the liver.In this study,we observed overexpression of iNOS and an increase in NO (as measured by levels of its nitrite and nitrate metabolites) in the liver of animals with TAA-induced SALF.Gln was able to reverse these parameters,reducing iNOS expression and nitrite and nitrate levels.In a study using the intestinal ischemia-reperfusion model,the authors observed a similar pattern of iNOS expression and NO levels in the liver of animals which Gln treatment[14].
Gln is an essential amino acid for several cell types,including hepatocytes.The mechanisms underlying the protective effects promoted by Gln include the antioxidant of GSH,which is dependent on a supply of glutamate—this,in turn,is synthesized from Gln.In addition,its protective role in the inflammatory process has been widely reported[47].Gln can attenuate oxidative stress and inflammation,consequently protecting against a variety of mechanisms of cell and tissue injury.In humans,Gln supplementation has been used in clinical practice especially in diseases of the gastrointestinal tract with daily doses that can reach 20 g,being administered preferably parentenally.
In conclusion,the results of this experimental study demonstrate that Gln administration had a protective role on the Nrf2-mediated antioxidant pathway and on inflammation activated by the TLR4/NFκB pathway in rats with TAA-induced SALF.As the molecular pathways involved in the pathophysiology of SALF are manifold and complex,further experimental studies are warranted to investigate other potential mechanisms of Gln action in this setting.

Table2 Effect of glutamine on enzyme activity of catalase,glutathione peroxidase,and glutathione S-transferase in rats with experimental severe acute liver failure

Table3 Effect of glutamine on levels of proinflammatory cytokines in the liver of rats with experimental severe acute liver failure
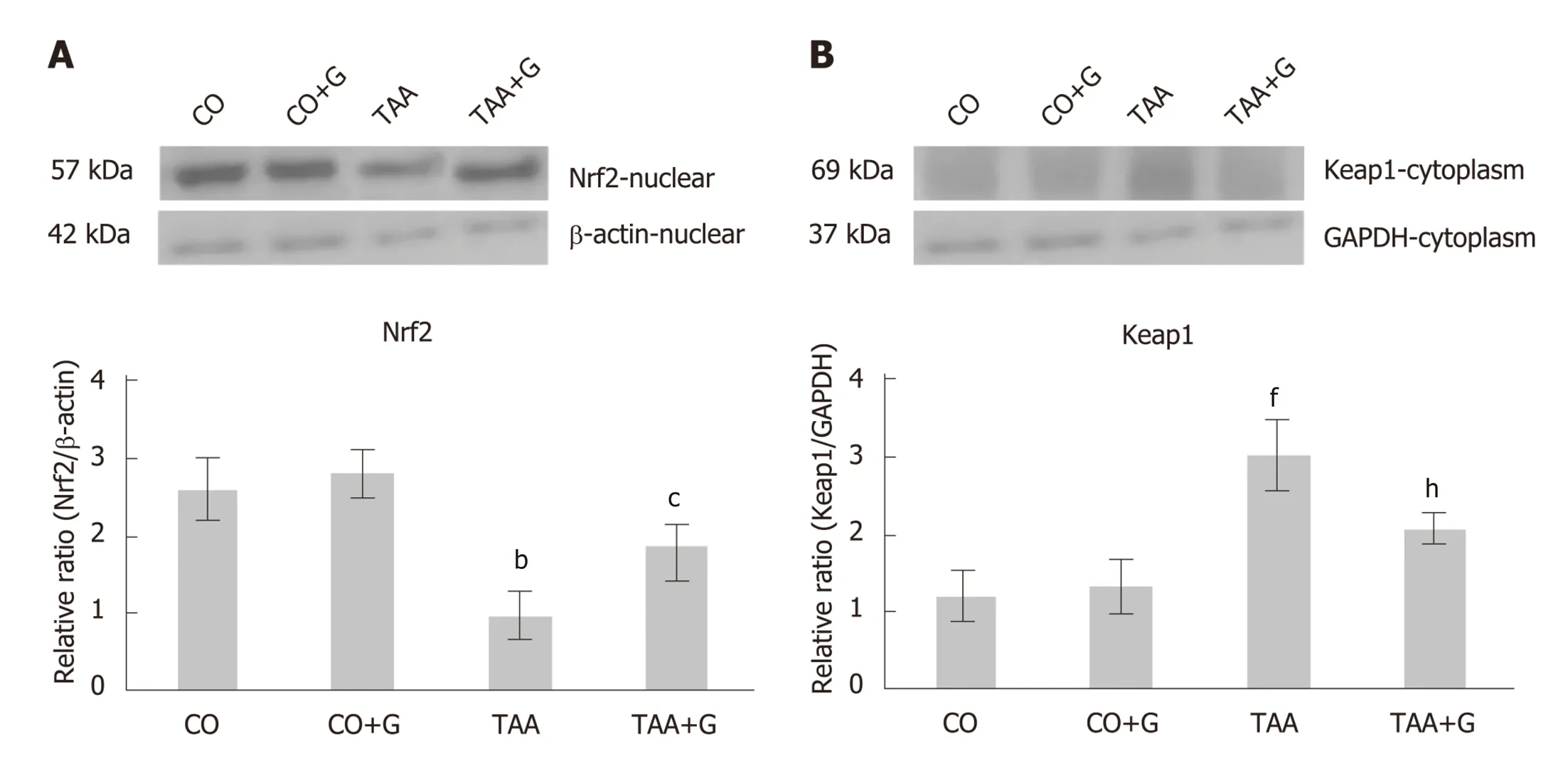
Figure3 Effect of glutamine in experimental severe acute liver failure.Western blot analysis of protein expression of (A) Nrf2 and (B) Keap1.Values expressed as mean ± standard error.bP < 0.01 TAA group vs groups CO and CO + G;cP < 0.05 TAA + G group vs the TAA group;fP < 0.001 TAA group vs groups CO and CO+ G.hP< 0.01 TAA + G group vs the TAA group.CO:Control;G:Glutamine;TAA:Thioacetamide.

Figure4 Effect of glutamine in experimental severe acute liver failure.Western blot analysis of protein expression of (A) NQO1 and (B) SOD.Values expressed as mean ± SE.aP < 0.05 TAA group vs groups CO and CO + G.bP < 0.01 TAA group vs groups CO and CO + G;dP < 0.01 TAA + G group vs the TAA group.CO:Control;G:Glutamine;TAA:Thioacetamide.

Figure5 Assessment of glutathione (GSH) levels in the liver of rats treated with glutamine after induction of severe acute liver failure.Values expressed as mean ± standard error.bP < 0.001 TAA group vs groups CO and CO + G;dP < 0.01 TAA + G group vs the TAA group.CO:Control;G:Glutamine;TAA:Thioacetamide.

Figure6 Effect of glutamine in experimental severe acute liver failure.Western blot analysis of protein expression of (A) TLR4 and (B) NFκB.Values expressed as mean ± SE.bP < 0.001 TAA group vs groups CO and CO + G;dP < 0.001 TAA + G group vs the TAA group.CO:Control;G:Glutamine;TAA:Thioacetamide.

Figure7 Effect of glutamine in experimental severe acute liver failure.Western blot analysis of protein expression of A:iNOS and B:COX-2.Values expressed as mean ± SE.bP < 0.001 TAA group vs groups CO and CO + G;dP < 0.01 TAA + G group vs the TAA group.CO:Control;G:Glutamine;TAA:Thioacetamide.

Figure8 Effect of glutamine on levels of nitric oxide metabolites (nitrites and nitrates) in the liver of rats with severe acute liver failure.Values expressed as mean ± SE.bP < 0.001,TAA group vs groups CO and CO + G;cP < 0.05,TAA + G group vs the TAA group.CO:Control;G:Glutamine;TAA:Thioacetamide.
ARTICLE HIGHLIGHTS
Research background
Severe acute liver failure (SALF) is a serious disease that does not have an effective treatment and liver transplantation is the only viable alternative.The mechanisms intrinsic to the pathophysiology of the disease are still not well understood and therefore,experimental studies are of fundamental importance in the attempt to elucidate the mechanisms and therapeutic agents.Glutamine is an amino acid used in the treatment of various diseases of the gastrointestinal tract and has been shown to be promising in previous studies of liver diseases.
Research motivation
In a previous study,the authors developed the experimental model of thioacetamide-induced SALF across different times.From the obtained data the best model to be studied was defined.It was evaluated the antioxidant action of glutamine that presented promising data.With the continuity of the research,we evaluated in this work the pathways of oxidative stress and inflammatory process at the molecular level,involved in the SALF.The results of this research are important indicators of the restorative role of glutamine in the SALF experimental model and will certainly provide a basis for a better understanding of the evaluated mechanisms contributing to the continuity of the studies.
Research objectives
The objective of this study was to investigate the action of glutamine on parameters of oxidative stress and inflammatory process in the experimental SALF.It is known that such parameters are implicated in the pathophysiology of various diseases.The understanding of the routes studied offers a basis for further studies that may evaluate other pathways involved in the disease,as well as the action of other therapeutic agents.
Research methods
To induce SALF in wistar rats the xenobiotic thioacetamide was used.The animals were randomized into different groups.In the treatment groups,the animals received doses of glutamine intraperitoneally.Blood tests were performed to evaluate hepatic integrity through a commercial kit.Liver portions were used for histopathological evaluation through hematoxylin and eosin staining.Techniques based on spectrophotometry were performed through protocols for the analysis of oxidative stress and inflammatory process.Protein expression was performed by Western Blot and cytokine analysis was done using multiplex technology based on magnetic beads through commercial kit.
Research results
Glutamine reduced hepatic integrity enzymes and restored the liver of the animals evidenced by decreased necrosis,balloonization and inflammation in the histopathological analysis.In addition,glutamine reduced lipid peroxidation and restored antioxidant enzyme activity.It was evidenced the decrease of the expression of proteins of the oxidative system and of the inflammatory process.Glutamine was able to increase the levels of interleukin 10,an important anti-inflammatory cytokine and also the expression of Nrf2,NQO1 and GSH levels,important proteins of the antioxidant protection system.
Research conclusions
When evaluating mechanisms implicated in the experimental SALF,studying parameters of oxidative stress and inflammatory process,it was possible to observe the protective action of glutamine in the liver of the animals.It is possible to confirm with this study that the oxidative stress and the inflammatory process play a primordial role in the progression of the disease,evidenced by the study of the molecular aspects.Glutamine has been shown to be effective by activating the antioxidant system and minimizing damage from the inflammatory process.Therefore,it presents as a viable alternative in the treatment of patients with severe hepatic insufficiency.Still,new studies confirming the efficacy of glutamine are needed so that in the future it may be part of the routine in the medical clinic.
Research perspectives
In this study the efficacy of the experimental model proposed by the authors in a previous study was confirmed.The pathophysiological mechanisms involved in SALF are diverse and complex.For further clarification of these mechanisms,other molecular routes should be investigated in an attempt to elucidate the aspects involved.We present the details of the involvement of the oxidative stress and the inflammatory process in this research and we hope that further studies can provide technical and theoretical data for the development of viable and effective strategies in the treatment of patients with SALF.
ACKNOWLEDGEMENTS
Funding was provided by Fundo de Incentivo à Pesquisa e Eventos (FIPE) do Hospital de Clínicas de Porto Alegre (HCPA);Universidade Federal do Rio Grande do Sul (UFRGS);Universidade Luterana do Brasil (ULBRA);the Coordination for the Improvement of Higher Education Personnel (CAPES);the National Council for Scientific and Technological Development (CNPq);and the Research Support Foundation of Rio Grande do Sul (FAPERGS).The authors thank Ceres Andréia Vieira Oliveira for her assistance with statistical analysis.
 World Journal of Hepatology2019年3期
World Journal of Hepatology2019年3期
- World Journal of Hepatology的其它文章
- Update on management of gastric varices
- Hepatocellular carcinoma recurrence after liver transplantation:Risk factors,screening and clinical presentation
- Extreme hyperbilirubinemia:An indicator of morbidity and mortality in sickle cell disease
- Angiogenesis of hepatocellular carcinoma: An immunohistochemistry study
- Preoperative immunonutrition in patients undergoing liver resection:A prospective randomized trial
- Intraperitoneal rupture of the hydatid cyst:Four case reports and literature review
