Comparison of intra-articular injection of parecoxib vs oral administration of celecoxib for the clinical efficacy in the treatment of early knee osteoarthritis
Lu Lu, Yu Xie, Ke Gan, Xiao-Wen Huang
Lu Lu, Yu Xie, Ke Gan, Department of Rheumatology, Affiliated Hospital of Nanjing University of Chinese Medicine (Jiangsu Province Hospital of Chinese Medicine), Nanjing 210029,Jiangsu Province, China
Xiao-Wen Huang, Department of Orthopedics, First Affiliated Hospital of Nanjing Medical University (Jiangsu Province Hospital), Nanjing 210029, Jiangsu Province, China
Abstract
Key words: Knee osteoarthritis; Intra-articular injection; Parecoxib; Non-steroid antiinflammatory drugs
INTRODUCTION
Osteoarthritis (OA) is a degenerative joint disease commonly found in elderly people[1-5].Early clinical symptoms of OA include joint pain and restricted mobility,and it may also cause joint deformities in later stages.The incidence of OA has been rising every year, which not only affects patient quality-of-life, but also causes considerable economic and mental burdens.The major treatments for OA include changing unhealthy lifestyles and modes of movement in the early stages,strengthening functional training of periarticular muscles and joints, physical therapy,and oral or external application of non-steroid anti-inflammatory drugs (NSAIDs) to relieve the symptoms[1-5].However, patients usually require joint replacement in late stages.In spite of the extensive clinical use of selective COX-2 inhibitors (e.g.,celecoxib and etoricoxib), the incidence of adverse gastrointestinal reactions remains high[6,7].Moreover, the cardiovascular risk of oral NSAIDs can never be underestimated, and the compliance with the long-term use of NSAIDs remains low[8].The analgesic effect of topical agents is poor, and adverse skin events may occur from time to time[1,2,4,5].Intra-articular medication is a treatment strategy worthy of wider clinical popularization.Parecoxib is a novel type of NSAID and can be injected intravenously.This agent can selectively inhibit the COX-2 pathway.Based on the pharmacokinetics of parecoxib, the plasma half-life of parecoxib is only 22 min because of its rapid conversion to valdecoxib.This blocks the synthesis of prostaglandins in peripheral and central regions, increases the pain threshold, inhibits hypersensitivity of the pain threshold, and produces anti-inflammatory and analgesic effects.Parecoxib is conventionally used for postoperative analgesia in anesthesiology and surgery departments[9,10].Intra-articular injection of parecoxib can block the inflammatory cascade in early OA by the same mechanism.However,it is undeniable that intra-articular injection has a series of possible adverse events, such as injection site inflammation, intra-articular hemorrhage, meniscus or cartilage damage, and even septic arthritis.Thus, this method is rarely discussed in the literature both at home and abroad[11].In this study, 36 cases with early knee OA (KOA) who received intra-articular injections of parecoxib from September 2016 to October 2017 were analyzed retrospectively, and the clinical efficacy was compared with those receiving other treatments.
MATERIALS AND METHODS
Subjects
From September 2016 to October 2017, 110 patients with early KOA (51 males and 59 females, aged 45- to 64-years-old, with an average age of 52.0 ± 4.1 years) who received treatment at the outpatient clinic of the orthopedics and rheumatology departments and had intact follow-up data were retrospectively reviewed according to the inclusion and exclusion criteria.Inclusion criteria:(1) Diagnosed as KOA according to the American Academy of Orthopedic Surgeons (AAOS) Guidelines for the Diagnosis and Treatment of Knee Osteoarthritis; (2) Receiving initial treatment with a medical history shorter than 6 mo; excluding those with moderate amounts of effusion in the joint upon musculoskeletal ultrasound); (3) Clinical staging:Kellgren-Lawrence (K-L) grade 0-II upon plain X-ray scan; (4) Age > 54 years old; (5) No history of severe knee joint trauma or ligament rupture, with intact bone and ligament structures; (6) Knee joint having at least 100° mobility, with varus and valgus deformity < 5°; and (7) Consenting to intra-articular puncture[1].Exclusion criteria:(1)Inflammatory lesions of the knee joint, such as rheumatoid arthritis, reactive arthritis,gout and ankylosing spondylitis; (2) Infectious arthritis; (3) Severe osteoporosis; (4)History of knee joint surgery; (5) Allergic to sulfonamides; (6) Severe neuropsychiatric disorders or organic diseases of the heart, liver and kidneys; and (7) Poor compliance and uncooperative with the follow-up.All patients provided informed consent and agreed to participate in the study.This study was approved by the ethics committee of our hospital.The ethical committee approval number is 2016NL-036-02.
According to the treatment modalities, these patients were divided into three groups:Basic treatment + oral glucosamine (group A,n= 37), oral celecoxib + basic treatment + oral glucosamine (group B,n= 37), and intra-articular injection of parecoxib + basic treatment + oral glucosamine (group C,n= 36).All patients were asked in detail about their medical history, and received X-ray scans and musculoskeletal ultrasounds.All of them signed the informed consent for intraarticular puncture.There were no significant differences between the three groups in terms of demographic information (gender, age, K-L grade, course of disease, body mass index) (Table 1).
Treatment modalities
All 37 patients in group A received basic treatment, which consisted of the following:health education, recommendations for changes to an unhealthy lifestyle and modes of movement, instructing the patients on the necessity of avoiding long-time running,jumping, and squatting, avoiding stair and mountain climbing as much as possible,and reducing weight.On this basis, they were given oral glucosamine sulfate (viatril-S, Rottapharm Ltd., CFDA approval No.H20090797) tid, at a dose of 0.5 each time before meals.The duration of medication was 12 consecutive wk.
Patients in group B received basic treatment, as in group A.On this basis, they took celecoxib orally (Celebrex, Pfizer Pharmaceuticals Limited, CDFA approval No.J20140072) bid, at a dose of 0.2 g each time, for 12 consecutive wk.Moreover, they were also given oral glucosamine sulfate using the same dose as group A.
Patients in group C received basic treatment, as in group A.After the informed consent and risk disclosure statement were signed, patients in group C received intraarticular injection of 40 mg parecoxib (Pfizer Pharmaceuticals Limited, CDFA approval No.J20130044).Before the injection, 40 mg parecoxib was dissolved in 3 mL of normal saline.The patients took a supine position with the affected lower limb straightened out naturally.The skin was disinfected with iodophor.After laying the sterile drape, the needle was inserted from the lateral side of the patella, with the needle tip inclining towards the middle.A small amount of synovial fluid was extracted after the intra-articular puncture was successful.Later, an intra-articular injection of 40 mg parecoxib was performed.At the end of the injection, the knee joint moved properly so that the injected liquid could be evenly distributed.Intra-articular injection of parecoxib was performed once every 2 wk, at a dose of 40 mg each time,for three times total.Moreover, they were also given oral glucosamine sulfate using the same dose as group A.Synovial fluid was extractedviaintra-articular puncture for all patients before treatment and at 3 mo after treatment.
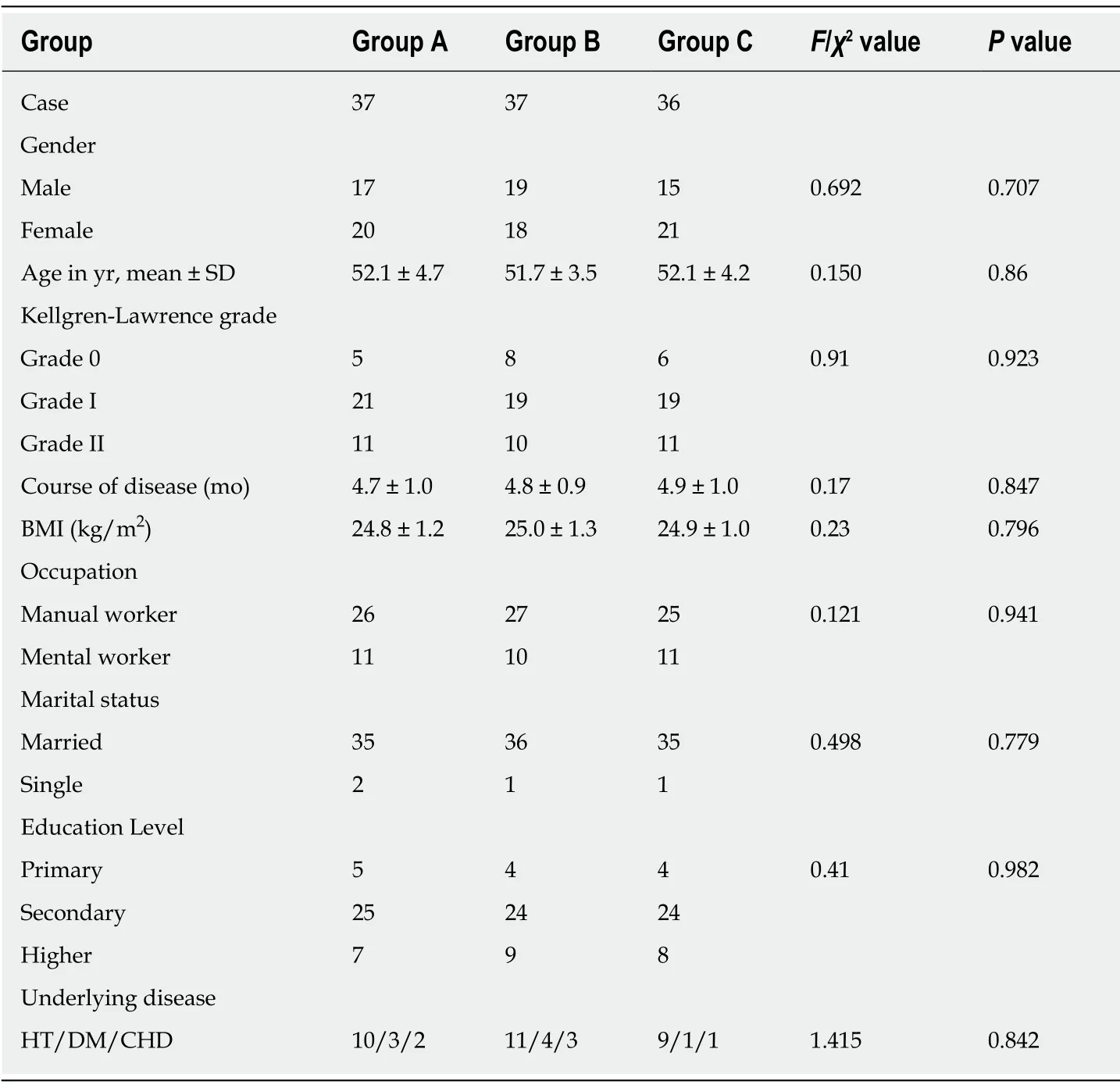
Table 1 Comparison of the baseline information of three groups
Observation indicators and methods
Visual analogue scale scores:Visual analogue scale (VAS) scores before treatment and at 12 mo after treatment were recorded for the three groups.This involved two senior physicians evaluating VAS scores as the patients climbed up and down stairs or were squatting.
Hospital for Special Surgery scores:Hospital for Special Surgery (HSS) scores before treatment and at 12 mo after treatment were recorded for the three groups (HSS Knee Score)[12].Knee joint function was separately evaluated by two senior physicians.
Determination of inflammatory cytokine levels in the synovial fluid:Before treatment and at 3 mo after treatment, synovial fluid was extracted for the three groups to determine the levels of inflammatory cytokines, including tumor necrosis factor α (TNF-α), interleukin (IL)-6 and IL-10.Detections were conducted by using an ELISA kit (R and D Systems, United States) in accordance with the manufacturer’s instruction.
Patient satisfaction:Patient satisfaction was recorded after administering the different treatments for 12 mo.
Efficacy evaluation criteria:Clinical efficacy was evaluated based on HSS scores of the knee joint from the following dimensions:Pain (30 points), function (22 points),mobility (18 points), muscle tone (10 points), flexion deformity (10 points) and stability (10 points).The total score was 100 points, and the score = 85 points was defined as excellent, 70-84 scores as good, 60-69 points as fair, and < 60 points as poor.
Statistical analysis
Statistical analyses were performed using STATA 15.0 software.Continuous numerical variables obeying normal distributions in the three groups were further analyzed by ANOVA, and an intergroup comparison was conducted using a Scheff test.Pairedt-tests were performed within each group before and after treatment.Categorical variables were analyzed by the chi-square test or Fisher’s exact test, with the significance level set at 0.05.
RESULTS
All patients were followed up for an average of 15.5 ± 2.7 mo, and none of the patients were lost during the follow-up.VAS scores of all patients decreased after treatment(Table 2), while HSS scores increased (Table 3).The levels of inflammatory cytokines TNF-α and IL-6 in the synovial fluid (Table 4) decreased, while the levels of IL-10 increased (Table 4).All of these changes were significant (P< 0.001), indicating that all treatments were effective against early KOA to a certain extent.After treatment, there were significant differences in VAS scores, HSS scores and levels of the inflammatory cytokines TNF-α, IL-6 and IL-10 in the synovial fluid among the three groups (P<0.001).The results of group C were significantly different from those of groups A and B (P< 0.001), and the results of group B were significantly different from those of group A (P< 0.001).As to patient satisfaction, there were significant differences among the three groups (P< 0.001), with the highest satisfaction found in group C.
DISCUSSION
OA is a degenerative joint disease that typically affects elderly people.This medical condition can greatly impair patient quality-of-life, and also cause significant economic and mental burdens for patient families.OA treatment should follow the principles of early diagnosis and early treatment, which is crucial for relieving symptoms, reducing complications, lowering disability rates, and lessening both family and social burdens.
The current guidelines for the diagnosis and treatment of OA provide detailed introduction for the ladder-like OA treatment strategy, which consists of basic treatment, medication therapy, surgery and traditional Chinese medicine[1,2,4,5].Medication therapy predominantly uses NSAIDs, either orally or topically.Topical NSAIDs take effect slowly, and may incur complications such as adverse skin events.Therefore, oral NSAIDs remain the mainstream treatment for early OA.Most OA patients are elderly people, who typically suffer from several diseases, particularly gastrointestinal, heart, liver and kidney diseases.Therefore, the selection of the type and dosage of medicine should be very carefully determined.Before the medication therapy begins, risk factors should first be evaluated, and medication safety should be prioritized to reduce adverse events.NSAIDs should be prescribed with caution for patients with a high cardiovascular risk, including selective COX-2 inhibitors (e.g.,celecoxib)[8].In clinical practice, patients with heart and gastrointestinal diseases cannot take NSAIDs orally, and therefore suffer from poor treatment efficacy.In addition, the long-term use of oral NSAIDs, even selective COX-2 inhibitors, may cause damage to the gastrointestinal tract.Patients usually show poor compliance with oral medications.Besides, the long-term use of oral drugs can be costly, which further leads to relapse or aggravation of the diseases.Patients are usually unsatisfied with treatment outcomes, and urgently need new solutions for the above problems.
Intra-articular injection of drugs is a more direct treatment, which promotes the enrichment of drugs at the target site, thereby relieving pain and improving joint function more effectively[3,13-15].However, as an invasive method, intra-articular injection may increase the risk of joint infection.The commonly used drugs for intraarticular injection include glucocorticoids, sodium hyaluronate, chitosan and plateletrich plasma.Hormone therapy has a potent anti-inflammatory effect, but may cause irreversible damage to the articular cartilage and hence have a poor long-term outcome.AAOS Guidelines for the Diagnosis and Treatment of Osteoarthritis does not recommend the intra-articular injection of sodium hyaluronate[1].In the 2008 version of the Guidelines, it is indicated that the efficacy of the treatment is uncertain[16].In the 2009 version of the Osteoarthritis Research Society International guidelines for OA, the intra-articular injection of sodium hyaluronate became “level 2” treatment for KOA, but was not recommended for individuals with polyarticular OA”[5].In the 2008 version of the guidelines for viscosupplementation for KOA, only cross-linked HA is allowed for single injections[3].Hence, the intra-articular injection of sodium hyaluronate is still controversial and largely used as a second-line treatment.Chitosan and platelet-rich plasma are less costly, and their efficacy is under further investigation.Guidelines do not mention the use of chitosan or platelet-richplasma, or only claim that their efficacy remains uncertain[1,2,4,5].There is a long way to go before the above drugs will be used in the clinic.Drugs with a definite antiinflammatory effect, no apparent side-effects and low cost are needed for intraarticular injections.In this study, a novel type of selective COX-2 inhibitor, parecoxib,was chosen for intra-articular injection.
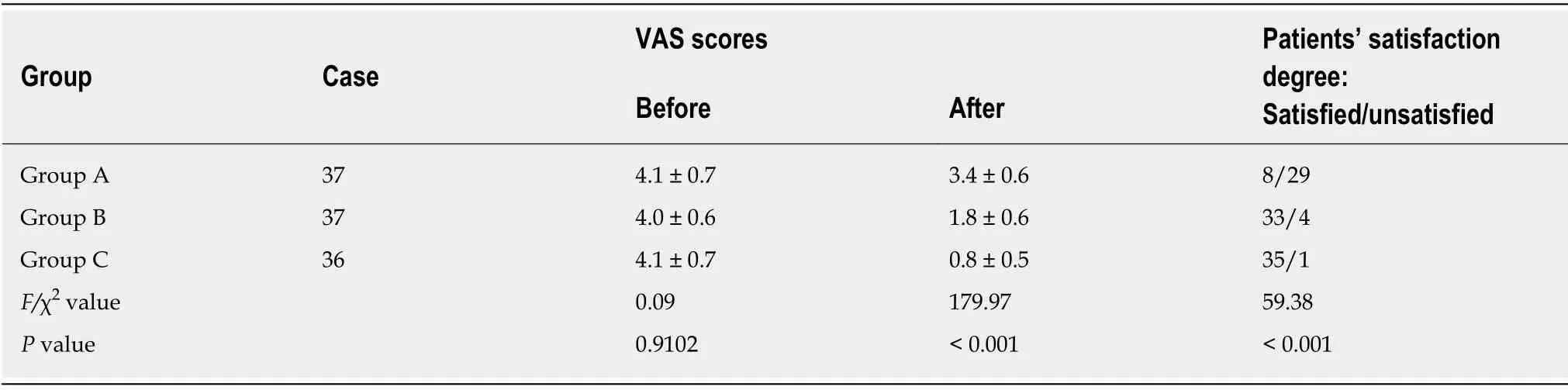
Table 2 Comparison of visual analogue scale scores and satisfaction of patients in the three groups before and after treatment
Parecoxib was once used in intravenous and intramuscular injections with high safety and reliability.Few reports are available as to its use in intra-articular injection[11].After local injection, parecoxib can be hydrolyzed into valdecoxib, which can further block the local synthesis of prostaglandin from arachidonic acid, thus achieving an anti-inflammatory and analgesic effect.Parecoxib’s inhibitory effect on COX-2 is 28,000 times stronger than its effect on COX-1, and it can achieve a potent anti-inflammatory and analgesic effect.As far as the pathogenesis of OA is concerned,inflammatory cytokines are involved in the regulation of the entire process.Inflammation and pain stimuli will cause the secretion of TNF-α by many cells.After binding to its receptors, TNF-α can rapidly trigger the NF-κB signaling pathway.As a result, proinflammatory cytokines such as IL-1, IL-6 and IL-8 will be secreted through a series of signal transduction steps.This further leads to inflammatory cascades and the activation of the inflammatory network, which significantly inhibits the production and release of anti-inflammatory cytokines such as IL-4, IL-10 and IL-13.IL-6 is the primary proinflammatory cytokine in inflammatory responses of the acute stage.It is directly related to OA inflammatory symptoms, such as pain and synovial edema[17,18].It has been reported that parecoxib not only inhibits the synthesis of prostaglandin, but also has potent anti-inflammatory and analgesic effects[19].Moreover, parecoxib can alleviate inflammatory responses by inhibiting activation of the NF-κB signaling pathway, and hence inhibit IL-6 transcription.Previous studies have shown that prostaglandin inhibits IL-10 synthesis, while parecoxib promotes IL-10 release and relieves the inflammatory response by inhibiting prostaglandin synthesis[19].The above studies on the anti-inflammatory mechanism agree with our measurements of inflammatory cytokine levels before and after treatment.These results explain the good clinical efficacy of the intra-articular injection of parecoxib for early KOA from the perspective of basic research.
In this study, we evaluated VAS scores and HSS scores before and after treatment,as well as the degree of patient satisfaction.It was preliminarily proven that the intraarticular injection of parecoxib outperformed oral celecoxib in the treatment of early KOA.After intra-articular injection, a large amount of liquid drugs rapidly accumulates in the articular cavity.Therefore, the local concentration of the drug at the target site is high.However, there are few blood vessels in the articular cavity of the knee, and drug absorption and metabolismviablood circulation are slow.For this reason, the anti-inflammatory and analgesic effects are more enduring.Local medication has very little systemic impact, and so this treatment also applies to elderly OA patients with cerebrovascular, cardiovascular and gastrointestinal diseases.Moreover, there is no need for long-term oral use, which greatly improves the compliance and reduces the economic burden for patients.Due to these benefits,we recommend the clinical use of intra-articular injection of parecoxib.
Our study has certain limitations.First, limited by the nature of the retrospective study design, it must be emphasized that patient selection and evaluation biases are inevitable.Second, our sample size remains small and the duration of follow-up was short.In future research, we should use strict prospective randomized clinical trials to verify the findings of our results through longer follow-ups.It should be noted that intra-articular injection is an invasive procedure, which carries a certain risk of joint infection, especially for elderly patients with such underlying diseases as diabetes.Given the limited number of literature reports in this respect at home and abroad,more research should be conducted on the dosage and frequency of parecoxib administration by intra-articular injection.In addition, it remains unclear whether local high-concentration NSAIDs will induce other joint complications, and also whether combined administration is feasible with sodium hyaluronate.
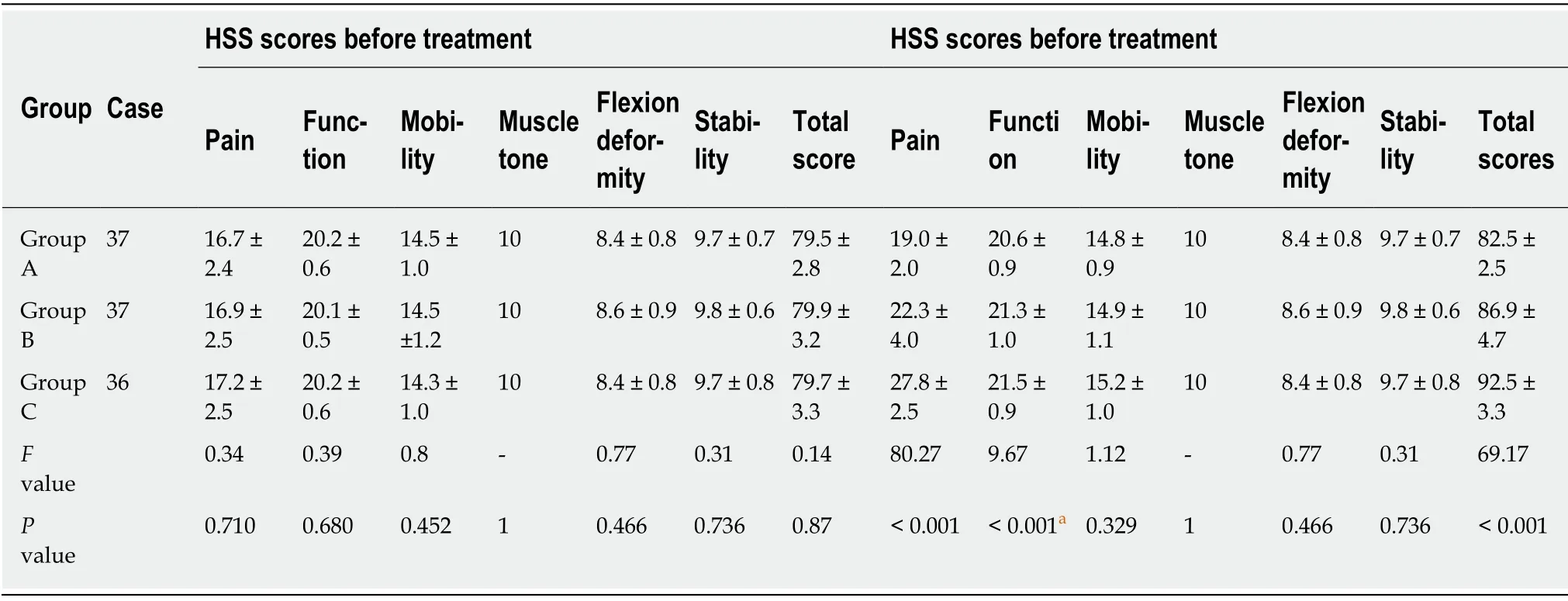
Table 3 Comparison of Hospital for Special Surgery Knee scores in the three groups before and after treatment
Taken together, among patients with early KOA, intra-articular injection of parecoxib can significantly improve clinical symptoms and prevent adverse events associated with the long-term oral use of NSAIDs.This treatment can be a suitable treatment alternative.
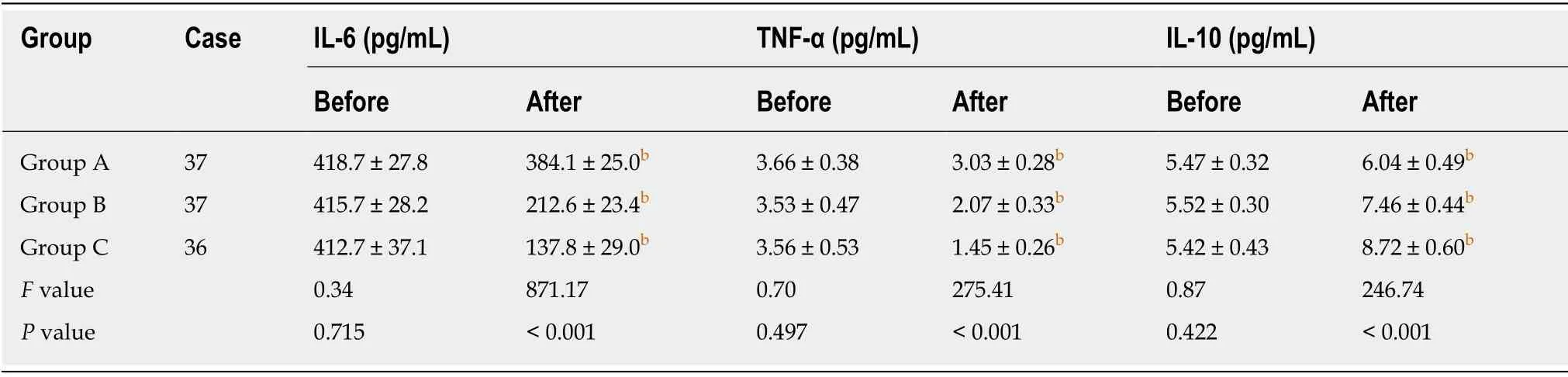
Table 4 Comparison of inflammatory cytokine levels in the synovial fluid in the three groups before and after treatment
ARTICLE HIGHLIGHTS
Research background
Oral non-steroid anti-inflammatory drugs (NSAIDs) are often used for the treatment of osteoarthritis.However, in elderly people, oral NSAIDs have certain side-effects especially in the incidence of adverse gastrointestinal reactions and cardiovascular risk.Meanwhile, the compliance with long-term use of NSAIDs is low in early osteoarthritis.
Research motivation
A new solution is needed for patients with early osteoarthritis, especially for those who cannot take NSAIDS orally.Intra-articular injection of drugs is a direct and effective treatment.It is a challenge to find suitable NSAIDs for intra-articular injection.Parecoxib is conventionally used for postoperative analgesia by an intravenous route of administration in the surgery department.The feasibility and effectiveness of the intra-articular injection of parecoxib should be explored.
Research objectives
A retrospective study was performed to observe and investigate the clinical efficacy of the intraarticular injection of Parecoxib to treat patients with early knee osteoarthritis (KOA).
Research methods
Three groups of patients (110 cases in total) underwent interventions of three separate treatment methods.By comparing visual analogue scale (VAS), Hospital for Special Surgery (HSS) scores and levels of different inflammatory cytokines in synovial fluid before and after treatment, the clinical efficacy of intra-articular injections of Parecoxib were evaluated.
Research results
The clinical efficacy of the intra-articular injection group in the treatment of early KOA was confirmed to be superior to the others, whether it be in terms of VAS and HSS scores or the variation of different inflammatory cytokine levels.
Research conclusions
Compared with oral NSAIDS, intra-articular injection of Parecoxib has better clinical efficacy in the treatment of early KOA.This method can avoid the side-effects associated with the long-term oral use of NSAIDs, and should be an option for clinical application.
Research perspectives
Osteoarthritis treatment methods require continual improvement and optimization.More randomized clinical trial studies are always necessary to improve and verify our findings.
 World Journal of Clinical Cases2019年23期
World Journal of Clinical Cases2019年23期
- World Journal of Clinical Cases的其它文章
- Pure squamous cell carcinoma of the gallbladder locally invading the liver and abdominal cavity:A case report and review of the literature
- Management of massive fistula bleeding after endoscopic ultrasound-guided pancreatic pseudocyst drainage using hemostatic forceps:A case report
- Fatal complications in a patient with severe multi-space infections in the oral and maxillofacial head and neck regions:A case report
- Bouveret syndrome:A case report
- Left armpit subcutaneous metastasis of gastric cancer:A case report
- Rigid esophagoscopy combined with angle endoscopy for treatment of superior mediastinal foreign bodies penetrating into the esophagus caused by neck trauma:A case report
