Resurrection of the Genus Leptomantis, with Description of a New Genus to the Family Rhacophoridae (Amphibia: Anura)
Dechun JIANG, Ke JIANG, Jinlong REN,2, Jun WU and Jiatang LI,4,5*
1 CAS Key Laboratory of Mountain Ecological Restoration and Bioresource Utilization & Ecological Restoration and Biodiversity Conservation Key Laboratory of Sichuan Province, Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, Sichuan, China
2 University of Chinese Academy of Sciences, Beijing 100049, China
3 Nanjing Institute of Environmental Sciences, Ministry of Ecology and Environment of China, Nanjing 210042, Jiangsu,China
4 CAS Center for Excellence in Animal Evolution and Genetics, Chinese Academy of Sciences, Kunming 650223,Yunnan, China
5 Southeast Asia Biodiversity Research Institute, Chinese Academy of Sciences, Yezin Nay Pyi Taw 05282, Myanmar
Abstract Genus Rhacophorus Kuhl and Van Hasselt, 1 822 is one of the most diverse genera of the family Rhacophoridae, and its taxonomy of genus Rhacophorus faces major challenges because of rapidly described new species and complex interspecies relations. In this study, we investigate the generic taxonomy within the genus Rhacophorus based on 1 972 bp of mitochondrial genes (12S rRNA, tRNA-val and 16S rRNA), containing 102 sequences from 58 species. The results reveal three well-supported and highly diverged matrilines that correspond with morphological characteristics and geographic distribution. Accordingly, we consider these three lineages as distinct genera:Rhacophorus sensu stricto, resurrected genus Leptomantis Peters, 1867, and the genus Zhangixalus gen. nov.
Keywords Rhacophorus, taxonomic revision, tree frog, Zhangixalus gen. nov.
1. Introduction
Old World Treefrogs, family Rhacophoridae, comprise 416 species in 18 recognized genera (Frost, 2018), of which 78 species in 12 genera are found in southern and southwestern China (AmphibiaChina, 2018). Of these,genus Rhacophorus Kuhl and Van Hassalt, 1 822 contains 92 species, distributed widely across China, Japan,India, and from the Philippines to Sulawesi (Frost, 2018;O’Connell et al., 2018). Genus Leptomantis Peters,1867 was established for the species L. bimaculata Peters, 1867, and Ahl (1931) subsequently synonymized it as a junior synonym of Rhacophorus. Later, Dubois(1987) made Leptomantis a subgenus of Rhacophorus,and divided subgenus Rhacophorus into ten species groups. Iskandar and Colijn (2000) subsequently raised Leptomantis to full genus rank, but Harvey et al. (2002)again synonymized Leptomantis with Rhacophorus,which Frost (2018) accepted. Fei (2012) established Huangixalus based on the type species Rhacophorus translineatus Wu, 1977, but treated as synonym of Rhacophorus by Frost (2018).
Recently, many new species of the genus Rhacophorus were revealed by virtue of molecular phylogenetic results (Matsui et al., 2013; Nguyen et al., 2017;Streicher et al., 2014). In addition, several phylogenetic works have shed new light on the generic phylogeny of Rhacophorus, which constantly recovered as three wellsupported lineages on the basis of molecular phylogenetic trees, although their phylogenetic relationships remain unresolved (Chan et al., 2018; Li et al., 2008, 2009,2012, 2013; O’Connell et al., 2018; Pan et al., 2017).
The three major clades show deep divergence with each other. The morphological characteristics of tree frog species within their respective clades differ substantially(Li et al., 2012; Yang, 2018). A clade that contains species from the Malay Peninsula to the Philippines agrees with the diagnosis of Leptomantis. On the basis of molecular phylogenetic results, morphological comparisons and distribution patterns, we recognize the validity of the genus Leptomantis. Additionally, resolved relationships require the further splitting of Rhacophorus and the erection of a new genus.
2. Material and Methods
2.1. Morphological characters and distribution collection Morphological characters used and their measurement methods followed Fei et al. (2009),morphological characters used as below: body size,snout-vent length (SVL); dermal folds along limbs,dermal folds or ridges along outer edge of forearm and tarsus; supracloacal fold, skin folds above cloaca;tarsal projection, a dermal projection on tibiotarsal articulation; upper eyelid projection, conical projection on upper eyelid. Morphological data of genera were obtained from previous studies (Abraham et al., 2013;Biju et al., 2010; Boulenger, 1882; Dubois, 1987;Fei et al., 2009; Jiang et al., 2016; Peters, 1867). The morphological descriptions, phylogentic assignments,and distributions of species of Rhacophorus were based on original descriptions and the following subsequent literature: Anderson (1871), Biju et al. (2013),Boulenger (1896), Das and Haas (2005), Dehling (2008;2015), Dehling and Grafe (2008), Hamidy and Kurniati(2015), Harvey et al. (2002), Hertwig et al. (2012),Inger (1954; 1966; 1999), Li et al. (2012), Malkmus and Brühl (2002), Matsui and Panha (2006), Matsui et al.(2013), Mo et al. (2008), Nguyen et al. (2017), Ohler and Delorme (2006), Onn and Ahmad (2009), Orlov et al. (2001, 2008, 2010, 2012), Ostroshabov et al. (2013),Rowley et al. (2012), and Streicher et al. (2014).
2.2. Phylogenetic analyses We downloaded 102 sequences for 55 species of Rhacophorus and three outgroups from GenBank. Chiromantis xerampelina Peters, 1854, Polypedates megacephalus Hallowell, 1861 and Polypedates leucomystax (Gravenhorst, 1829) were selected as outgroups for phylogenetic analyses (Li et al., 2008, 2009). The respective gene partitions were 12S rRNA, tRNA-val and 16S rRNA (1 972 bp alignment totally). Details on specimen voucher, GenBank accession codes and sampling sites were listed in Table S1.
The mitochondrial gene fragments were aligned by using the FasParser software package (Sun, 2017).After initial comparison, we partitioned the data sets and assigned substitution models as suggested by the Bayesian Information Criterion (BIC) as calculated in PartitionFinder v2.1.1 (Lanfear et al., 2012). This resulted in one partition for the data set with a GTR+I+G substitution model. The Bayesian phylogenetic relationships were conducted using MrBayes v3.2.6 (Ronquist et al., 2012).Two independent runs of Markov Chains for 10 000 000 generations were summarized, and sampled every 100 generations. The first 25 000 sampled trees were discarded as a conservative burn-in and convergence was investigated in Tracer v1.6 (Rambaut et al., 2013). The frequency of nodal resolution, termed a Bayesian posterior probability (BPP), was determined to assess con fi dence of the topology. Nodes were considered strongly supported when BPP ≥ 0.95. A maximum likelihood (ML) tree was conducted with RAxML v8.0.17 (Stamatakis, 2006) using a GTR+I+G model for our final likelihood search during fast bootstrapping with 1000 pseudoreplicates, bootstrap proportions (BSP) were assessed to test the node support,where nodes with BSP ≥ 70 were supported significantly.The alignment was partitioned for each locus.
3. Results
The two phylogenetic methods (BI, ML) resolved each major clade with strong support (Figure 1; Figure S1). The genus Rhacophorus was recovered to be a monophyletic group with strong support (BPP = 1.00; BSP = 100);Rhacophorus contained three strongly supported clades(Clades A, B, C), that were highly diverged with long basal branch lengths. Detailed content of these clades as follows:Clade A contained the following species: R. orlovi Ziegler and Köhler, 2001; R. calcaneus Smith, 1924; R.verrucopus Huang, 1983; R. robertingeri Orlov, Poyarkov,Vassilieva, Ananjeva, Nguyen, Sang, and Geissler, 2012;R. translineatus Wu, 1977; R. annamensis Smith, 1924;R. exechopygus Inger, Orlov, and Darevsky, 1999; R.baluensis Inger, 1954; R. rhodopus Liu and Hu, 1960;R. reinwardtii (Schlegel, 1840); R. norhayatii Chan and Grismer, 2010; R. borneensis Matsui, Shimada, and Sudin,2013; R. bipunctatus Ahl, 1927; R. kio Ohler and Delorme,2006; R. helenae Rowley, Tran, Hoang, and Le, 2012; R.pardalis Günther, 1858; R. malabaricus Jerdon, 1870;R. pseudomalabaricus Vasudevan and Dutta, 2000; R.lateralis Boulenger, 1883; R. nigropalmatus Boulenger,1895; R. bengkuluensis Streicher, Hamidy, Harvey, Anders,Shaney, Kurniawan, and Smith, 2014; R. margaritifer(Schlegel, 1837); R. modestus Boulenger, 1920; R.poecilonotus Boulenger, 1920; R. indonesiensis Hamidy and Kurniati, 2015; R. catamitus Harvey, Pemberton, and Smith, 2002 (BPP = 1.00). Among them, R. rhodopus from four areas (Longchuan, Lvchun, Mengyang and Medog)formed a well-supported lineage (BPP = 1.00; BSP = 86).Rhacophorus kio from Yunnan, China and Vietnam formed a lineage, which was sister to R. helenae from southern Vietnam (BPP = 1.00; BSP = 98).

Figure 1 Phylogenetic relationships of species of Rhacophorus based on three mitochondrial genes: 12S rRNA, tRNA-val and 16S rRNA.Circles on nodes correspond to three clades: Clade A (green), Clade B (orange), Clade C (blue). Numbers beside the nodes are given as Bayesian posterior probabilities (BPP) (≥0.90 retained)/bootstrap proportions (BSP) for maximum likelihood analyses (≥70 retained); ‘--’represents BPP and BSP lower than 90% and 70, respectively. Photos were taken by Ke JIANG, Cheng LI, Jin-Long REN.
Clade B contained the following species: R. gauni(Inger, 1966); R. penanorum Dehling, 2008; R.angulirostris Ahl, 1927; and R. monticola Boulenger, 1896(BPP = 1.00; BSP = 98). This clade had species widely distributed in maritime Southeast Asia, from Peninsular Malaysia to the Philippines.
Clade C contained the following species: R. feae Boulenger, 1893; R. moltrechti Boulenger, 1908; R.duboisi Ohler, Marquis, Swan, and Grosjean, 2000; R.omeimontis (Stejneger, 1924); R. burmanus (Andersson,1939); R. dorsoviridis Bourret, 1937; R. zhoukaiyae Pan,Zhang, and Zhang, 2017; R. lishuiensis Liu, Wang, and Jiang, 2017; R. hui Liu, 1945; R. dugritei (David, 1872);R. hungfuensis Liu and Hu, 1961; R. wui Li, Liu, Chen,Wu, Murphy, Zhao, Wang, and Zhang, 2012; R. minimus Rao, Wilkinson, and Liu, 2006; R. hongchibaensis Li,Liu, Chen, Wu, Murphy, Zhao, Wang, and Zhang, 2012;R. puerensis (He, 1999); R. schlegelii (Günther, 1858);R. arboreus (Okada and Kawano, 1924); R. chenfui Liu,1945; R. nigropunctatus Liu, Hu, and Yang, 1962; R.dennysi Blanford, 1881; R. smaragdinus (Blyth, 1852);R. dulitensis Boulenger, 1892; R. prominanus Smith,1924; and R. achantharrhena Harvey, Pemberton, and Smith, 2002 (BPP= 1.00). Rhacophorus zhoukaiyae from Anhui, China and R. lishuiensis from Zhejiang, China,formed a well-supported lineage (BPP = 1.00). Previous studies resolved R. dorsoviridis as the sister-species of R. zhoukaiyae (Pan et al., 2017), and also was the sisterspecies of R. lishuiensis (Liu et al., 2017). Because our data set included both R. lishuiensis and R. zhoukaiyae, the result indicated that R. lishuiensis had closer relationship with R. zhoukaiyae than R. dorsoviridis (BPP = 1.00; BSP= 97).
Distinct morphological differences diagnosed the three clades, including body sizes, dermal folds along limbs,tarsal projections, and dorsal coloration (Table 1). Further,differences in distribution among three clades were also obtained. Although Clade A covered all of Southeast Asia,clades B and C were rather isolated (Figure 2).
4. Discussion
Previous studies shed light on the phylogenetic resolution and systematics of Asian tree frogs. The molecular phylogeny of family Rhacophoridae resolved a monophyletic Rhacophorus and generally with three major clades (Chan et al., 2018; Li et al., 2008, 2009,2013). Several studies obtained species delimitations in Rhacophorus and all results showed a topology of three clades (Li et al., 2012; O’Connell et al., 2018; Pan et al., 2017). However, the BPP values of root lineages remained low, which suggested uncertain phylogenetic relationships among these three clades. The genusLeptomantis was established by Peters (1867) but its taxonomic status has long not been evaluated until now.

Table 1 Comparisons of morphology, distribution patterns, and reproduction models among the genera, Rhacophorus, Leptomantis, and Zhangixalus gen. nov.

Figure 2 Comparison of distributions among three clades. Circles on nodes correspond to three clades: Rhacophorus (green), Leptomantis(orange), Zhangixalus gen. nov. (blue).
Our interpretations of Rhacophorus focus mainly on support values of root lineages. The molecular phylogeny depicts three well-supported matrilines, and these are consistent with the results of Li et al. (2012),although phylogenetic relationships of three clade differ.On the basis of highly supported molecular trees, distinct differences of morphological characteristics, and mostly nonoverlapping geographic distribution, it is evident that these three lineages show deep evolutionary divergences. Therefore, in order to better reflect the phylogenetic relationships and biogeographic history of these frogs, we propose to recognize each of the three lineages as a distinct genus: Clade A represents genus Rhacophorus sensu stricto; Clade B is valid genus Leptomantis, which consistent with the results of Iskandar and Colijn (2000); and Clade C requires erection of a new genus that we name here. Combined with this study and previous literature as shown before, we currently recognized 39 species in Rhacophorus sensu stricto, 14 species in Leptomantis, and 36 species in the new genus, respectively.
Taxonomic account
Rhacophorus Kuhl and Van Hasselt, 1822
Type species: Rhacophorus reinwardtii (Schlegel, 1840)
Diagnosis: (1) Body size relatively moderate or large(SVL 30-100 mm, above 40 mm in most species); (2)presence of intercalary cartilage between terminal and penultimate phalanges of digits; (3) terminal phalanges of fi nger and toes Y-shaped; (4) tip of the digits expanded into large disks bearing circummarginal grooves; (5) webbed fingers; (6) skin not co-ossified to skull; (7) upper eyelid projections absent, tarsal projections present in most species; (8) dermal folds along forearm or tarsus present;(9) pupil horizontal; (10) iris without “X” shaped pattern;(11) white foam nests or jelly-encapsulated eggs produced by breeding pairs; and (12) distributed mainly in Indochina.
Phylogenetic definition: Genus Rhacophorus includes species that share a more recent common ancestor with Rhacophorus reinwardtii than with Leptomantis bimaculata and Zhangixalus dugritei.
Etymology: The generic name presumably derived from the Greek noon rhakos, meaning rag or tatter and the suf fi xphorus, meaning bearer. The English common name of the genus is “Flying Frogs” or “Parachuting Frogs”, and we suggest the Chinese name “Shu Wa Shu (树蛙属 )”. The gender of this genus is masculine.
Content: We currently recognized 39 species in the genus Rhacophorus as follows: R. annamensis Smith, 1924; R.baluensis Inger, 1954; R. barisani Harvey, Pemberton,and Smith, 2002; R. bengkuluensis Streicher, Hamidy,Harvey, Anders, Shaney, Kurniawan, and Smith, 2014;R. bifasciatus Van Kampen, 1923; R. bipunctatus Ahl,1927; R. borneensis Matsui, Shimada, and Sudin, 2013;R. calcadensis Ahl, 1927; R. calcaneus Smith, 1924;R. catamitus Harvey, Pemberton, and Smith, 2002; R.exechopygus Inger, Orlov, and Darevsky, 1999; R. helenae Rowley, Tran, Hoang, and Le, 2012; R. hoabinhensis Nguyen, Pham, Nguyen, Ninh, and Ziegler, 2017; R.hoanglienensis Orlov, Lathrop, Murphy, and Ho, 2001;
R. indonesiensis Hamidy and Kurniati, 2015; R. kio Ohler and Delorme, 2006; R. laoshan Mo, Jiang, Xie, and Ohler,2008; R. larissae Ostroshabov, Orlov, and Nguyen, 2013;R. lateralis Boulenger, 1883; R. malabaricus Jerdon, 1870;R. margaritifer (Schlegel, 1837); R. marmoridorsum Orlov,2008; R. modestus Boulenger, 1920; R. nigropalmatus Boulenger, 1895; R. norhayatii Chan and Grismer, 2010; R.orlovi Ziegler and Köhler, 2001; R. pardalis Günther, 1858;R. poecilonotus Boulenger, 1920; R. pseudomalabaricus Vasudevan and Dutta, 2000; R. reinwardtii; R. rhodopus Liu and Hu, 1960; R. robertingeri Orlov, Poyarkov, Vassilieva,Ananjeva, Nguyen, Sang, and Geissler, 2012; R. spelaeus Orlov, Gnophanxay, Phimminith, and Phomphoumy, 2010;R. subansiriensis Mathew and Sen, 2009; R. translineatus Wu, 1977; R. tuberculatus (Anderson, 1871); R. vampyrus Rowley, Le, Thi, Stuart, and Hoang, 2010; R. verrucopus Huang, 1983; and R. viridimaculatus Ostroshabov, Orlov,and Nguyen, 2013.
Distribution: Distributed widely across Southeast Asia,including India, Bangladesh, Vietnam, Laos, Thailand,Cambodia, Malaysia, Indonesia, and Brunei, as well as extreme southern and southwestern China (mainly in Hainan, Guangxi Zhuang Autonomous Region, Yunnan,and Tibetan Autonomous Region).
Leptomantis Peters, 1867
Type species: Leptomantis bimaculata Peters, 1867
Diagnosis: (1) Body size relatively small (SVL 30-80 mm, about 30-50 mm in most species); (2) snout pointed or obtusely pointed; (3) terminal phalanges of finger and toes Y-shaped; (4) snout projections absent, upper eyelid projections present or not, tarsal projections absent in most species; (5) dermal folds along forearm or tarsus absent;(6) skin of dorsal surfaces smooth or finely shagreened;(7) webbed fi ngers; (8) dorsal coloration usually light tan or reddish brown; (9) iris without “X” shaped pattern; (10)white foam nests produced by breeding pairs; and (11)distributed in maritime Southeast Asia.
Phylogenetic de fi nition: Leptomantis includes species that share a more recent common ancestor with Leptomantis bimaculata than with Zhangixalus dugritei and Rhacophorus reinwardtii.
Etymology: The generic name derived from the Greek adjective leptos, meaning thin or small and Greek noun mantis, meaning treefrogs. We suggest the English common name of the genus to be “Slim Treefrogs”, and“Shou Shu Wa Shu (瘦树蛙属 )” in Chinese. The gender of this genus is masculine.
Content: We currently recognized 14 species in the genus Leptomantis as follows: L. angulirostris (Ahl, 1927); L.belalongensis (Dehling and Grafe, 2008); L. bimaculatus Peters, 1867; L. cyanopunctatus (Manthey and Steiof,1998); L. fasciatus (Boulenger, 1895); L. gadingensis (Das and Haas, 2005); L. gauni (Inger, 1966); L. harrissoni(Inger and Haile, 1959); L. malkmusi (Dehling, 2015); L.monticola (Boulenger, 1896); L. penanorum (Dehling,2008); L. pseudacutirostris (Dehling, 2011); and L.robinsonii (Boulenger, 1903); L. ru fi pes (Inger, 1966).
Distribution: Mainly maritime Southeast Asia, Malaysia,Singapore, Brunei, Indonesia, The Philippines, and southern Thailand.
Zhangixalus gen. nov. Li, Jiang, Ren, Jiang
Type species: Polypedates dugritei David, 1872
Diagnosis: (1) Body size relatively large (SVL 30-120 mm, above 50 mm in most species); (2) snout rounded; (3)snout, upper eyelid and tarsal projections absent; (4)dermal folds along forearm or tarsus absent; (5) terminal phalanges of fi nger and toes Y-shaped; (6) skin of dorsal surfaces smooth, or scattered with small tubercles; (7)webbed fingers; (8) dorsal coloration green in most species; (9) iris without “X” shaped pattern; (10)white foam nests produced by breeding pairs; and (11)distributed in eastern Asia and northern Indochina.
Phylogenetic definition: Genus Zhangixalus gen. nov.includes species share a more recent common ancestor with Zhangixalus dugritei than with Leptomantis bimaculata and Rhacophorus reinwardtii.
Etymology: The generic nomen of Zhangixalus gen.nov. is named after Dr. Ya-Ping Zhang, Vice President of Chinese Academy of Sciences, for using his family name“Zhang”, and ixalus, a common generic root for treefrogs.Dr. Zhang has contributed greatly to the promotion and development of biodiversity and evolutionary studies in China, and we acknowledge his support and encouragement to us, especially for Li’s rhacophorid study. We suggest the English common name of the new genus as “Zhang’s Treefrogs”, and “Zhang Shu Wa Shu( 张 树 蛙 属 )” in Chinese. To avoid possible confusion with regard to administration and conservation in China,we also suggest the Chinese common name of each species remains unchanged, which consistent with previous usages,e.g. Zhangixalus dugritei (宝兴树蛙). The gender of this genus is masculine and is named by Jia-Tang Li, Ke Jiang,Jin-Long Ren and Dechun Jiang.
Content: We currently recognized 36 species in the genus Zhangixalus gen. nov. as follows: Z. achantharrhena(Harvey, Pemberton, and Smith, 2002) comb. nov.;Z. arboreus (Okada and Kawano, 1924) comb. nov.;Z. arvalis (Lue, Lai, and Chen, 1995) comb. nov.; Z.aurantiventris (Lue, Lai, and Chen, 1994) comb. nov.;Z. burmanus (Andersson, 1939) comb. nov.; Z. chenfui(Liu, 1945) comb. nov.; Z. dennysi (Blanford, 1881)comb. nov.; Z. dorsoviridis (Bourret, 1937) comb. nov.; Z.duboisi (Ohler, Marquis, Swan, and Grosjean, 2000) comb.nov.; Z. dugritei (David, 1872) comb. nov.; Z. dulitensis(Boulenger, 1892) comb. nov.; Z. feae (Boulenger, 1893)comb. nov.; Z. hongchibaensis (Li, Liu, Chen, Wu,Murphy, Zhao, Wang, and Zhang, 2012) comb. nov.; Z.hui (Liu, 1945) comb. nov.; Z. hungfuensis (Liu and Hu,1961) comb. nov.; Z. jarujini (Matsui and Panha, 2006)comb. nov.; Z. leucofasciatus (Liu and Hu, 1962) comb.nov.; Z. lishuiensis (Liu, Wang, and Jiang, 2017) comb.nov.; Z. minimus (Rao, Wilkinson, and Liu, 2006) comb.nov.; Z. moltrechti (Boulenger, 1908) comb. nov.; Z.nigropunctatus (Liu, Hu, and Yang, 1962) comb. nov.;Z. omeimontis (Stejneger, 1924) comb. nov.; Z. owstoni(Stejneger, 1907) comb. nov.; Z. pinglongensis (Mo, Chen,Liao, and Zhou, 2016) comb. nov.; Z. prasinatus (Mou,Risch, and Lue, 1983) comb. nov.; Z. prominanus (Smith,1924) comb. nov.; Z. puerensis (He, 1999) comb. nov.;Z. schlegelii (Günther, 1858) comb. nov.; Z. smaragdinus(Blyth, 1852) comb. nov.; Z. suffry (Bordoloi, Bortamuli,and Ohler, 2007) comb. nov.; Z. taipeianus (Liang and Wang, 1978) comb. nov.; Z. viridis (Hallowell, 1861)comb. nov.; Z. wui (Li, Liu, Chen, Wu, Murphy, Zhao,Wang, and Zhang, 2012) comb. nov.; Z. yaoshanensis (Liu and Hu, 1962) comb. nov.; Z. yinggelingensis (Chou, Lau,and Chan, 2007) comb. nov.; Z. zhoukaiyae (Pan, Zhang,and Zhang, 2017) comb. nov.
Distribution: Occurs in southern and southwestern China mainly, as well as the southern Japan, southern slope of Himalayas, and northern part of Myanmar, Thailand, Laos,and Vietnam.
Comparison: Zhangixalus gen. nov. differs from Rhacophorus by the absence of dermal folds along limbs and tarsal projections (vs. present), the absence of supracloacal fold (vs. present or not); differs from Leptomantis by the relatively larger body size (SVL 30-120 mm, above 50 mm in most species vs. SVL 30-80 mm, within 30-50 mm in most species), rather rounded snout (vs. snout pointed), the absence of tarsal and upper eyelid projections (vs. tarsal projections, upper eyelid projections present or not), and different dorsal coloration(mostly green vs. light tan or reddish brown); differs from Buergeria by having “Y” shaped phalange (vs. absent);differs from Theloderma by relatively smooth dorsal skin(vs. dorsal surfaces with developed tubercles); differs from Kurixalus by the absence of prominent tubercles on outer edge of tarsus (vs. present); differs from Nasutixalus by the absence of a pale “X” shaped pattern on iris and raised canthus rostralis (vs. a pale “X” shaped pattern on iris and the raised canthus rostralis present); differs from Polypedates by webbing between fingers (vs. no web);differs from Taruga by the absence of snout and tarsal projections (vs. snout projection or tarsal projections present); differs from Chiromantis, Feihyla, and Liuixalus by the larger body size (SVL 30-120 mm, above 50 mm in most species vs. SVL below 40 mm in most species);and differs from Beddomixalus, Mercurana, Nyctixalus,Philautus, Pseudophilautus and Raochestes by the different reproductive modes (foam nests produced by breeding pairs vs. without foam nests).
Although the taxonomic statuses of the three wellsupported and highly diverged lineages of Rhacophorus sensu lato were preliminarily solved herein, numerous lineages within this species-rich group still remained understudied (Chan et al., 2018). For example, the generic placement of a further deeply divergent clade that includes Z. achantharrhena, Z. dulitensis, and Z. prominanus within genus Zhangixalus requires further study. The clade that includes Z. achantharrhena, Z. dulitensis, and Z. prominanus was recovered as the sister-group of all the remaining species of Zhangixalus, which can be diagnosed readily from latter clade in the presence of dermal folds along limbs and tarsal projections (vs. absent). The three species in this clade also differ from Rhacophorus and Leptomantis by a suit of morphological characters,including body relatively small, SVL 36-50 mm, the presence of dermal folds along limbs, well-developed supracloacal folds, the presence of tarsal projections and green dorsal coloration. However, we cannot evaluate the taxonomic status of Z. achantharrhena, Z. dulitensis, and Z. prominanus due to unavailable material, and tentatively assign them as members of genus Zhangixalus, pending further study.
Key to the genera of Rhacophorus sensu lato
In the absence of a key to all genera of Rhacophoridae,morphological diagnosis of some genera should be de fi ned in the further study. Following the new taxonomic arrangement in this work, the diagnostic key to all genera of Rhacophorus sensu lato was provided, which including three genera, i.e. Rhacophorus sensu stricto, Leptomantis,and Zhangixalus gen. nov.
1 Dermal folds along limbs present................Rhacophorus
- Dermal folds along limbs absent......................................2
2 Tarsal projections absent; body size relatively moderate or large, SVL 30-120 mm (mostly above 50 mm); dorsal coloration mostly green; eastern Asia and northern Indochina distributed......................Zhangixalus gen. nov.
- Tarsal projections present or not; body size relatively
small, SVL 30-80 mm (mostly within 30-50 mm); dorsal
coloration mostly light tan or reddish brown; Maritime
Southeast Asia distributed..............................Leptomantis
Lastly, for the lack of both molecular and morphological data, the taxonomic status of “Rhacophorus” edentulus Müller, 1894, “Rhacophorus” georgii Roux, 1904, and“Rhacophorus” turpes Smith, 1940 remains uncertain.The unavailability of further materials for certain species hampers further taxonomic work. Possible misidentifications, undescribed new taxa, and nonmonophyly of some species also reflect the need of continuous taxonomic analyses on this group (Chan et al.,2018).
Acknowledgements We thank Mr. Hanren LIU and Ms. Tong YANG for their kind helps in the molecular data collection; Dr. Robert W. MURPHY for polishing English of the manuscript; and Mr. Cheng LI for photos. This work was supported by National Key R&D Program of China (2016YFC1200705); the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB31000000); the National Natural Science Foundation of China (31722049, 31772434); Key Research Program of Frontier Sciences, CAS (QYZDBSSW-SMC058); the Youth Innovation Promotion Association of CAS; Southeast Asia Biodiversity Research Institute (Y4ZK111B01); and the CAS “Light of West China” Program (2018XBZG_JCTD_001).
Appendix

Figure S1 Bayesian phylogenetic relationships of species of Rhacophorus, based on three mitochondrial genes: 12S rRNA, tRNA-val and 16S rRNA. Circles on nodes correspond to three clades: Clade A (green), Clade B (orange), Clade C (blue). Numbers beside the nodes are given as Bayesian posterior probabilities (BPP) (≥0.90 retained).

Table S1 Samples, with sampling site, museum voucher nos., and GenBank accession Nos. of corresponding sequences. “-” represents missing data.

(Continud Table S1)
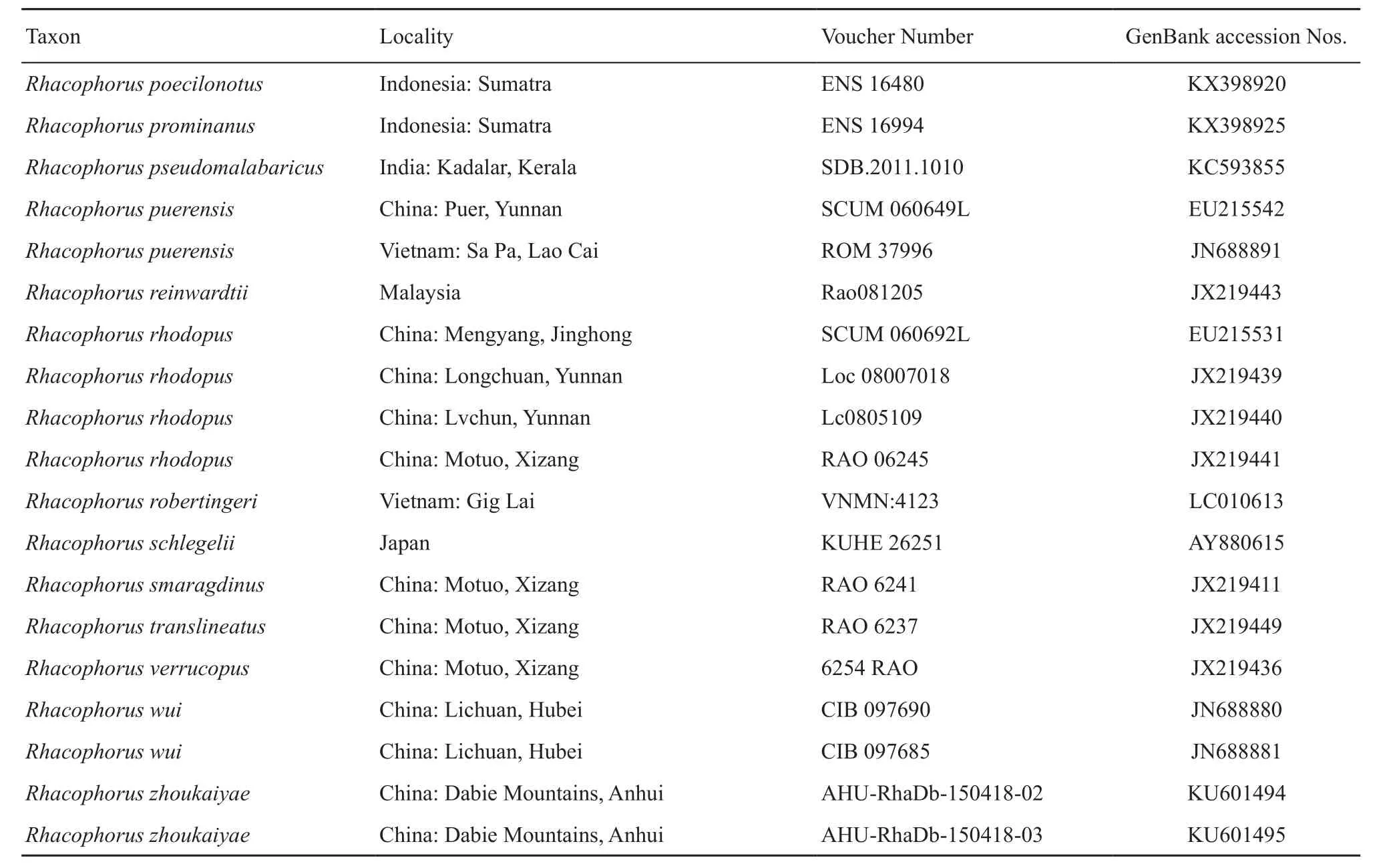
(Continud Table S1)
 Asian Herpetological Research2019年1期
Asian Herpetological Research2019年1期
- Asian Herpetological Research的其它文章
- Investigating the Effectiveness of Road-related Mitigation Measures under Semi-controlled Conditions: A Case Study on Asian Amphibians
- Embryonic Growth and Yolk Depletion during Incubation in the Chinese Skink, Plestiodon chinensis
- Microhabitat Segregation of Parapatric Frogs in the Qinling Mountains
- Macroecological Patterns of Climatic Niche Breadth Variation in Lacertid Lizards
- Female-biased Dispersal of the Emei Moustache Toad(Leptobrachium boringii) under Local Resource Competition
- A New Species of the Genus Trimeresurus from Southwest China(Squamata: Viperidae)
