基于5-氯烟酸的镍Ⅱ和锌Ⅱ配位聚合物的合成、晶体结构、荧光和磁性质
陈金伟 黎 彧 邹训重 邱文达 成晓玲
(1广东轻工职业技术学院轻化工技术学院,广州 510300)
(2广东轻工职业技术学院广东省特种建筑材料及其绿色制备工程技术研究中心/佛山市特种功能性建筑材料及其绿色制备技术工程中心,广州 510300)
(3广东工业大学轻工化工学院,广州 510006)
0 Introduction
The design and hydrothermal syntheses of metalorganic coordination polymers have attracted great interest in the field of coordination chemistry and organic chemistry owing to their intriguing architectures and topologies,as well as potential applications in catalysis,magnetism,luminescence and gas absorption[1-6].There are many factors,such as the coordination geometry of the metal centers,type and connectivity of organic ligands,stoichiometry,reaction conditions,template effect,presence of auxiliary ligands,and pH values influencing the structures of target coordination polymers during self-assembly[7-12].Among these factors,organic ligands play a very importantrole in constructing coordination polymers.
As we known,the carboxylate ligands have been certified to be of great significance as constructors due to their abundant coordination modes,which could satisfy differentgeometric requirementsofmetal centers[13-18]. Apart from carboxylate ligands,1,10-phenanthroline (phen)and 2,2′-biimidazole(H2biim)have often been used as secondary N,N-donor building blocks to construct and stabilize new structures,on account of their effective π…π stacking and/or weak H-bonding interactions[15-18].As a continuation of our research in this field,we have tested the hydrothermal self-assembly reactions of nickel and zinc chlorides with 5-chloronicotinic acid(5-Clnic)as a main building block and 1,10-phenanthroline or 2,2′-biimidazole as N,N-donor anxiliary ligands in view of the following considerations:(A)an availability of pyridyl N and carboxylate O atoms for the coordination to a metal center, (B)the presence of Cl-functionality that is capable of taking part in Cl…Cl interaction,(C)facilitating the crystallization ofcompounds and stabilization of their structures by the introduction of phen and H2biim ligands.
Taking into consideration the above discussion,we herein report the syntheses,crystal structures,magnetic and luminescent properties of four NiⅡand ZincⅡ coordination polymers constructed from 5-chloronicotinic acid ligand.
1 Experimental
1.1 Reagents and physical measurement
All chemicals and solvents were of AR grade and used without further purification.Carbon,hydrogen and nitrogen were determined using an Elementar Vario EL elemental analyzer.IR spectra were recorded usingKBrpelletsandaBrukerEQUINOX 55 spectrometer.Thermogravimetric analysis (TGA)data were collected on a LINSEIS STA PT1600 thermal analyzer with a heating rate of 10℃·min-1.Magnetic susceptibility data were collected in a temperature range of 2~300 K with a Quantum Design SQUID Magnetometer MPMS XL-7 with a field of 0.1 T.A correction was made for the diamagnetic contribution prior to data analysis.Excitation and emission spectra were recorded for the solid samples on an Edinburgh FLS920 fluorescence spectrometer at room temperature.
1.2 Synthesis of{[Ni(μ-5-Clnic)(μ3-5-Clnic)(μ-H2O)0.5]·1.5H2O}n(1)
A mixture of NiCl2·6H2O (0.025 g,0.10 mmol),5-ClnicH(0.032 g,0.20 mmol),NaOH(0.008 g,0.20 mmol),and H2O(10 mL)was stirred at room temperature for 15 min,and then sealed in a 25 mL Teflonlined stainless steel vessel,and heated at 160℃for 3 days,followed by cooling to room temperature at a rate of 10℃·h-1.Green block-shaped crystals of 1 were isolated manually,and washed with distilled water.Yield:71%(based on 5-ClnicH).Anal.Calcd.for C12H10Cl2NiN2O6(%): C 35.34,H 2.47,N 6.87;Found(%):C 35.22,H 2.46,N 6.91.IR (KBr,cm-1):3 434w,3 125w,1 632m,1 562 w,1 528w,1 457w,1 394s,1 289w,1 236w,1 131w,1 097w,1 026w,909w,782w,746m,688w,589w.
1.3 Synthesis of[Ni(5-Clnic)(μ-5-Clnic)(H2biim)]n (2)
The synthesis of complex 2 is same to 1 except that H2biim(0.014 g,0.1 mmol)is added.Blue blockshaped crystals of 2 were isolated manually,and washed with distilled water.Yield:62% (based on 5-ClnicH). Anal. Calcd.for C18H12Cl2NiN6O4(%):C 42.73,H 2.39,N 16.61;Found(%):C 42.61,H 2.41,N 16.85.IR (KBr,cm-1):1 609s,1 551w,1 452w,1 388s,1 370m,1 335w,1 277w,1 189w,1 125m,1 026w,991w,927w,898w,782m,752w,688w,595w.
1.4 Synthesis of[Zn(5-Clnic)(μ-5-Clnic)(H2biim)]n(3)
Synthesis of 3 was similar to 2 except using ZnCl2(0.014 g,0.10 mmol)instead of NiCl2·6H2O.Colorless block-shaped crystals of 3 were isolated manually,and washed with distilled water.Yield 55%(based on Zn).Colorless block-shaped crystals of 3 were isolated manually,and washed with distilled water.Yield:65%(based on 5-ClnicH).Anal.Calcd.for C18H12Cl2ZnN6O4(%):C 42.17,H 2.36,N 16.39;Found(%):C 42.34,H 2.37,N 16.53.IR(KBr,cm-1):1 609s,1 556w,1 440w,1 394s,1 370s,1 329w,1 283w,1 189w,1 125m,1 032 w,991w,898w,782m,752w,729w,688w.
1.5 Synthesis of{[Zn(5-Clnic)(μ-5-Clnic)(phen)]·2H2O}n(4)
Synthesis of 4 was similar to 3 except using phen(0.020 g,0.10 mmol)instead of H2biim.Colorless block-shaped crystals of 4 were isolated manually,and washed with distilled water.Yield:60% (based on 5-ClnicH).Anal.Calcd.for C24H18Cl2ZnN4O6(%):C 48.47,H 3.05,N 9.42;Found(%):C 48.35,H 3.07,N 9.50.IR(KBr,cm-1):3 438w,3 097w,1 628s,1 561m,1 510w,1 423w,1 393s,1 291w,1 225w,1 184w,1 133w,1 102 w,1 026w,898w,847w,786w,750w,724m,684w,638w.The compounds are insoluble in water and common organic solvents,such as methanol,ethanol,acetone,and DMF.
1.6 Structure determination
Four single crystals with dimensions of 0.26 mm×0.21 mm×0.20 mm (1),0.28 mm×0.26 mm×0.25 mm(2),0.27 mm×0.23 mm×0.22 mm (3),and 0.25 mm×0.23 mm×0.21 mm(4)were collected at 293(2)K on a Bruker SMART APEXⅡCCD diffractometer with Mo Kα radiation(λ=0.071 073 nm).The structures were solved by direct methods and refined by full matrix least-square on F2using the SHELXTL-2014 program[19].All non-hydrogen atoms were refined anisotropically.All the hydrogen atoms were positioned geometrically and refined using a riding model.Some lattice solvent moleculesin 1 arehighlydisordered and were removed using the SQUEEZE routine in PLATON[20].The number of solvent H2O molecules was obtained on the basis of elemental and thermogravimetric analyses.A summary of the crystallography data and structure refinements for 1~4 is given in Table 1.The selected bond lengths and angles for compounds 1~4 are listed in Table 2.Hydrogen bond parameters of compounds 1~4 are given in Table 3.
CCDC:1859598,1;1859599,2;1859600,3;1859601,4.
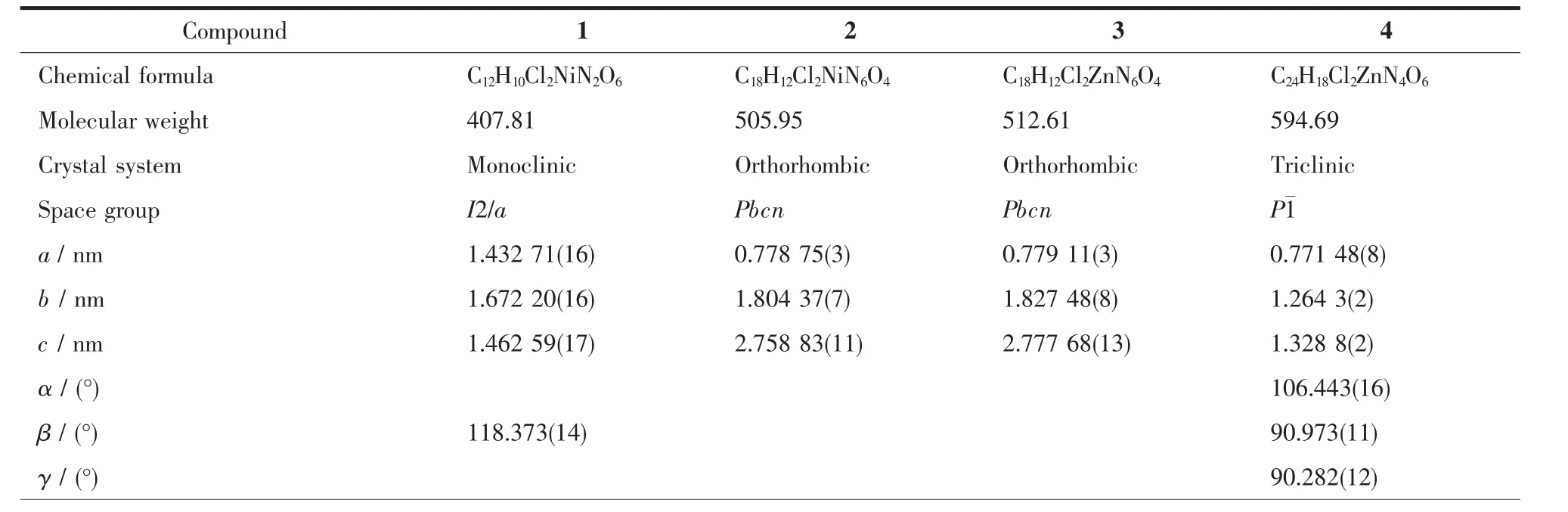
Table 1 Crystal data for compounds 1~4
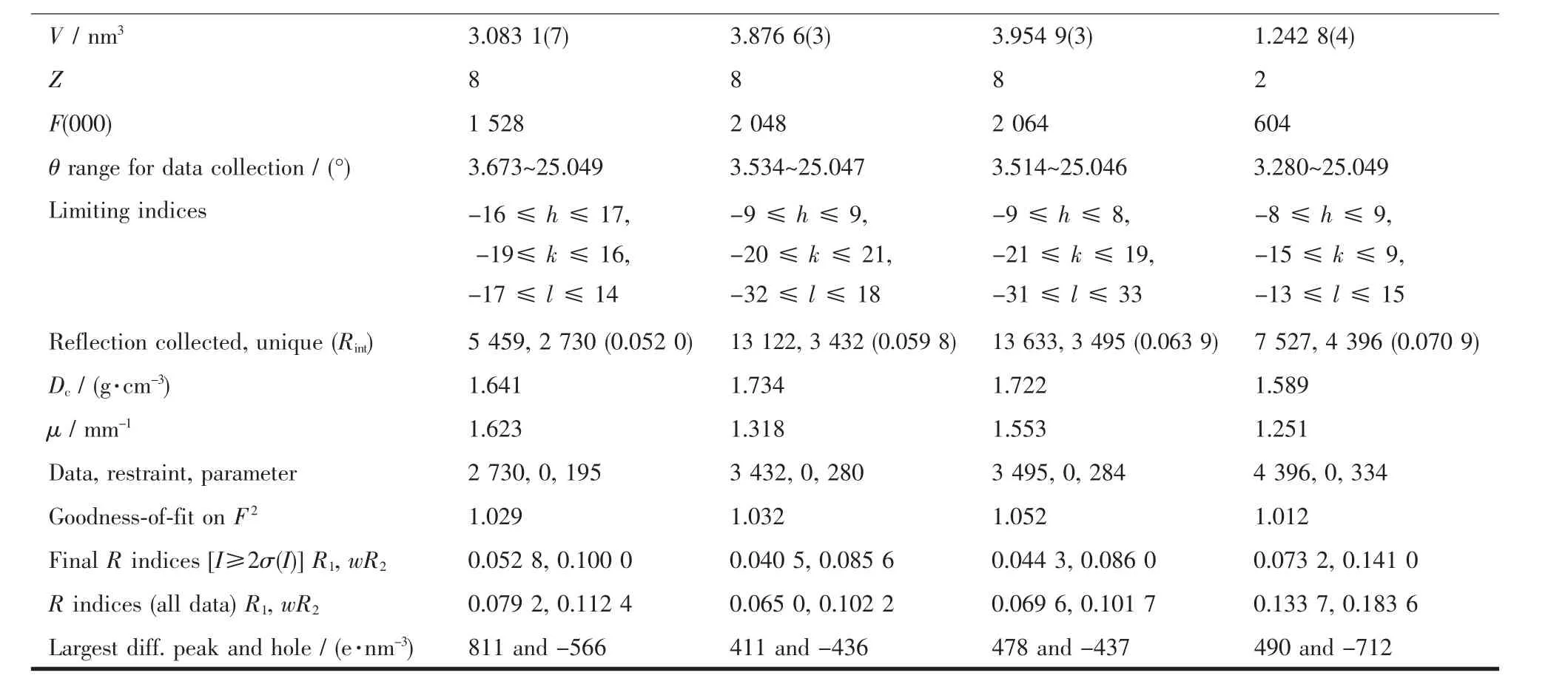
Continued Table 1
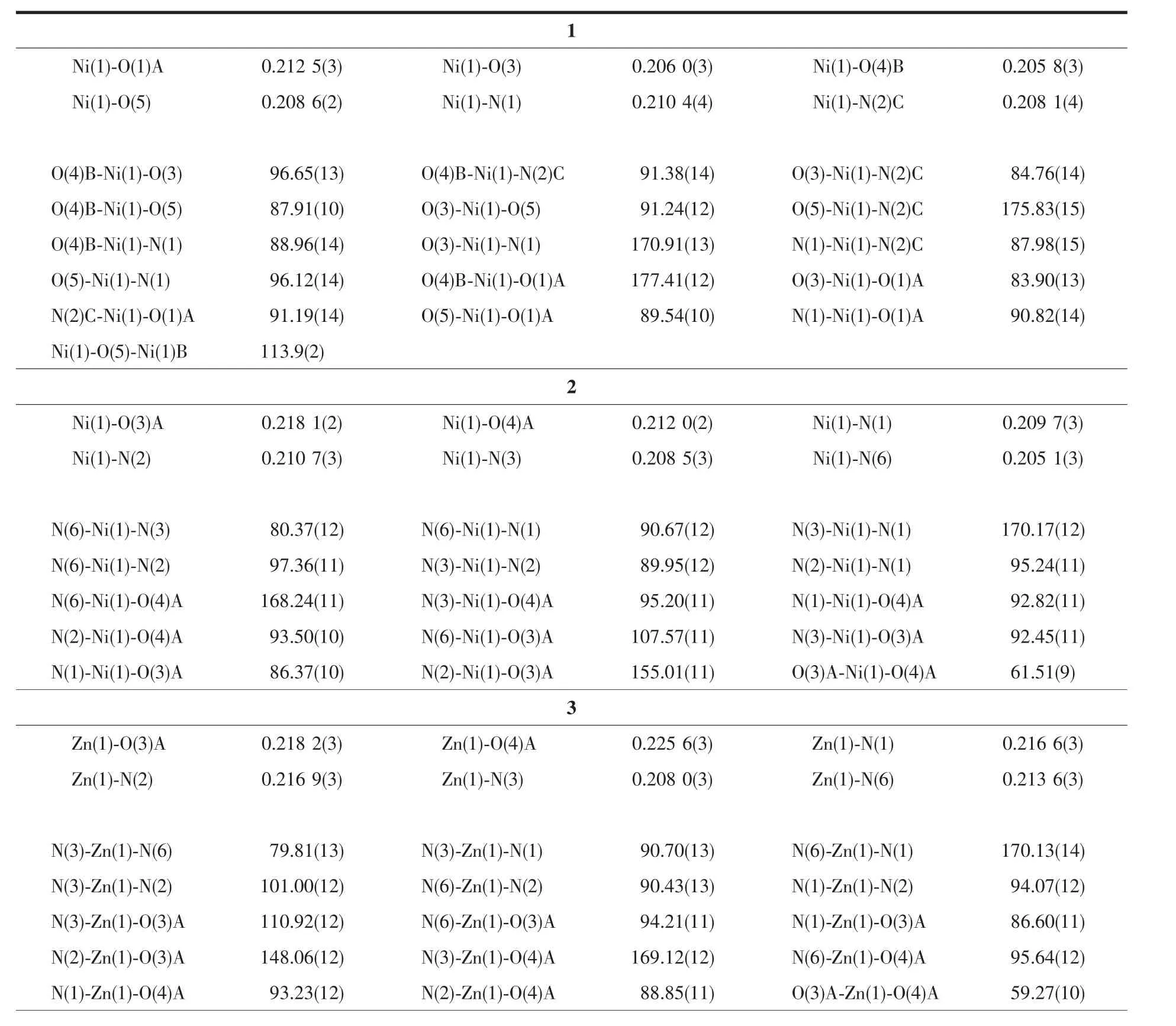
Table 2 Selected bond distances(nm)and bond angles(°)for compounds 1~4
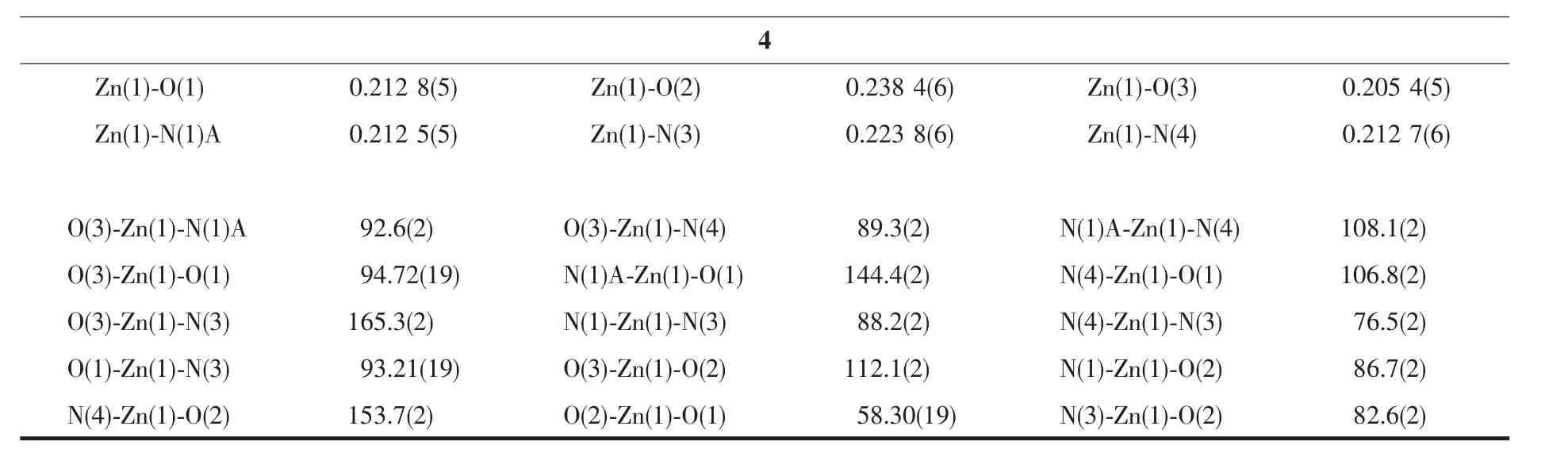
Continued Table 2

Table 3 Hydrogen bond lengths(nm)and angles(°)of compounds 1 and 2
2 Results and discussion
2.1 Description of the structure
2.1.1 {[Ni(μ-5-Clnic)(μ3-5-Clnic)(μ-H2O)0.5]·1.5H2O}n(1)
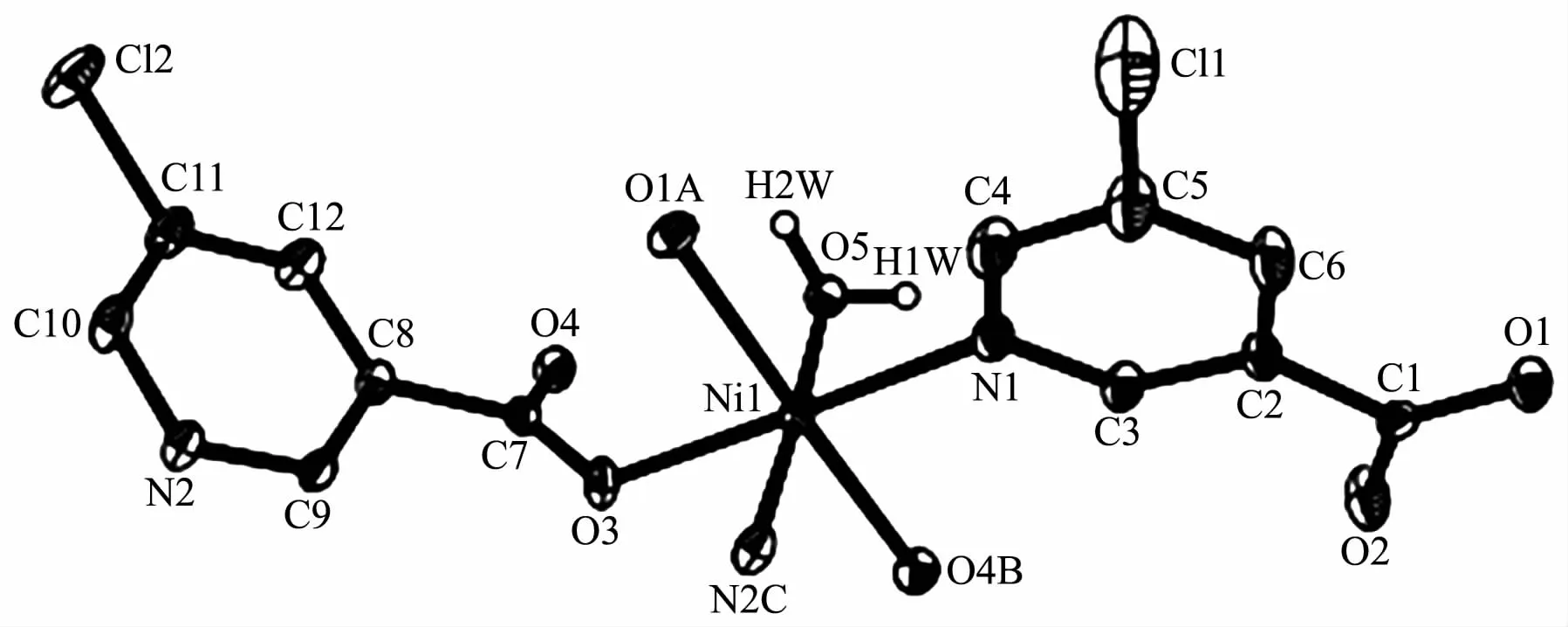
Fig.1 Drawing of asymmetric unit of compound 1 with 30%probability thermal ellipsoids
Compound 1 has a 3D metal-organic framework structure.The asymmetric unit of 1 (Fig.1)comprises one NiⅡ ion,two μ-5-Clnic-and μ3-5-Clnic-ligands,and a half of H2O ligand that is positioned on a 2-fold rotation axis.The six-coordinated Ni1 ion is surrounded by three O atoms of three different 5-Clnic-blocks,one O atom of H2O ligand,and two N atoms of two individual 5-Clnic-moieties,constructing a slightly distorted {NiN2O4}octahedral geometry.The Ni-O(0.205 8(3)~0.212 5(3)nm)and Ni-N(0.208 1(4)~0.210 4(4)nm)bond lengths are in good agreement withthoseobservedinsomeotherNiⅡcompounds[17-18,21].In 1,the 5-Clnic-blocks take μ-and μ3-coordination modes (modesⅠandⅡ,Scheme 1),in which the carboxylate groups act in η1∶η0monodentate and μ2-η1∶η1bidentate modes,respectively.Two Ni1 centers are bridged by two carboxylate groups and one μ-H2O ligand,giving rise to a binuclear Ni2unit(Fig.2)with a Ni…Ni separation of 0.349 7(4)nm and a Ni-Owater-Ni angle of 113.9(2)°.The adjacent Ni2units are multiply interlinked by the 5-Clnic-blocks to form an intricate 3D framework (Fig.3).Interestingly,in the MOF 1 the distance between the adjacent Cl atoms is 0.342 4(4)nm,which is shorter than the sum of the van der Waals radii of the two Cl atoms(ca.0.350 nm)[22],thus indicating the existence ofthe Cl…Cl interactions(Fig.3).

Fig.2 Di-nickelⅡunit of 1

Fig.3 Perspective of a 3D framework of 1 parallel to bc plane
2.1.2 [Ni(5-Clnic)(μ-5-Clnic)(H2biim)]n(2)and[Zn(5-Clnic)(μ-5-Clnic)(H2biim)]n(3)
Compounds 2 and 3 are isostructural and feature a 1D metal-organic chain.As a representative example,the structure of 2 is described in detail(Fig.4).The asymmetric unit of compound 2 contains one crystallographically unique NiⅡion,two distinct 5-Clnic-and μ-5-Clnic-ligands,and one H2biim moiety.As depicted in Fig.4,each NiⅡion is six-coordinated and adopts a distorted octahedral {NiN4O2}geometry formed by two carboxylate O atoms of one μ-5-Clnic-block,two N atoms of two different 5-Clnic-and μ-5-Clnic-ligands as well as two N atoms of one H2biim moiety.The Ni-O(0.212 0(2)~0.218 1(2)nm)and Ni-N(0.205 1(3)~0.210 7(3)nm)bond lengths are comparable to the literature data[15,17,21].In 2,the 5-Clnic-ligands exhibit two different terminal and μ-coordination modes(modesⅢandⅣ,Scheme 1),in which the carboxylate group either shows a η1∶η1bidentate mode or remains uncoordinated.The H2biim ligand actsin a bidentate chelating mode;the dihedral angle of two imidazole groups is 1.17°.The μ-5-Clnic-moieties alternatively link the adjacent NiⅡcenters to form a linear 1D metal-organic chain with the Ni…Ni separation of 0.778 7(3)nm(Fig.5).The neighboring chains are assembled into 2D supramolecular sheetmotifs through the N-H…O hydrogen bonds(Fig.6).
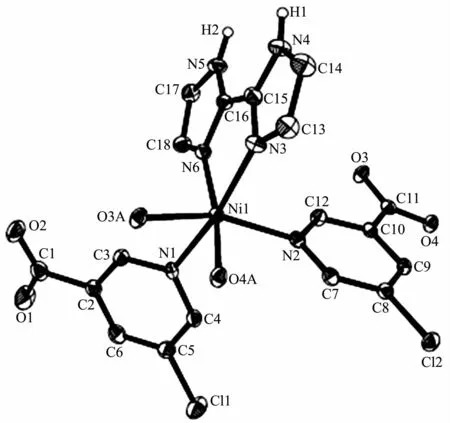
Fig.4 Drawing of asymmetric unit of compound 2 with 30%probability thermal ellipsoids
2.1.3 {[Zn(5-Clnic)( μ-5-Clnic)(phen)]·2H2O}n(4)
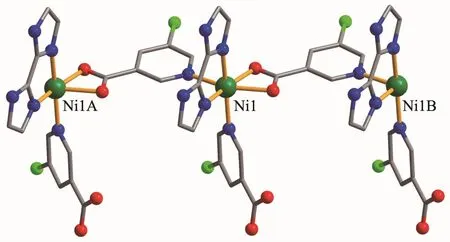
Fig.5 Perspective view of 1D chain along c axis
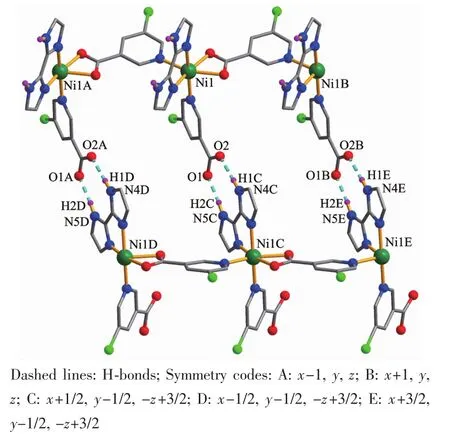
Fig.6 Perspective of a 2D supramolecular network along a axis
The compound 4 crystallizes in the triclinic space group P1 and also shows a linear 1D metalorganic chain structure.The asymmetric unit bears one crystallographically independent ZnⅡion,one terminal 5-Clnic-and one μ-5-Clnic-block,one phen ligand,and two lattice water molecules.As depicted in Fig.7,the six-coordinated ZnⅡion adopts a distorted octahedral{ZnN3O3}geometry taken by three O atoms from the two distinct 5-Clnic-moieties and three N atoms from one 5-Clnic-and one phen ligand.The Zn-O and Zn-N bond distances are in ranges of 0.205 4(5)~0.238 4(6)nm and 0.212 5(5)~0.223 8(6)nm,which are within typical values for the ZnⅡderivatives[16,23-24].In 4,the 5-Clnic-ligands show two different coordination modes (modesⅣ andⅤ,Scheme 1),in which the carboxylate groups are either η1∶η0monodentate or η1∶η1bidentate.It should be mentioned that the N atom of 5-Clnic-remains uncoordinated in the modeⅤ .The μ-5-Clnic-moieties alternately bridge the adjacent ZnⅡcenters to form a linear 1D metalorganic chain(Fig.8).The neighboring chains are sewed up into 3D supramolecular framework through Cl…Cl(0.331 6(5)nm)interactions and O-H…O hydrogen bonds involving lattice water molecules(Fig.9).
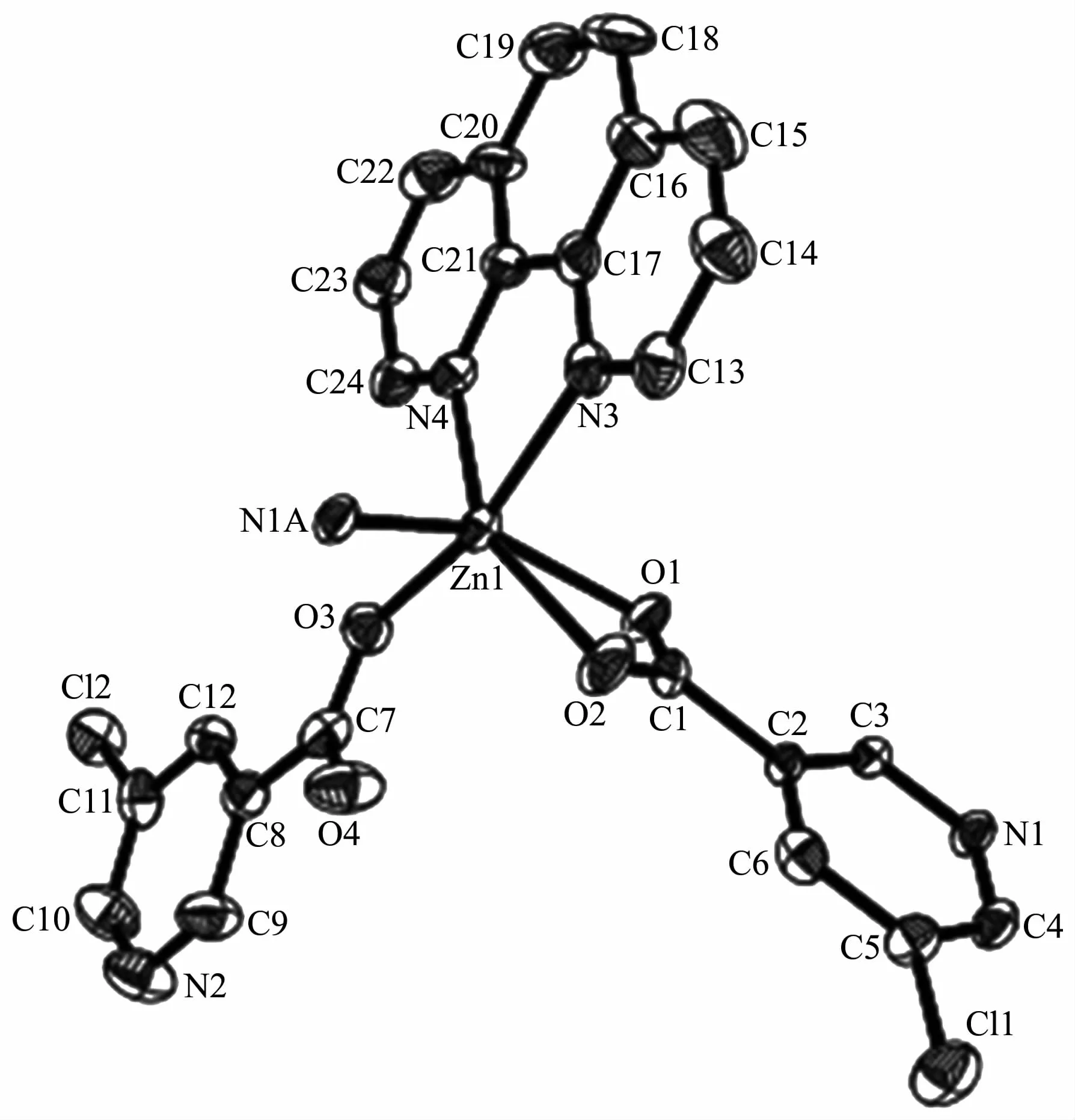
Fig.7 Drawing of asymmetric unit of compound 4 with 30%probability thermal ellipsoids
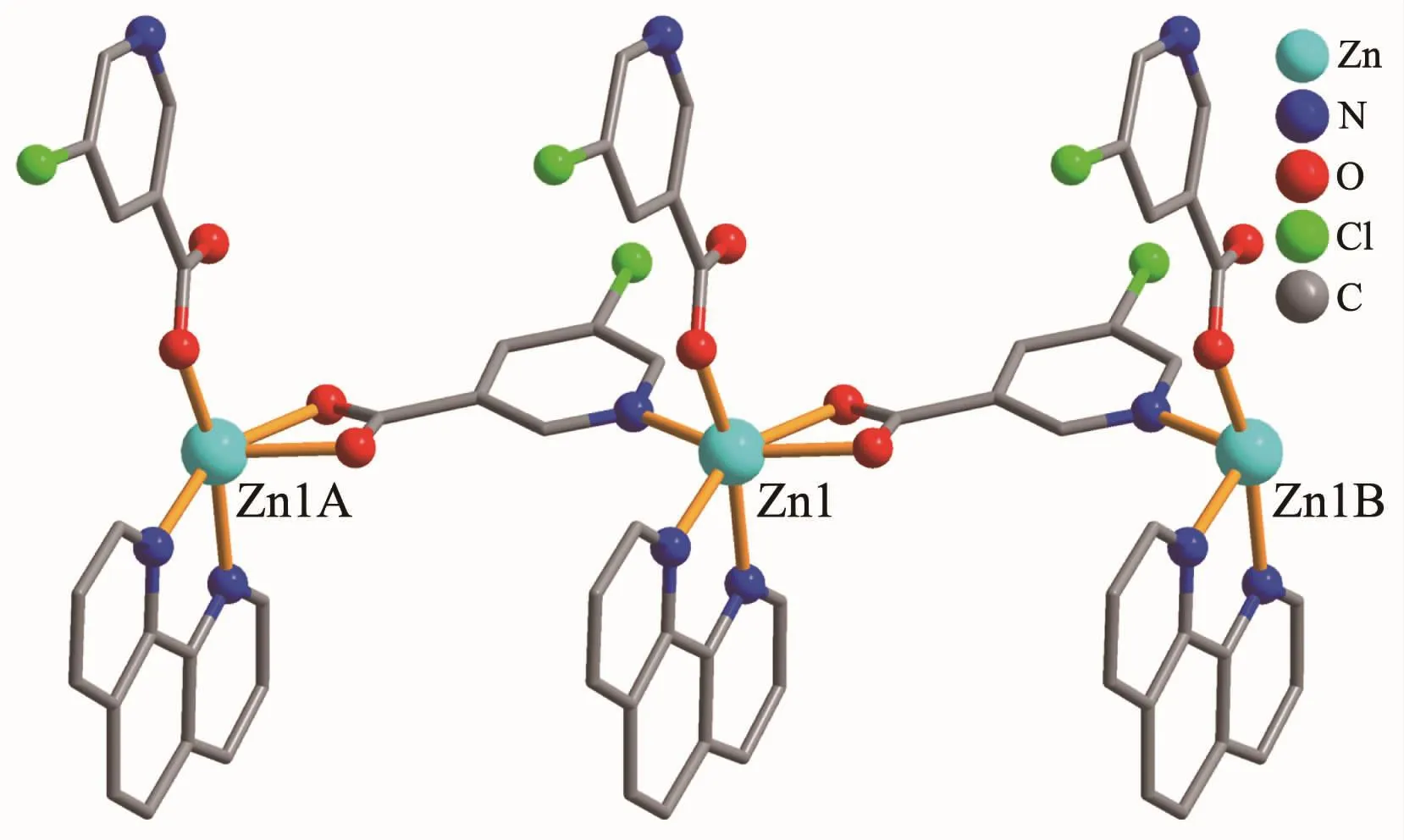
Fig.8 One dimensional chain in compound 4
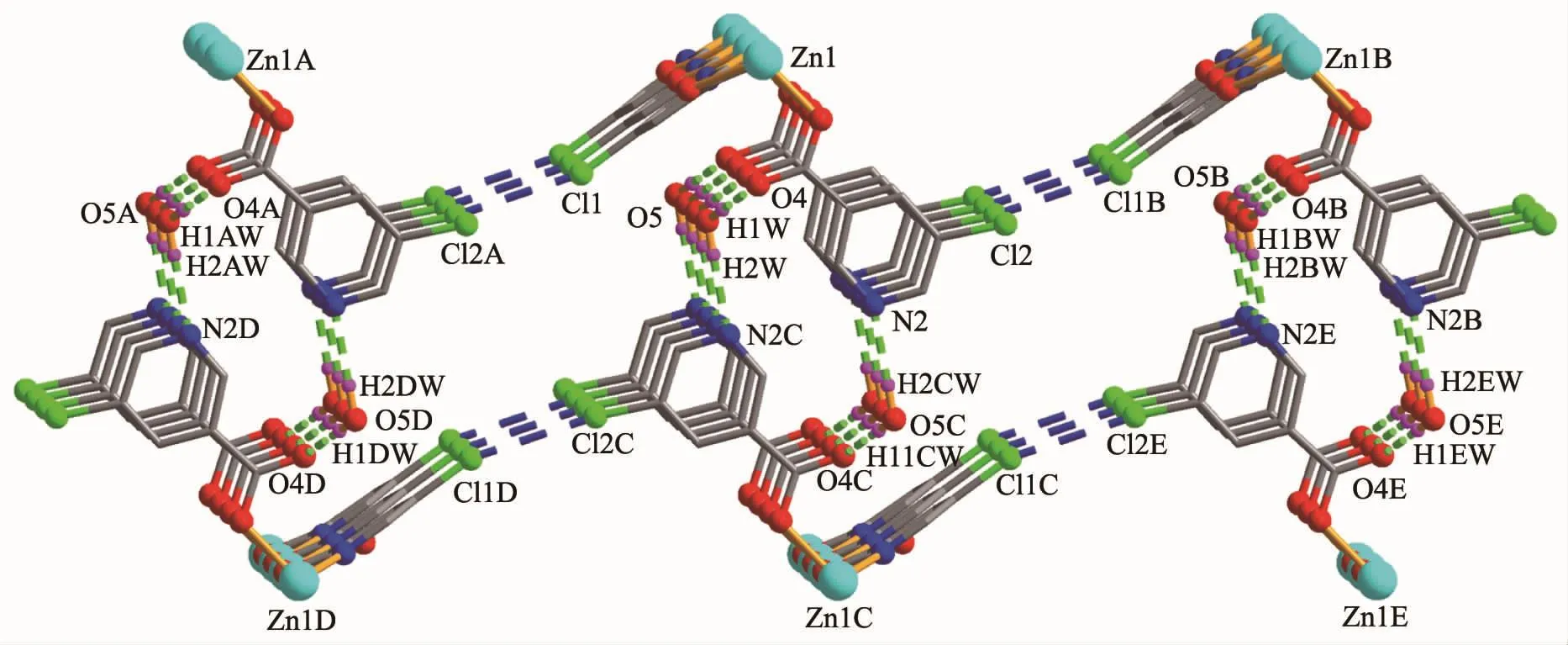
Fig.9 Perspective of a 3D supramolecular framework along a axis
2.2 TGA analysis
To determine the thermal stability of compounds 1~4,their thermal behaviors were investigated under nitrogen atmosphere by thermogravimetric analysis(TGA).As shown in Fig.10,polymer 1 lost its one and a half of lattice water molecules as well as a half of one H2O ligand from 30 to 160℃ (Obsd.8.7%;Calcd.8.8%),followed by the decomposition at 329℃.For polymers 2 and 3,the TGA curves revealed that their samples were stable up to 348℃and 260℃,respectively,followed by a decomposition on further heating.The TGA curve of 4 showed that two lattice water molecules were released from 29 to 79℃(Obsd.5.8%;Calcd.6.0%),and the dehydrated solid began to decompose at 278℃.
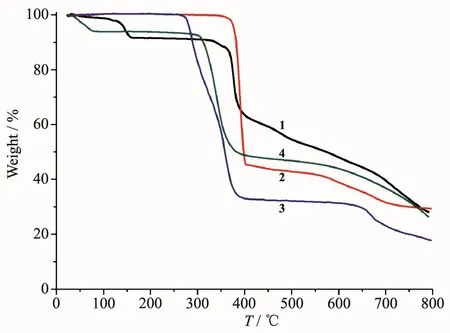
Fig.10 TGA curves of compounds 1~4
2.3 Magnetic properties
Variable-temperature magnetic susceptibility studies were carried out on powder samples of 1 and 2 in a temperature range of 2~300 K.For the NiⅡMOF 1,the χMT value at 300 K was 1.16 cm3·mol-1·K,which was higher than the spin only value of 1.00 cm3·mol-1·K for one magnetically isolated NiⅡ center(SNi=1,g=2.0).Upon cooling,the χMT value droped down very slowly from 1.16 cm3·mol-1·K at 300 K to 1.12 cm3·mol-1·K at 37 K and then decreased steeply to 0.26 cm3·mol-1·K at 2 K (Fig.11).The χM-1vs T plot for 1 in the 3~300 K interval obeyed the Curie-Weiss law with a Weiss constant θ of-10.32 K and a Curie constant C of 1.19 cm3·mol-1·K,suggesting a weak antiferromagnetic interaction between the NiⅡions.Because of the long separation between the adjacent Ni2units,only the coupling interactions within the di-nickelⅡblocks were considered.
We tried to fit the magnetic data of 1 using the following expression[25]for a dinuclear NiⅡunit:
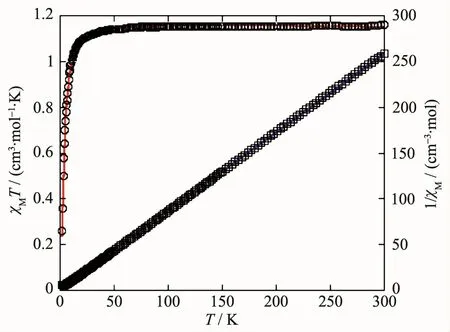
Fig.11 Temperature dependence of χMT(○)and 1/χM(□)vs T for compound 1
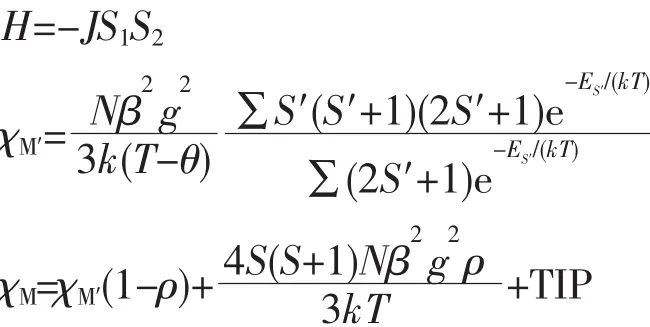
where ρ is a paramagnetic impurity fraction and TIP is temperature independent paramagnetism.Using this model,the susceptibility for 1 above 2.0 K was simulated,leading to the values of J=-2.53 cm-1,g=2.11,ρ=0.005,and TIP=9.04×10-6cm3·mol-1,with the agreement factor R=9.38×10-4(R=∑(Tobs-Tcalc)2/∑(Tobs)2).The negative J parameter confirms that a weak antiferromagnetic exchange coupling exists between the adjacent NiⅡcenters,which is in agreement with a negative θ value.In the structure of 1 (Fig.2),there are two types of magnetic exchange pathways within the dinuclear units,namely via the μ-H2O and μ-η1∶η1-carboxylate(syn-syn)bridges.According to previous studies,the magnetic interaction is highly sensitive to the value of the Ni-O-Ni bridging angle,showing the domination of the Ni-Ni ferromagnetic coupling when the Ni-O-Ni angles are(90±14)°[26].The larger Ni-O-Ni angles in the Ni2unit(113.9(2)°)might suggest that the O bridges could be responsible for an antiferromagnetic exchange component.Meanwhile,the syn-syn carboxylate group bridging is likely to have a dominant antiferromagnetic effect.Hence,the small-J value observed for 1(-2.53 cm-1)could well be a result of two compatible exchange effects.
For 2,the room temperature value of χMT,1.10 cm3·mol-1·K,was close to that of 1.00 cm3·mol-1·K expected for one magnetically isolated high-spin NiⅡion (S=1,g=2.0).Upon cooling,the χMT value droped down very slowly from 1.10 cm3·mol-1·K at 300 K to 1.07 cm3·mol-1·K at 45 K and then decreased steeply to 0.53 cm3·mol-1·K at 2 K (Fig.12).Between 2 and 300 K,the magnetic susceptibilities can be fitted to the Curie-Weiss law with C=1.12 cm3·mol-1·K and θ=-9.14 K.These results indicate an antiferromagnetic interaction between the adjacent NiⅡ centers in compound 2.An empirical(Weng′s)formula can be applied to analyze the 1D systems with S=1,using numerical procedures[27-28]:
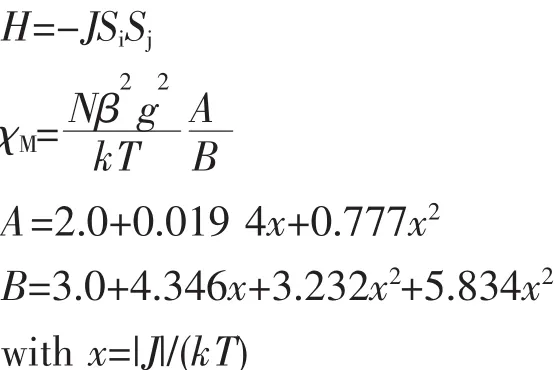
Using this method,the best-fit parameters for 2 were obtained:J=-1.91 cm-1,g=2.05 and R=3.37×10-5.A negative J parameter indicates a weak antiferromagnetic exchange coupling between the adjacent NiⅡcenters in 2,which is in agreement with a negative θ value.

Fig.12 Temperature dependence of χMT(○)and 1/χM(□)vs T for compound 2
2.4 Luminescence properties
The emission spectra of 5-ClnicH and compounds 3 and 4 were measured in the solid state at room temperature,as depicted in Fig.13.The free 5-ClnicH ligand displayed a weak photoluminescence with an emission maximum at 452 nm.For compounds 3 and 4,more intense emission bands are observed with maximum at 522 nm for 3 and 451 nm for 4(λex=305 nm in all cases).All bands can be assigned to an intraligand(π*→n or π*→π)emission[11-13].
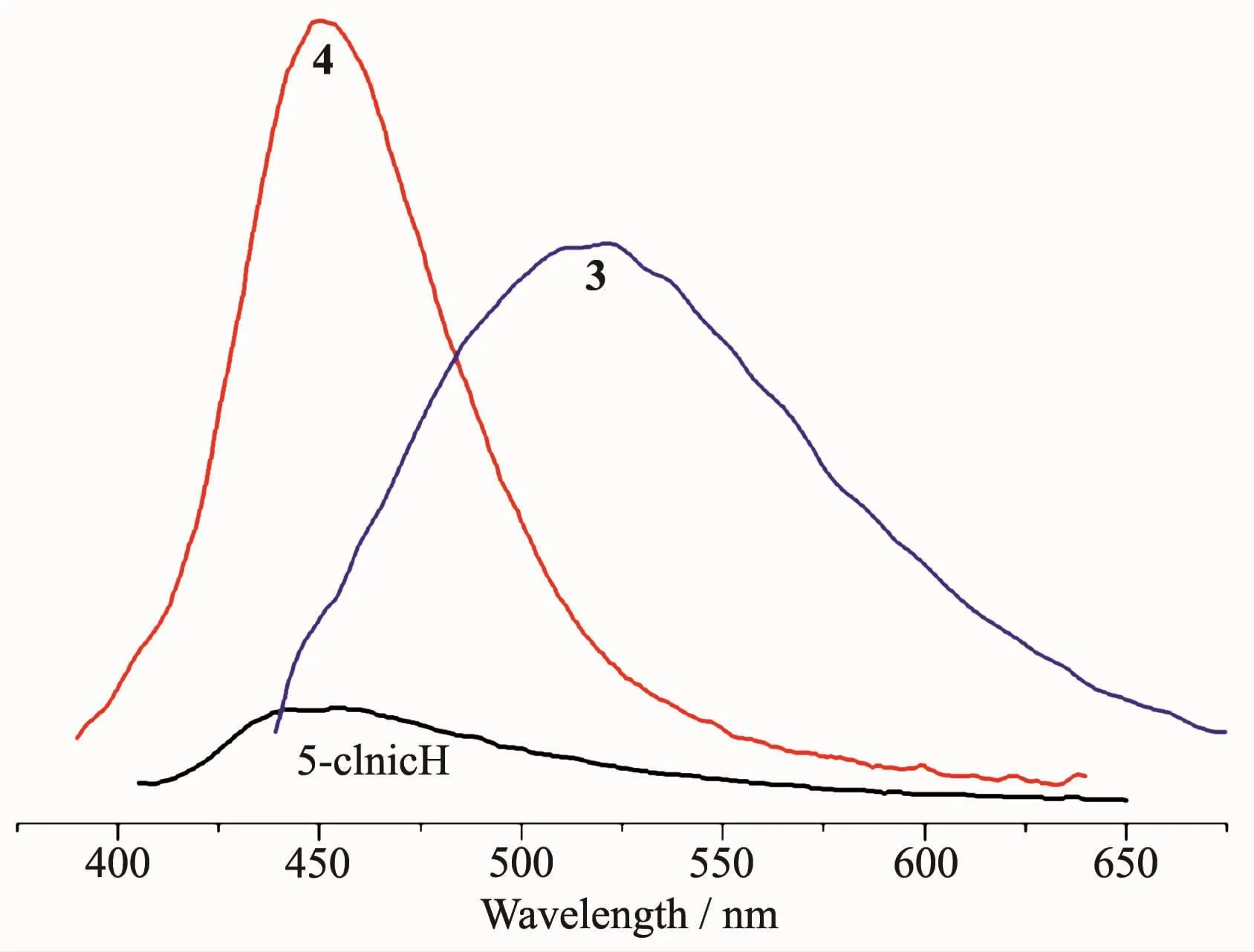
Fig.13 Solid state emission spectra of 5-ClnicH and compounds 3 and 4
3 Conclusions
In thiswork,we applied an aqueous medium self-assembly method for the hydrothermal generation of four new coordination polymers derived from 5-chloronicotinic acid as a main building block.The obtained compounds were fully characterized and their structures range from 3D metal-organic framework(1)to 1D chain (2~4).The low dimensionality of 2~4 arises from the introduction of 2,2′-biimidazole and 1,10-phenanthroline as auxiliary ligands.Besides,the magnetic(for 1 and 2)and Luminescent(for 3 and 4)properties were also investigated and discussed.The results show that such simple,low-cost,and watersoluble carboxylic acid can be used as a versatile multifunctional building block toward the generation of new coordination polymers.

