Low-frequency HIFU induced cancer immunotherapy:tempting challenges and potential opportunities
Guilian Shi, Mingchuan Zhong, Fuli Ye, Xiaoming Zhang
1School of Biomedical Engineering, Hubei University of Science and Technology, Xianning 437100, China; 2Department of Radiology, Mayo Clinic College of Medicine, Rochester 55905, MN, USA
ABSTRACT Immunotherapy is playing an increasingly important role in the treatment of tumors. Different from the traditional direct killing or excision therapies, immunotherapy depends on autologous immunity to kill tumor cells and tissues by activating or enhancing the body’s immune system. Large numbers of recent studies suggest that low-frequency HIFU can not only enhance the intensity of the body’s anti-cancer immune response, but also improve the efficiency of immunotherapy drug delivery to strengthen the effects of tumor immunotherapy. The focused ultrasound (FUS) destructs the tumor and simultaneously generates tumor debris and tumor-associated antigens, which enhances the immunogenicity of the tumor and stimulates the immune cells, inducing the body’s immune response. Microbubbles are clinically used as a contrast. As a matter of fact, the addition of microbubbles can reinforce the destructive effect of FUS on the tumor and activate a stronger immune response. The combined application of ultrasound and microbubbles can more effectively open the blood brain barrier (BBB), which is beneficial to improving the intake of immune cells or immunotherapy drugs and exerting a positive influence in the lesion area. Currently, microbubbles and nanoparticles are commonly used as gene and drug carriers. Using ultrasound, the immune-related gene or antigen delivery itself can enhance the immune response and improve the efficacy of the immunotherapy.
KEYWORDS Immunotherapy; low-frequency HIFU; biological effect; immune response; combination therapy
Introduction
Malignant tumors are one of the main diseases that seriously threaten human life. The therapeutic regimens commonly used in clinic include surgery excision, radiotherapy, and chemotherapy. As several traditional treatment means, they can treat the primary cancers very well although their drawbacks, such as uncontrolled recurrence and metastasis,cannot be ignored in the practical application, so new cancer therapeutic methods need to be developed urgently1.Immunotherapy, a new type of cancer treatment, does not kill the cancer cells directly. By activating or enhancing the human immune system, the cancer immunotherapy eradicates the cancer cells or tissues depending on autoimmune function2. The target of this method aims at the autologous immune system instead of the cancer cells or tissues3. Cancer immunotherapy has received wide attention because of its great clinical application value4.
The functions of the immune system are mainly manifested in three aspects: defense, stabilization and immune surveillance. Once these functions are out of balance, immunopathological reactions occur5. Immunity of the body can be roughly divided into two kinds: specific immunity and non-specific immunity. Non-specific components need not be exposed beforehand and can respond immediately, effectively preventing the invasion of various pathogens. Specific immunity is developed in the lifetime of the body, and is specific to a certain pathogen6.Dendritic cells (DCs) are the most powerful antigen presenting cells and can efficiently absorb, process and present antigens. Immature DCs have strong migration ability. Mature DCs are at the center of initiation, regulation and maintenance of immune response, and can activate the initial T cells effectively7. An effective immune response includes three procedures8: (1) The DCs capture tumor antigens and process them inside acquiring “mature signals”to activate the immune response for the antigens. (2) DCs present antigenic information to the immature T cells in lymphoid tissue and make them activated, which initiates the specific immunity of the human body. If the DCs presenting antigens are activated by immature signals, the T cells will be induced to create tolerance and consequently resist the immune response. (3) The activated T cells infiltrate the cancer tissue, specifically recognize and kill cancer cells.However, tumor cells possess the nature of immune escape,which makes them avoid the recognition and attack from the immune system by multiple mechanisms, so it is very difficult to eradicate the tumor cells or tissues via the autoimmune system of the human body. Eradication of tumors by means of stimulating the autoimmune system is very promising for anticancer treatment9,10. Currently, tumor immunotherapy mainly includes the following types:enhancing a specific immune system11, tumor vaccine12,13,and adoptive cell transfer (ACT)14.
Ultrasound (US) is defined as a kind of mechanical wave with a frequency over 20 kHz and cannot be detected by the human ear. Aside from medical imaging, US also performed well in the treatment field15. In recent years, many studies indicate that US can be used to promote the anti-cancer immune response of the human body16,17. Focused ultrasound (FUS) can not only create thermal effects but also create mechanical effects or cavitation effects18. The mechanical effects or cavitation effects can increase the anticancer immune response of the host body19. Theoretically,these effects can be classified by various US parameters.Cavitation effect, as one of the main mechanical effects,usually occurs at low frequency, high intensity and low duty cycle20. The thermal effects of US often occur at high to moderate intensity and longer duty cycles, and they are generally used in combination with thermosensitive formulations21. Genes and antigens are delivered into the cancer cells or tissues adopting US, which can also activate the anti-cancer immune response20,22. The force exerted on the cell membrane when the microbubble is broken by US directly delivers the substance to the cell, evading each kind of natural barrier23. By this means, one can deliver the cancer antigen and the antigen-encoding gene to immune cells, and the gene that stimulates the immune response can be also delivered to the cancer cells24,25. In this paper, we focused on activation of the anti-tumor immune response using lowfrequency high-intensity focused ultrasound (HIFU).
Low-frequency HIFU
Biological effect of low-frequency HIFU
US is generally considered safe for imaging in vivo except for two side effects26-28: thermal and mechanical effects(including sonoporation). However, these two adverse factors for medical imaging are very important to treatment using US. Thermal effects depend on the absorption and accumulation of US energy. The intensity of US, irradiation time, and biological properties of tissue are the main three factors that determines the amount of heat29. The dose of US has a very strong relevance with thermal and mechanical effects. At a low intensity, the ratio of apoptosis to lysis is high, and with the increasing of intensity, lysis becomes predominant over apoptosis and directly causes cell to death.More effective induction of apoptosis is obtained if paused modulation is used with a longer pause than the irradiation time30,31. Accounting for the degree of membrane damage and the capacity of repairing the damage, the death of a cell can be divided into three modes, instant lysis, necrosis, and apoptosis32,33. Figure 1 shows the correlation between the degree of membrane damage caused by US and the corresponding cell death mode34,35. Although some of the damaged cells can successfully self-repair and eventually survive, the process of US irradiation will speed their apoptosis or necrosis36.
Cavitation effect
The mutual effects between microbubbles and US can easily lead to the cavitation effect37. Microbubbles oscillate symmetrically and linearly at a low powered US field, which implies an opposite tendency of the expansion and compression of a microbubble38. According to the different dynamic behaviors of bubbles, cavitation effects of an US wave can be divided into stable cavitation and inertial cavitation39,40. If the sound intensity of US is weak, the cavitation nucleus is enlarged in the negative pressure stage of sound pressure, and then compressed and reduced in the positive pressure stage41. In other words, the bubble vibrates radially with the balanced radius of sound frequency, which is called steady state cavitation. When the bubbles are compressed under greater pressure to a certain degree, the density of mixed gas inside the bubbles will increase, and it is difficult for the bubbles to continue being compressed42.However, because of the inertial push from the surrounding fluid, the increasing speed of pressure on the bubble wall is still very fast, even surpassing the compression speed of gas inside the bubble. Under such a condition, bubbles will collapse, and the intensity is determined by the inertia of inward-pushing fluid43-45. That is defined as inertial cavitation. The cavitation effect induced by ultrasoundmediated microbubble destruction can directly damage tumor cells and cause obvious ultrastructural changes in tumor neovascularization, which can lead to mitochondrial swelling, myeloid degeneration, and cytoplasmic cavitation of endothelial cells promoting apoptosis46,47. If the cavitation effect is very strong, the walls of small tumor blood vessels can be damaged, which will activate endogenous or exogenous coagulation, and induce thrombosis in blood vessels leading to large-area capillary embolization, blocking the nutritional supply of cancerous tissue cells, and then causing the necrosis of local tumor cell48. Lethal cavitation effects can directly lead to lysis and death of tumor cell. All these provide a theoretical basis for the direct treatments of tumor with ultrasonic microbubbles49-51.
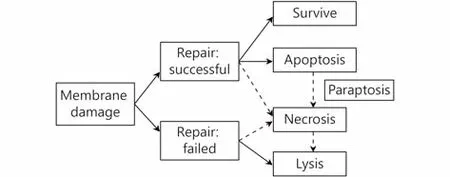
Figure 1 Schematic presentation shows the correlation of membrane damage, repair or not, and mode of cell death.
Medical application of low-frequency HIFU
Low-frequency HIFU is widely used clinically for drug delivery, fractured bone and cartilage, nerve stimulation,inflamed tendons, wound healing and ligaments repairing52,53. Compared with the thermal therapy of highenergy US, the non-thermal effects of low-frequency HIFU mainly display in the mechanical stimulation induced by microbubbles, microjets, cavitation and acoustic steaming54-57.In fact, low-frequency FUS can reach deep into the body,which allows precise local treatment and avoids possible disadvantageous side effects to surrounding healthy tissues58.In the nanomedicine field, US stimulus has shown great application prospects such as strengthening extravasation of nanoparticles through blood capillaries, increasing cell membrane permeation, inducing an anti-tumor immunity and so on59,60. Immunotherapy can be classified into active immunotherapy and passive immunotherapy61. Both these two disease treatment strategies can be reinforced by lowfrequency HIFU62.
The blood brain barrier (BBB) is a protective barrier system between blood and brain tissue, which is composed of endothelial cells, basal membrane, and glial cell podocytes of brain capillaries63. The BBB allows nutrients needed by brain tissue to pass through, avoids causing brain damage by effectively preventing some foreign bodies and large molecules from passing through, and stopping harmful substances in the blood from invading the brain64. Because of the filtering effect, the BBB affects the deliver ability of drugs and antibodies to brain tissue in almost all intracranial neurological diseases, which limits many drugs and antibodies to low concentrations when entering the brain from the blood, making it difficult to apply the due effect65,66.Currently, there are three main strategies for delivering drugs to the brain67. One is to compound new small molecules that can reach the brain tissue, but only a few diseases can be treated with small molecules. The second is to deliver drugs to the brain by using invasive catheters, but a local puncture can easily damage brain tissue. Third, noninvasive and reversible opening of the BBB, and such an idea has attracted increasing attention. It has been one of the research focuses for scientists to open the BBB by combination of FUS and MB68. The mechanism can be explained as follows69,70:(1) US waves cause microbubbles to expand and collide in capillaries, and the larger microbubbles expand and fill the capillary lumens, leading to the mechanical dilation of blood vessels, which results in the opening of tight connections.(2) Pressure changes in capillaries induce biochemical reactions that trigger the opening of the BBB. (3) Microbubble vibration can reduce local blood flow and lead to transient ischemia, triggering the opening of the BBB. (4) US irradiation causes the burst of micro-bubbles, resulting in local high-speed turbulence and jet flow, and these mechanical effects also participate in the opening of the BBB71. Figure 2 shows the FUS-induced BBB opening and its potential effect in CNS immune modulation and immunotherapy72. So far, there have been many clinical reports about using low-frequency HIFU to open the BBB,such as the delivery of tumor-targeted drugs, gene vectors and analgesics73,74.
Low-frequency HIFU induced tumor immune response
The primary mechanisms of low-frequency HIFU mediated immune response are as follow75,76: (1) After the irradiation of low-frequency HIFU, both the tumor debris and the released relating cancer antigens can work as a cancer vaccine, enhancing the immunogenicity. (2) The treatment of low-frequency HIFU on the tumor lesion can induce Th1 reaction, which leads to significant changes of cellular immunity, strengthening the activity of DC and cytotoxic lymphocytes. (3) The treatment of low-frequency HIFU can balance the immunosuppressive action induced by the tumor microenvironment. The three effectors above can effectively stimulate the anti-cancer immune response of the human body. Currently, there is abundant literature for preparing tumor lysates from tumor samples aiming to activate the anti-tumor response77. The tumor lysates are loaded on cytotoxic T lymphocytes (CTLs), which will enhance the killing activity aiming to cancer cells. Many literatures have given the detection and analysis methods of related immune parameters, such as cytokine detection, chemokine detection and so on78.
Compared with high-frequency HIFU, low-frequency HIFU can reserve the antigens more efficiently, stimulate the DCs’ infiltration and maturity in situ, which triggers a stronger immune response79. In addition, the different scanning ways of low-frequency HIFU also affect the immunotherapy effect78. In diseased tissue, the sparse scanning mode can reserve antigens better, and be more effective than the intensive scanning mode59,80. Yang et al.81discovered very early that FUS can stimulate the anticancer immune response. They treated neuroblastoma C1300 mice with HIFU, and the same cancer cells were inoculated again in the mice after the ablation of cancer tissue. Comparing with mice that were not inoculated for cancer cells in the initial stage or the mice that were not treated with HIFU, they found that the proliferation rate of the re-inoculated tumor cell after HIFU treatment is significantly decreased. Many subsequent studies also indicated that the tumor debris treated with FUS can induce the tumor specific immune response, so the tumor debris after FUS treatment can be taken as effective anti-tumor vaccine56,60,81-88. A summary of the HIFU anti-cancer response is displayed in Figure 389.
Studies showed that the adoptive transfer of immune cells activated with low-frequency HIFU also has very good effect90. After treating H22 cancer-bearing mice with lowfrequency HIFU for 14 days, the T cells were taken out and then adoptively transferred into other mice bearing H22 cancer91. The experimental data indicates that CD3+, CD4+,the ratio of CD4+/CD8+, CTL cytotoxicity, and the secretion of IFN-γ and IFN-α of the mice after low-frequency HIFU treatment all increase significantly. After transferring the activated T lymphocytes to the cancer-bearing mice, both the tumor infiltration T lymphocytes and IFN-γ secretory cells increased significantly. Table 1 shows the overview of recent clinical research on immune effects after the irradiation of FUS92-99.
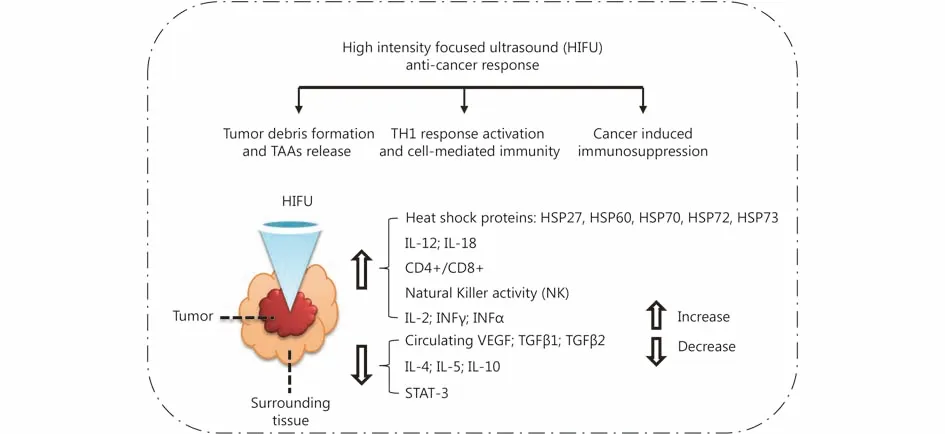
Figure 3 Summary of HIFU-induced anti-cancer response, in which STAT means signal transducer and activator of transcription, and TAA means tumor-associated antigens. Adapted from reference 89.
Combined treatment strategies
Immunotherapy assisted by microbubbles and low-frequency HIFU
Cavitation effect is the important physical foundation of USenhanced drug delivery and US-guided gene transfection100.One can deliver drugs or transfect genes by using the cavitation effect of US, and assist the tumor cellular immunotherapy101. Microbubbles are commonly used as gene or drug barriers. The US microbubble contrast agent can enhance the cavitation effect, improve drug delivery efficiency, and increase local drug concentration when the drug is loaded on the microbubbles102. The simplest method using microbubbles to deliver the active substance is coinjection103. Microbubbles and the active substance are mixed in some solution in vitro, and US irradiation promotes the delivery in vivo. However, the potential problem of coinjection is that the distribution of microbubbles and active substance in vivo is not exactly the same, which leads to the decrease of drug availability104. A better method is to conjugate the microbubbles with the active substance, and this method can ensure the same bio-distribution of two conjugating objects, improve the local concentration of the active substance, and decrease drug dosage105. The combination of targeted microbubbles and US afford a more complex and efficient delivery system. Such a therapeutic system possesses site specificity and cell specificity, which activates the immune system more efficiently104,106. Figure 4 shows the use of US with mRNA-loaded microbubbles, in which the mRNA-loaded microbubbles implode upon exposure to US and sonoporate the DCs107. As a result, both antigen and DC-modulating proteins are produced by DCs,which can lead to antigen presentation and T-cell activation.Many of the studies on microbubbles for drug and gene delivery have used commercially available US imaging contrast agents or similar bubbles equipped with targeting ligands on the surface or bubbles complexed with active substances108,109.
Even without any active agent, microbubbles still possess potential as immune response triggers110. In recent research,the effect of SonoVue microbubbles was examined in combination with focused US on solid CT-26 tumors in mice111. Intravenous injection of microbubbles came first,and then irradiation of US on the tumor followed immediately. Compared with results treated with only US,the combined therapeutic strategy distinctly decreased the tumor growth. Infiltration of immune cells increased in the tumor tissue, in which the CD8+CTL and CD4+non-Treg levels are included. As for the immune cells, microbubbles have also been commonly used to promote the permeability of the BBB under the irradiation of US112. And the natural killer cells moving through the BBB come at a much higher extent than if only US was used113,114.
Microbubbles, usually taken as carriers of delivering genes and antigens in immunotherapy, also have somedisadvantages115,116. Firstly, the diameter of microbubbles is generally in the range 2-5 μm, the tumor tissue can be penetrated effectively without the irradiation of US.Secondly, the half-life period of microbubbles is relatively short in vivo, which is not beneficial to gene or drug delivery.One other potential problem is their complex preparation method, and much pretreatment is needed before use. The disadvantages above have limited the application of microbubbles in immunotherapy to a certain extent117.
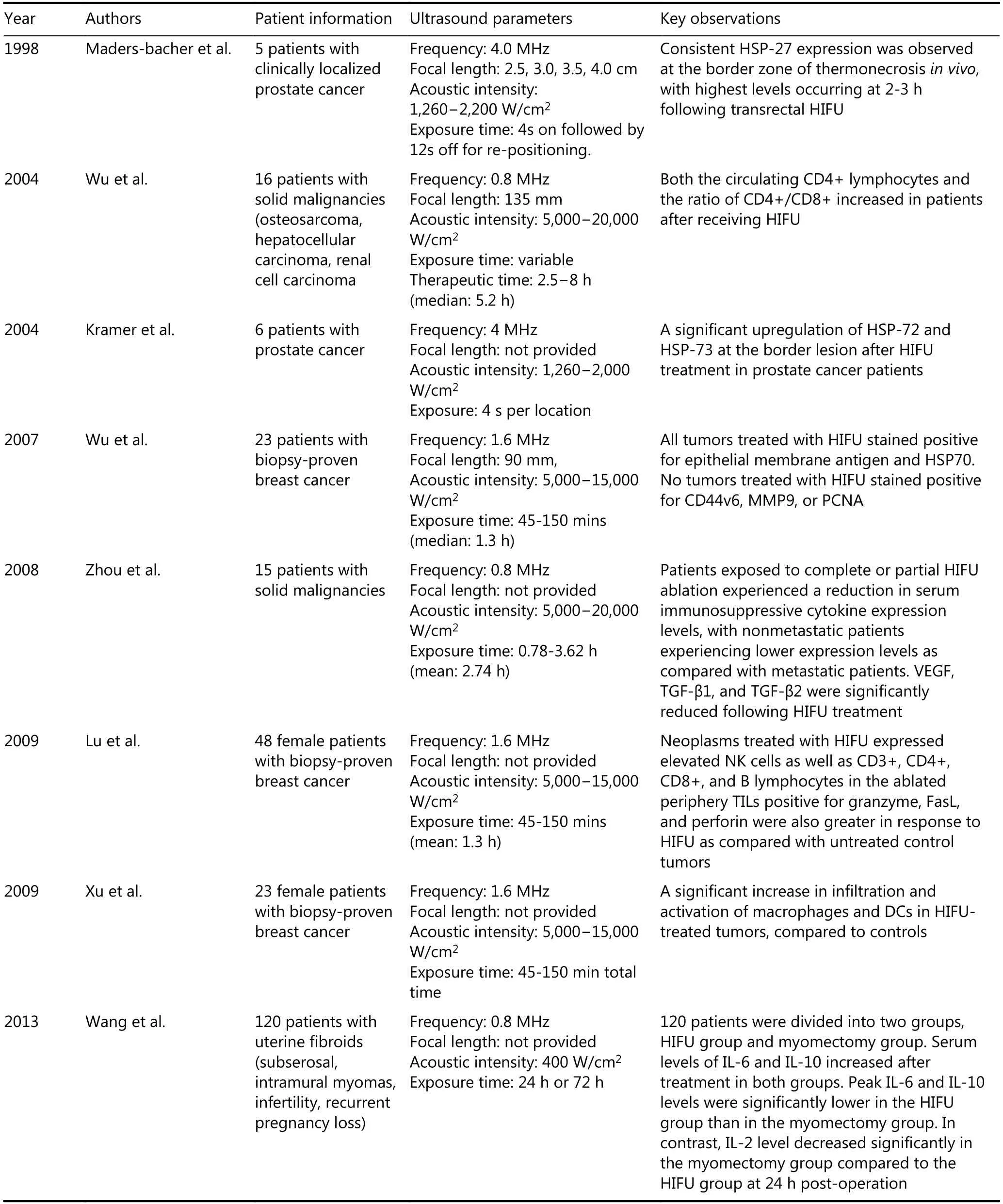
Table 1 Overview of described immune effects after irradiation of FUS in clinical studies
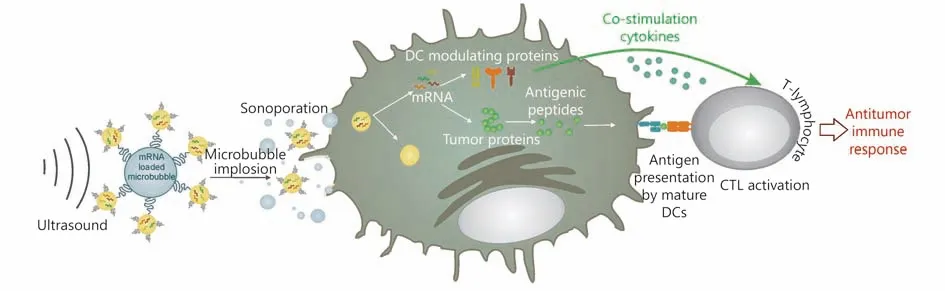
Figure 4 Schematic of the use of US with mRNA-loaded microbubbles. Adapted from reference 107.
Nanoparticles for low-frequency HIFU cancer immunotherapy
Nanoparticles have high surface area to volume ratio and advantageous delivery kinetics, and can be well used in clinical diagnosis and treatment including tumor immunotherapy according to their unique physical and chemical properties118. The size of nanoparticles are ranging from 1nm to 100nm119. The design of nanoparticles can be customized for a special application through modulating the properties of nanoparticles such as size, shape and charge120.According to the permeability and retention effect of nanoparticles, early researchers payed much attention to nanoparticle delivery on tumors, which could be further reinforced by conjugating tumor-targeting antibodies to the nanoparticles120-122. Nowadays these delivery methods are still commonly adopted in clinical application, and many research groups also put the natural bio-distribution of nanoparticle into use for cancer immunotherapy123.
The release of the drug can be triggered from different nanocarriers via the cavitation effects or radiation forces induced by US waves124. Nanoparticles have played an important role in ultrasound-mediated drug and gene delivery125,126. Compared with microbubbles, the major advantage of nanoparticles is that they can be made small enough to extravasate effectively from the leaky vasculature of some tumors, and the main disadvantages are their complex preparation, instability and toxicity127. Figure 5 shows the schematic of targeted gold nanoparticle (GNP)drug releasing and the enhancement of delivery through the BBB in US irradiation128.
Many experimental results show that targeted US microbubbles can greatly improve the accuracy of tumor diagnosis, having the advantages of safety, efficiency, good targeting, and strong controllability in the treatment of tumors129. Although the application prospect of targeted microbubbles is exciting, there are still several points worth paying attention to: (1) Strengthening the binding ability between microbubbles and ligands, and reinforcing the binding strength between ligands and receptors are the basis for microbubbles to be targeted130. Although the antibiotinbiotin complex is currently the most effective targeted binding system, antibiotin is subject to endogenous biotin competition131. The main source is egg white or bacteria and other extrinsic proteins with immunogenicity, which may lead to a rejection reaction in clinical application. In addition, antibiotin is a kind of large molecular cation that is prone to form immune complexes in the renal basement membrane with high anion concentration in the body132.Therefore, a more ideal ligand connection method is needed133. (2) The construction of targeted microbubbles is tedious and time-consuming, and their stability and target seeking efficiency in vivo needs to be improved134. (3) The key technology of targeted microbubbles carrying drugs and genes needs to be improved135,136. It is necessary to better protect bioactive substances from shear flow or enzyme degradation during microbubble rupture before entering cells137. (4) In order to improve the targeting ability of microbubbles, researches on tumor immunity should be further improved138. (5) There are some dangers in the application of ultrasonic microbubbles139. The drug particles carried by microbubbles are easily ingested by the liver and spleen140. In this case, adverse reactions of different drugs may be relatively large141,142. In addition to microbubbles that may cause microembolism and toxicity in blood circulation, the potential risks caused by cavitation nuclei and cavitation effects should not be ignored143.

Figure 5 Schematic of the targeted GNP-drug release and the enhanced delivery through the BBB in US irradiation. Adapted from reference 128.
The advent of targeted US microbubbles is a revolution in the development of US medicine144. With the continuous improvement and optimization of construction technology,and the rapid development of related imaging technology,targeted US microbubbles will be increasingly accelerated into clinical applications, and drugs and gene therapy for tumors will certainly make gratifying progress145,146.
Low-frequency HIFU enhanced effect of checkpoint inhibitor therapy
Checkpoint blockade antibodies, such as PD-1 and CTLA-4,have demonstrated high efficacy for some extracranial tumors147-150. Preclinical and anecdotal clinical evidence showed benefits from treatment of brain malignancies by using checkpoint blockade antibodies151. FUS has been used for delivery of antibodies to the brain to treat some diseases,thus, it can be arguably concluded that FUS also has the potential to promote the concentrations of immunemodulating antibodies at the desired site148,152-154. Besides the delivery of antibodies, the BBB can be opened with FUS to deliver larger vehicles for drugs and genes, such as liposomes,polymers, polymeric nanoparticles, and virus. The capability of targeted delivery of gene vectors opens up possibilities for altering immune stimuli within the diseased tissue155.
Checkpoint inhibitor (CI) immunotherapy is playing an important role in the treatment of cancer, but for a subgroup of patients, this treatment is ineffective or even a failure because of drug resistance156-158. To improve the CI therapy effect, many concerted efforts are conducted through the use of multiple CIs or use of CIs in combination with other anticancer agents. In 2017, the important first report on the efficacy of combining US with CI immunotherapy was published by Silvestrini et al159. Their work examined the impact of ablation coupled with aPD-1 and toll-like receptor agonist therapy (CpG). For the case of a single treated tumor,the growth rate of distal tumor is faster using combination therapy than that of aPD-1+CpG only, unless the drugs were administered before ablation. In this paper, an finding was that when two tumors were treated rather than one, abscopal effects were achieved such that the additional non-ablated tumor underwent regression, and survival was improved relative to drug-only treatment. In addition to suggesting the potential of local thermal ablation to affect the treatment of metastatic disease, this highlights the complexity of local treatment on immune effects in that they can be deleterious as well as complementary.
In 2019, Bulner et al.160reported the use of “anti-vascular”ultrasound-stimulated microbubble (USMB) treatment in combination with anti-PD-1 CI therapy. Longitudinal growth studies along with acute experiments were conducted by using colorectal cancer cell line CT26 to assess ultrasoundinduced anti-tumor immune responses. The results indicated that USMB+anti-PD-1 treatments significantly reinforced tumor growth inhibition and animal survival compared with monotherapies. The ability of anti-vascular USMBs increased the anti-tumor effects of CI therapy, but did not clearly support a T cell-dependent mechanism for the reinforcement.
Conclusion and perspectives
Tumor is a systemic disease. The ideal method of cancer treatment is to remove local tumors without damaging normal tissues, and to activate the whole body's anti-tumor immune response161. In recent years, many exciting research results have been achieved in the field of tumor immunotherapy, which bring great hope for the advanced or terminal cancer patient162. Due to the high degree of heterogeneity and specificity of the human immune system,current immunotherapy is not perfect yet, and cannot be commonly applied in clinical treatment163,164.
US has a good prospect in the treatment of diseases,especially in tumor ablation and drug delivery165. The use of US alone or the delivery of immune stimulants by US can induce an anti-tumor immune response166. The mechanical and cavitation effects produced by FUS can enhance the host's anti-tumor immune response, and deliver genes and antigens to cells to activate the anti-tumor immune response101. Compared with HIFU at high temperature aiming for ablating cancer tumor, low-frequency HIFU at low temperature can protect antigen more effectively,stimulate invasion and maturation of DCs in situ, and trigger stronger immune response167. Non-thermal effect of lowfrequency HIFU can inhibit the growth and reproduction of cancer cells, damage DNA, and promote apoptosis. After the treatment with low-frequency HIFU, DCs could be recruited to aggregate into the damaged tumor areas, and mainly concentrated in the surrounding of the denatured areas168.The combination of low-frequency HIFU and immunotherapy has better results than immunotherapy alone169.
Many mechanisms of ultrasound-mediated immunotherapy have not been fully understood and need to be further studied78,170. First, many factors should be taken into consideration in tumor immunotherapy, such as the patient’s cancer type, genetic background, gender, age and so on. According to the action sites, indications and mechanism of different therapeutic drugs, appropriate drug combinations can be reasonably designed in the treatment of diseases. Secondly, low-frequency HIFU at a lower temperature can induce a stronger immune response171,172.However, the high-temperature HIFU is more effective for tumor ablation and curing primary diseases, so the advantages and disadvantages of tumor ablation and immunotherapy should be carefully weighed when using HIFU. In addition, as a carrier of genes or antigens in immunotherapy, microbubbles are also complicated to prepare with full consideration of the half-life and penetration efficiency of carriers162. It is undeniable that with the gradual thorough research on the mechanisms of immunotherapy, HIFU ablation, microbubble-mediated drug delivery and other mechanisms, the combined treatment of US and immunotherapy has a very broad prospect.
Acknowledgments
This study was supported by the Scientific Research Development Fund of Hubei University of Science and Technology (Grant No. 2019-21GP11) and the National College Students' Innovation and Entrepreneurship Training Project (Grant No. 201810927021S). We thank Mrs. Jennifer Poston for editing this manuscript.
Conflict of interest statement
No potential conflicts of interest are disclosed.
 Cancer Biology & Medicine2019年4期
Cancer Biology & Medicine2019年4期
- Cancer Biology & Medicine的其它文章
- Interpretation of breast cancer screening guideline for Chinese women
- Breast cancer screening guideline for Chinese women
- Erratum to Simultaneous inhibition of PI3Kα and CDK4/6 synergistically suppresses KRAS-mutated non-small cell lung cancer
- The correlation and overlaps between PD-L1 expression and classical genomic aberrations in Chinese lung adenocarcinoma patients: a single center case series
- Nomogram based on albumin-bilirubin grade to predict outcome of the patients with hepatitis C virus-related hepatocellular carcinoma after microwave ablation
- Omics-based integrated analysis identified ATRX as a biomarker associated with glioma diagnosis and prognosis
