Prevalence of Wolbachia in 10 Tenebrionidae stored-product insects and spatiotemporal infection dynamics in Tribolium confusum(Jaquelin Du Val)(Coleoptera:Tenebrionidae)
Yujie Lu,Shiyun Mio,Zhengyn Wng,Sibo Wngb College of Food Science nd Technology,Henn University of Technology,Zhengzhou 450001,Chin
b Institute of Plant Physiology&Ecology,Shanghai Institutes of Biological Sciences,Chinese Academy of Sciences,Shanghai 200032,China
Keywords:
Wolbachia
Tenebrionidae
Tribolium spp.
Stored product insects
Endosymbiont
Infection density
Tetracycline
A B S T R A C T
Insect symbionts Wolbachia used for pest population control has focused on vector pest species and agricultural insects while rare reports in Chinese stored-product insect samples.In this paper,we surveyed the prevalence of Wolbachia using a PCR detection method in ten Tenebrionidae stored-product insects.Subsequently, the spatiotemporal Wolbachia infection dynamics in Tribolium confusum and Wolbachia elimination patterns using tetracycline treatment were investigated in detail by TaqMan®probe real-time quantitative PCR,and host reproductive fitness parameters were compared.T.confusum was the only Wolbachia infected species in all the surveyed species.Wolbachia infection density consistently increased with the development of T.confusum and plateaued at 3.7×107 wsp copies per individual insect at the young adult stage.Wolbachia densities in females were higher than that in males with a significant difference at the pupae stage and varied among different tissues and organs.Uninfected female beetles were completely incapable of producing mature progenies when crossed with Wolbachia infected males.Embryogenesis and egg hatch rate were specifically inhibited after Wolbachia elimination,while other traits,including the number of eggs,pupation rate and sex ratio,remained unaffected by tetracycline treatment.Our results show that the TaqMan®probe qPCR is a reliable detection method for quantifying the density of Wolbachia as compared to qualitative detection of wsp gene by PCR and relatively quantified by real-time qPCR.The fitness results indicated that Wolbachia infection was not an obligate symbiont and benefited the host confused flour beetle.
1.Introduction
The endosymbiotic bacteria Wolbachia infects about 20%~80%of arthropod species[1-4].In recent years,increasing reports have focused on the role of Wolbachia manipulate host insects'reproduction,which could throughout as alternative methods to control these pests[1].Wolbachia infection has been reported in 13 of 39 Coleoptera species,4 of 7 Lepidoptera species,2 of 4 Hymenoptera species[5,6]and 2 species in Liposcelis spp.,among stored-product insects[7-9],while the information of Wolbachia infection status in Chinese stored product insect samples is still rare.
Tenebrionidae stored-product insects are considerable cosmopolitan pests of wheat flour,starch,and other stored-products,especially in flour mills and grain-processing and storage facilities[10].In previous reports,it has been demonstrated that the confused flour beetle, T.confusum(Jaquelin Du Val) (Coleoptera: Tenebrionidae) was naturally infected with an endosymbiont Wolbachia[5,6,11-15].Complete unidirectional incompatibility(CI)was induced when uninfected females mated with infected males[11,13,16].
Wolbachia strains are strictly intracellular bacteria and are inherited solely through the maternal lineage of the host by trans-ovarian transmission,so they are expected to be associated with the reproductive tissues of the host organisms[17,18].It has been recognized that Wolbachia infections were also found in a variety of somatic tissues in various insects[17,18].The density of Wolbachia has been studied for the whole body of Drosophila spp.[19], the eggs of Nasonia vitripennis [20] and the sperm cysts of Drosophila simulans[21].Several studies have suggested that the density of Wolbachia in a host is positively correlated with the intensity of CI expression [19,20].For example, Wolbachia infection in Drosophila melanogaster and extensive infection of the somatic tissues of adult insects resulted in a striking reduction in the lifespan of the insects[22].Wolbachia infection densities in various hosts were detected by qPCR method using wsp gene that was relative quantification method which had a reference housekeeping gene(e.g.COII,EF-2,translation elongation factor 2)[16,17,23].However,the sensibility and accuracy of this method were lower than the qPCR with Taqman®probe that was an absolute quantification method for diagnostic detection and measurement[24-26].To screen an absolute quantification of Wolbachia infection in the various tissues and organs of T.confusum throughout the growth,the real-time qPCR with Taqman®probe was used in our study.
The purposes of this paper were to survey the Wolbachia infection in various geographical populations of stored-product pests in China and to clarify the infection density dynamics and reproductive effects of Wolbachia on the host to obtain basic information that had the potential to be applied for future stored pest management strategies.
2.Materials and methods
2.1.Insect strains collection and rearing
Ten species (18 geographical populations) of Tenebrionidae storedproduct pests were collected from flour mills and warehouses.Insects were taken to the Stored-product Insects Ecology Laboratory,Henan University of Technology (Zhengzhou, China).Insects were reared in jars with a feeding medium that contained 5%(W/W)brewer yeast in whole wheat flour and were maintained at 30±1°C and 75%±5%RH.
Different life stages of T.confusum samples were collected as described below.Mated females were allowed to oviposit on black filter paper with a small amount of wheat flour in Petri dishes.The eggs were collected 24 h after the start of oviposition.To collect different instar larvae,eggs were gently collected from the substratum and allowed to hatch in a Petri dish.Based on developing time and size,1st to 6th instar larvae(7,9,12,15,18,21 d after collecting the eggs at 30±1°C and 75%±5%RH),prepupae and pupae were separated.Beetles were sexed at the pupal stage based on their genital lobe morphology[16].Adult insects were collected within 1 day after emergence as new adults,5-10 days after emergence as young adults,and over 90 days after emergence as old adults.For the collection of various tissues,adult insects were dissected with forceps under a dissecting microscope,and the head,thorax,abdomen,middle gut,malpighian tubule, testis, and ovary tissues were dissected and immediately used for DNA extraction.
2.2.DNA extraction
DNA from samples(the whole body of larvae,pupae,adults,and body parts and tissues)was extracted using a Takara MiniBest bacteria genome DNA extraction kit(Takara Biotechnology(Dalian)Co.,Ltd.),according to the manufacturer's instructions.DNA was preferentially extracted from female pupa or adults when possible since they tended to have higher titers of Wolbachia[17].Extracted DNA was eluted using 100 μL of TE buffer and stored at −20°C until amplified using a diagnostic PCR assay and real-time quantitative PCR.
2.3.Diagnostic PCR
The diagnostic primer set was wsp(Wolbachia surface protein gene)universal primers wsp81F (5′-TGGTCCAATAAGTGATGAAGAAAC-3′) and wsp691R(5′-AAAATTAAA CGCTACTCCA-3′)[27].The amplification reactions were initiated by incubation at 95°C for 5 min,followed by 30 cycles of 94°C for 1 min,53°C for 30 s and 72°C for 1 min,with a final elongation step at 72°C for 10 min and a final hold at 4°C.The extracted DNA of the Wolbachia-infected Callosobruchus chinensis Linnaeus(Coleoptera:Bruchidae)was used as the positive control[28]and RNase-free PCR-grade water as a negative control for each test.The 600-bp PCR products of the wsp gene were electrophoresed in a 1% agarose gel, stained with Golden View II(Solarbio), observed on a UV transilluminator, and excised and purified using a Gel Extraction Kit(Axygen,Corning,Inc.).Purified PCR products were cloned into a TA cloning vector,pMD19,and transfected into Escherichia coli JM109 competent cells;ampicillin and X-gal and IPTG were used for blue-white plaque selection.
2.4.Real-time quantitative PCR
To estimate Wolbachia densities,a real-time quantitative PCR assay based on a single-copy gene wsp encoding a surface protein of Wolbachia was used to determine Wolbachia copy number in the hosts in a Thermal cycler(Applied Biosystems 7500,Life Biotechnology Inc.).Primers were specifically designed to detect the wCon strain and amplified 332-to 513-bp regions of the wsp gene(wspF,5′-GCAGCATATATC AGCAATCCTTCAA-3′;wspR,5′-GCATCATCCTTAGCCGCCTTAT-3′)[16].The amplification reaction was monitored using a set of fluorescent probes specific to the PCR product.A specific TaqMan®probe (5′-FAM-TGTTAGCTATGATGTAAC TCCAGAATAMRA-3′) for the central region of the PCR product was designed and used to measure the Wolbachia infection density.PCR was performed under the following conditions: 30 s at 95 °C, followed by 40 cycles of 95°C for 5 s and 60°C for 34 s.The PCR in a 20 μL reaction system was well-optimized as follows:10 μL of Premix Ex Taq(Probe qPCR)(Takara),the 0.2 μmol/L concentration of each primer, 0.4 μmol/L of TaqMan®probe,2 μL of template DNA,and 8.4 μL of DNase/RNase-free water.
The 182-bp PCR amplification products of the Wolbachia wsp gene were electrophoresed on a 1%agarose gel,excised,purified and cloned into a TA cloning vector, pMD-19 T, and transfected into Escherichia coli JM109-competent cells.A standard curve was constructed using wCon amplicons that were previously cloned into a pMD-19 T vector(Takara),linearized with Hind III,and quantified as the template.Three replicates were performed and averaged per sample.Strain-specific primers for wCon were applied to all samples, and the total Wolbachia population density was calculated by integrating both numbers based on a single-copy gene wsp copy number[29].
2.5.Tetracycline treatment on T.confusum
A serial solution of tetracycline(0.3,0.5,1.5,3.0 mg/mL)was prepared by diluting a prepared stock tetracycline solution(5 mg/mL).These solutions were added to the feed medium (whole wheat flour and brewer yeast,19:1,W/W),and redundant water was evaporated by cold air flows to keep the ultimate feed moisture content at 13%-14%.Medium with tetracycline supplement was made up and homogenized by a mill,at a total concentration of 0.3,0.5,1.5,and 3.0 mg/g(tetracycline in feed medium)[30].Mated females were allowed to oviposit on the tetracycline medium.The fourth-instar larvae,pupae,and adults were separated after 2,3 and 4 weeks of tetracycline treatment,respectively,to examine the density of Wolbachia.Pupae were sexed and separated after 3 weeks of tetracycline treatment.Experiments were conducted at 30±1°C and 70%±5%RH.
2.6.Crossing experiments and reproductive parameters of T.confusum
Beetles with PCR-positive results for Wolbachia were designated w+(Wolbachia positive),while those in which Wolbachia was eliminated by tetracycline treatment were designated w−(Wolbachia negative).Four crossing couples were performed in 6-well cell culture plates; every well contained a pair of virgin adults of each crossing couple and 1 g diet.The total number of eggs laid was counted every two days by sieving the diet by two sieves of 100 mesh(0.15 mm)and 18 mesh(1.0 mm)separating the adults and eggs respectively,and the eggs were transferred to 24-well cell culture plates to observe and calculate the hatching percentage;the pupation rate and sex ratio were calculated.Sixteen replicates of each crossing couples in every trial were conducted.
3.Results
3.1.Wolbachia infection rate detection in Tenebrionidae stored-product insects
In ten Tenebrionidae stored-product insects,only the two strains of T.confusum species were detected Wolbachia infection (Table 1).Positive PCR results for the Wolbachia-specific wsp gene showed that females and males of all T.confusum strains were infected with Wolbachia.In addition,there was no Wolbachia infection in two Tenebrio species,three Alphitobius species,and three Palorus species.
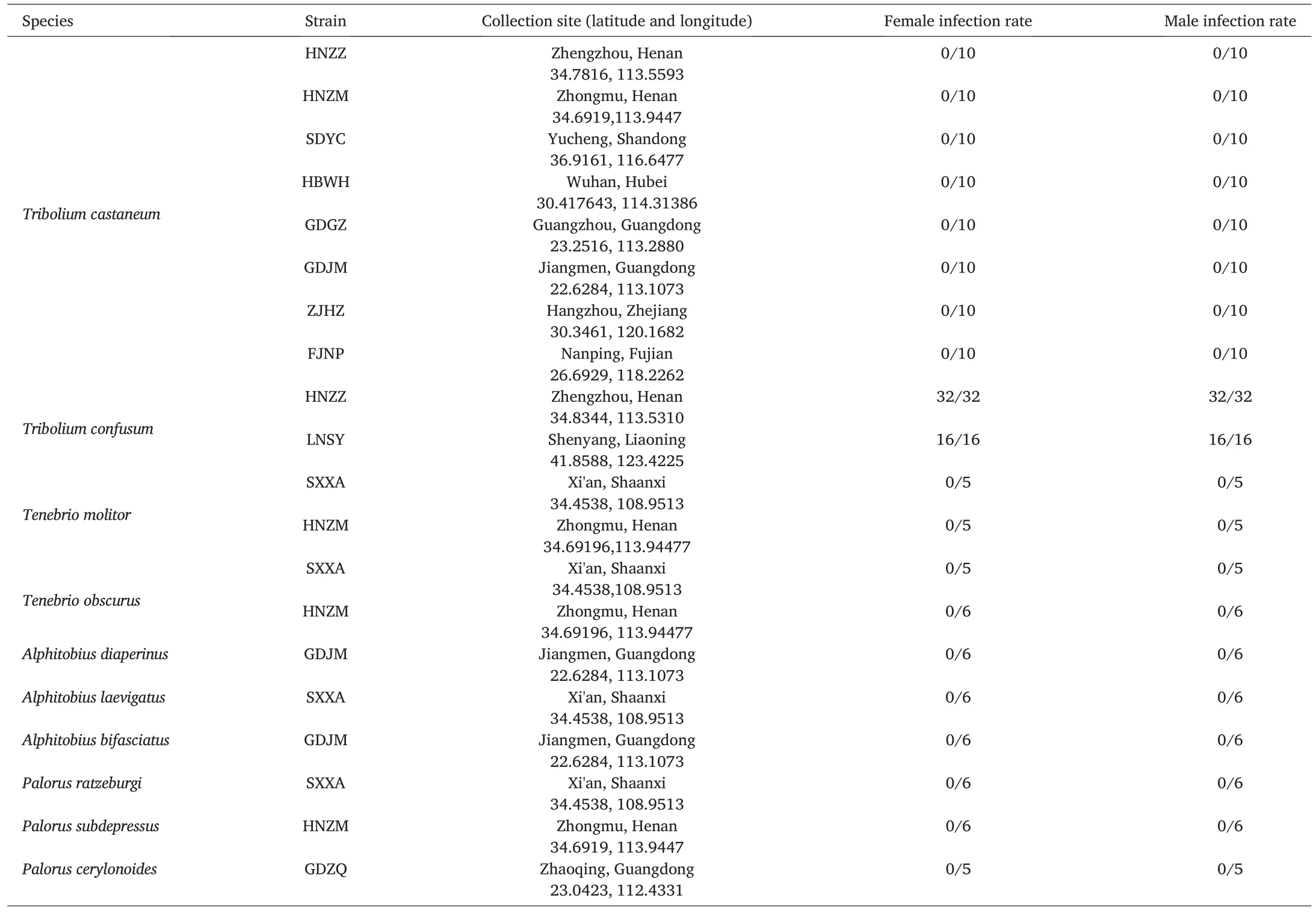
Table 1 Wolbachia infection status in Tenebrionidae stored-product pests in China.
3.2.Wolbachia density in the different developing stage of T.confusum
The Wolbachia infection dynamics in terms of wsp copy number per insect were examined throughout the development of T.confusum using a TaqMan®probe quantitative PCR technique(Fig.1).The results indicated that the population of Wolbachia consistently increased with host development.The Wolbachia infection density was highest in the young adults(5 days post-eclosion),reached 3.7×107wsp copies per insect.Wolbachia density showed a gender difference:females had a higher Wolbachia density than males,especially in the pupae stage(Fig.2).
3.3.Wolbachia density in different tissues of T.confusum
Amounts of Wolbachia infection density quantified by TaqMan®probe qPCR were apparently higher in the abdomen than in the thorax and head (Fig.3).For the tissues and organs examination, the density of Wolbachia differed strikingly among tissues and organs.Higher Wolbachia infection was detected in the reproductive tissue and fat body than in the middle gut and Malpighian tubules(Fig.4).
3.4.Effect of tetracycline treatment on Wolbachia density in T.confusum
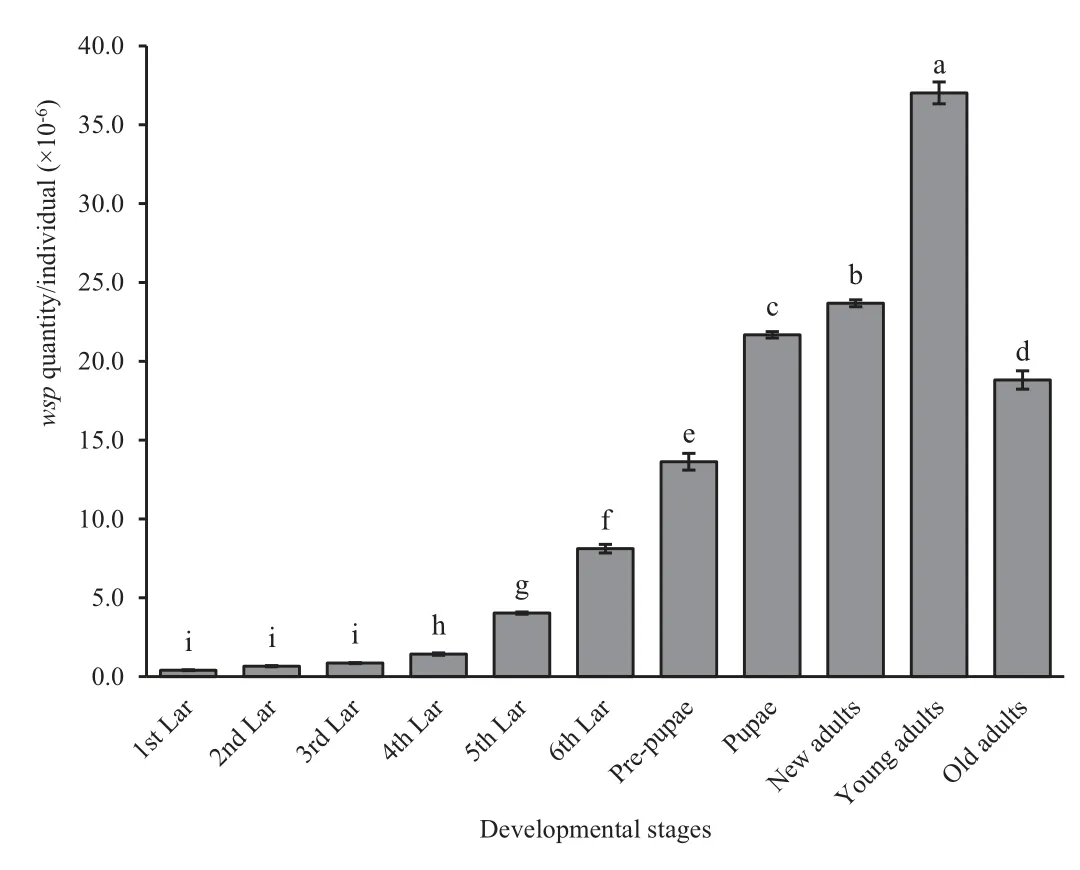
Fig.1.Dynamics of Wolbachia infection during the development of Tribolium confusum.
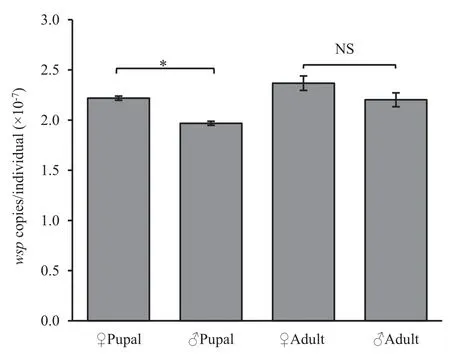
Fig.2.Comparison of Wolbachia infection density in Tribolium confusum male and female pupae and adults.
The clear relationship between the total Wolbachia density and tetracycline treatment doses and time is shown in Fig.5.An increase in tetracycline dose resulted in a significant reduction in estimated densities of Wolbachia in T.confusum.Tetracycline treatments specifically inhibit Wolbachia density in the flour beetle.The density elimination rate of Wolbachia showed sex-related differences in the pupae stage (treat for 3 weeks).The Wolbachia density was higher in females than in males,which means that it is easier to remove Wolbachia in males than in females with a relatively low dosage of tetracycline treatment(Fig.5c).
3.5.Effect of Wolbachia on the fitness of T.confusum population development
Tetracycline treatments specifically inhibit reproductive fitness in the flour beetle T.confusum population.Apo-symbiotic(w−)female beetles were completely incapable of producing mature embryogenesis after crossing with Wolbachia-infected males and therefore could not reproduce,even though they could produce sterile eggs.There were no significant differences(P>0.05)in the total egg number,percentage of pupal survival and percentage of adult survival expect for the egg hatch rate (P<0.05) in(Table 2).In addition,the sex ratio of female/male offspring did not significantly deviate from 1:1,and no significant differences were found between the number of males and females(P>0.05).Total cytoplasmic incompatibility was expressed when w+males mated with w−females (Table 2),resulting in few or no viable offspring produced,but the other two possible crosses(♂w−×♀w+and ♂w+×♀w−)were compatible.
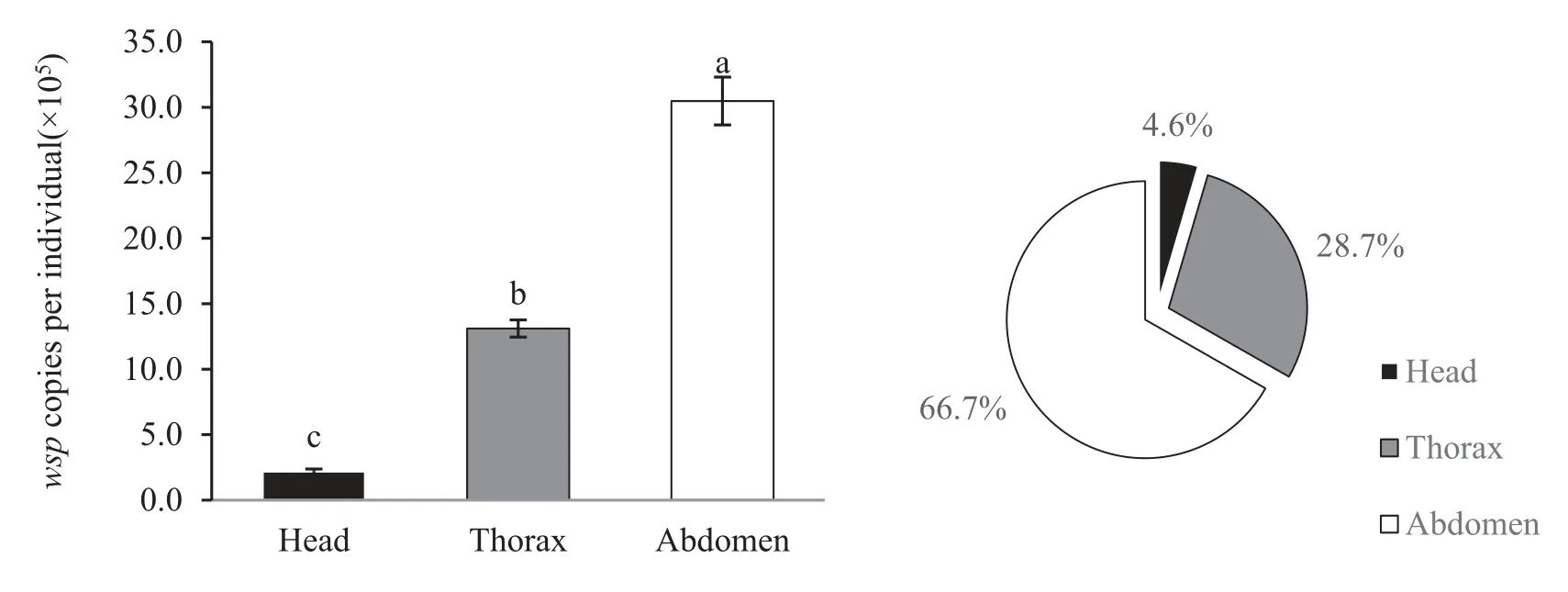
Fig.3.Relative amounts of Wolbachia population density in the head,thorax and abdomen of T.confusum.
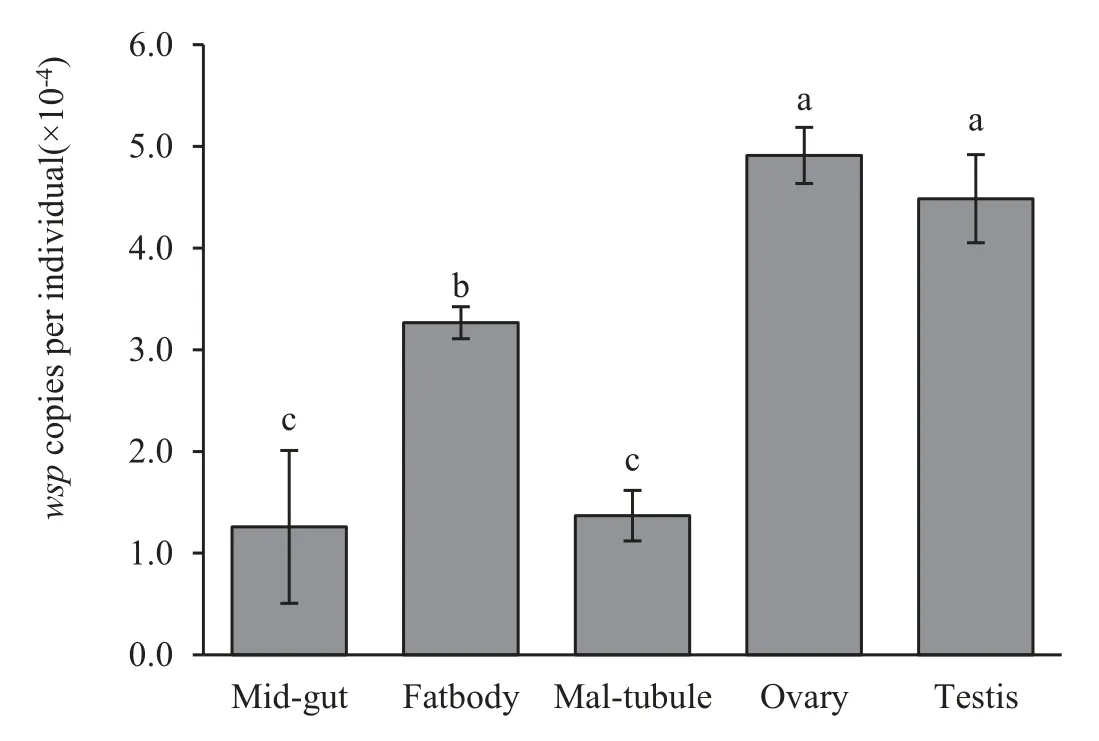
Fig.4.Comparison of the population density of Wolbachia in different tissues of T.confusum.
4.Discussion
The detection and characterization of the Wolbachia strains in storedproduct insect pests were attempted to obtain basic information that had the potential to be used for future pest management strategies.In this study,we found that Wolbachia infection was detected from T.confusum of 10 Tenebrionidae stored-product insect strains(18 populations)from wild and laboratory strains in China.All T.confusum sample individuals,female and male,were infected with Wolbachia(Table 1).We confirmed that there was a monophyletic Wolbachia infection in T.confusum in two strains(LNSY and HNZZ) in China.This report coincided with the results of Kageyama and Li et al.[5,6]who surveyed Wolbachia prevalence and diversity in stored-product insect species in Canada,Denmark,England,France,Germany,Greece,Italy,Portugal,Spain,the USA and Japan.In addition,there was no Wolbachia infection in three Alphitobius species and three Palorus species.There were positive PCR results in the isolated heads,thoraxes,middle guts,fat-bodies and Malpighian tubules(females and males)(n=10),demonstrating the presence of Wolbachia in digestive,immune and excretive tissues in addition to reproductive tissues.This result is consistent with studies of the adzuki bean beetle C.chinensis and a parasitic wasp Asobara tabida[17,31].In our study,the density of Wolbachia was measured by an absolute quantification method using a TaqMan®probe of real-time qPCR and expressed as the copy number of the wsp gene per individual or tissue rather than body or tissue mass,for the guts and reproductive tissues were too small to weigh[23].Since qPCR with TaqMan®probe is the gold standard for DNA copy number analysis,any compromise inaccuracy will be a major concern for PCR applications such as pathogen detection [25], food regulation [24], and scientific research [32].It had a significant impact on the accuracy of absolute quantification.
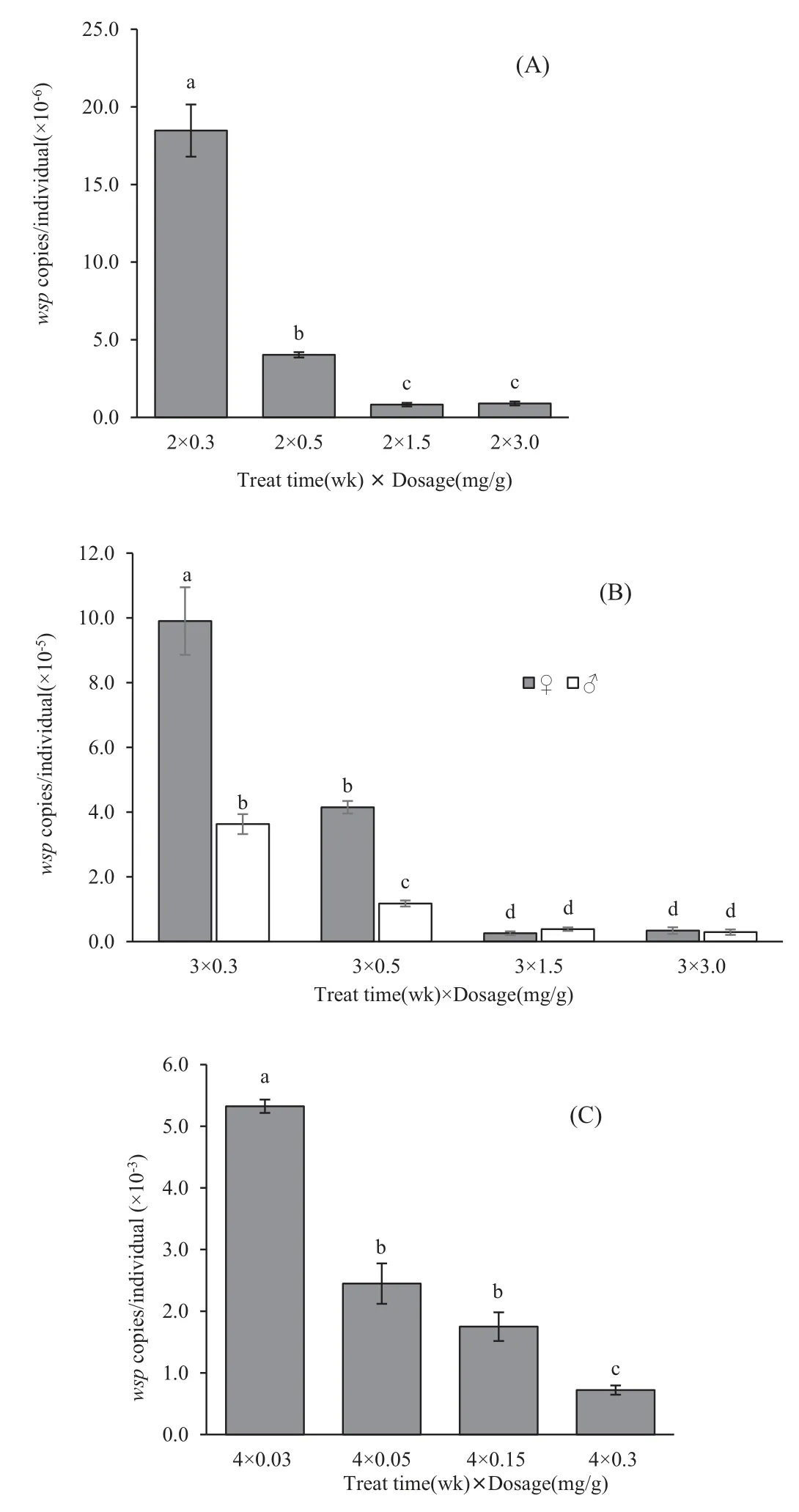
Fig.5.Comparison of population density of Wolbachia in T.confusum with different concentrations of tetracycline treatment for 2,3 and 4 weeks.
Our results show that Wolbachia density in the host varies with tetracycline fed dosage and time of treatment.This correlation allows the use of tetracycline treatment to investigate the effect of Wolbachia density on host fitness.The elimination of Wolbachia could be accessed by fed on low dosage tetracycline medium for a long time, which indicated that Wolbachia in T.confusum could be eliminated by this antirickettsial antibiotic as the continued fed on antibiotic diet(>1 month),and the resultant Wolbachia-uninfected T.confusum could be obtained and used for matingand crossing experiments[33,34].When Wolbachia density reduced to an undetectable level,which was <1000 copies per individual,it could not be detected by conventional PCR amplification.The real-time PCR with TaqMan®probe could detect the 101to 108DNA copies.The sensitive and accuracy of this method were better than the relative quantification[24].
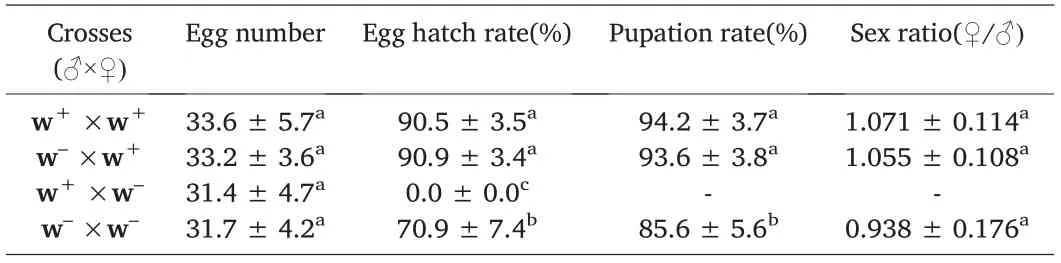
Table 2 CI expression and reproduction parameters of antibiotic-treated(w-)and untreated(w+)Tribolium confusum
Wolbachia infection could generate strong reproductive incompatibilities between uninfected females and infected males(cytoplasmic incompatibility CI)and significantly reduce the reproductive success of females and males [18].The impact of Wolbachia on the mating behavior in D.melanogaster and D.simulans showed that infected males mated at a higher rate than uninfected males in both species[35].D.simulans males exhibited some preference for mating with females of the same infection status[35,36].According to the authors'observation and the mate choice experiments reported by Ming et al.[16],infected and uninfected males of T.confusum did not have an obvious preference for a mating partner in mate choice tests.This may indicate that Wolbachia infection had no influence on mate preference in T.confusum[16].
It is generally accepted that vertically transmitted microorganisms should tend to evolve toward a benign state,or even to be beneficial to their hosts, for their fitness is inextricably linked to host performance[37-39].However,in arthropods,Wolbachia is rarely found to be beneficial to their hosts.Wolbachia strains can maintain themselves in arthropod populations through induced modifications to host reproductive biology[22,40].Moreover,even if many studies have failed to detect the negative effect of infection and a few studies have shown a slight enhancement of reproductive success in infected individuals[41],the reproductive manipulation effects of Wolbachia are still not well-demonstrated.The equality of sex ratio and the higher fecundity encouraged CI expression and Wolbachia prevalence in the flour beetle population by two beneficial outcomes:infected females increase infected offspring and infected males decrease uninfected offspring in subsequent generations.
There are several other symbionts (e.g.Arsenophonus, Cardinium,Flavobacteria,Rickettsia,Spiroplasma)that can act as reproductive parasites and may cause other similar effects in arthropods[42,43].Perhaps there are several other symbionts infection in T.confusum.We only detected the Wolbachia prevalence using special gene wsp with qPCR and screened the eliminating effect of tetracycline at low concentrations on Wolbachia and its role on the reproductive of T.confusum.The role of other special symbionts on reproductive parasites should be researched by detection of its infection and its density by designing the species-specific genes[44]to detect its infestation and its density in T.confusum with real-time PCR.It is very interesting to illuminate the role of another symbionts tor its interaction with Wolbachia on reproductive or development parasites.
5.Conclusions
In this study,the aposymbiotic strain was established in the confused flour beetle T.confusum by feeding tetracycline diets.Wolbachia induced the complete CI and decreased the fitness of the host.Nevertheless,a higher fecundity was found among all crosses in the w+females.A slight positive rather than the negative effect of Wolbachia was found on fecundity,survival,or reproductive rate of its host T.confusum.All these results indicate that Wolbachia infection is not obligate but benefits the confused flour beetle.
Conflicts of interest
The authors declare no conflicts of interest in relation to this work.
Acknowledgements
We thank Dr.Bai Liang for assistance with molecular biology techniques (Institute of Plant Physiology and Ecology, SIBS, CAS), Dr.Chen Yunfang and Dr.Zheng Sizhu (Suzhou Entry-Exit Inspection and Quarantine Bureau)for providing the instruments and Zhao Yaru(China Agricultural University)for insect strain collection and experimental assistance in this study.Dr.Paul G Fields(University of Manitoba) critically read the manuscript and give the comments.This research was supported by National Natural Science Fund Project(No.31601890) and the Fundamental Research Funds for the Henan Provincial Colleges and Universities in Henan University of Technology(No.2016XTCX01).
 Grain & Oil Science and Technology2019年4期
Grain & Oil Science and Technology2019年4期
- Grain & Oil Science and Technology的其它文章
- Production of biscuits by substitution with different ratios of yellow pea flour
- Effect of wheat bran insoluble dietary fiber with different particle size on the texture properties,protein secondary structure,and microstructureof noodles
- Antimicrobial and cytotoxic potential of seeds and flowers crude extracts of sunflower
- Health benefits of black rice-A review
