头孢洛宁乳房注入剂(干乳期)在牛奶中的残留消除
闫超群,李帅鹏,张申,谢顺,魏开赟,黄显会
头孢洛宁乳房注入剂(干乳期)在牛奶中的残留消除
闫超群1,李帅鹏2,张申1,谢顺1,魏开赟1,黄显会1
(1华南农业大学兽医学院/广东省兽药研制与安全评价重点实验室,广州 510642;2内蒙古金河动物药业有限公司,内蒙古托克托 010200)
【目的】头孢洛宁为第一代半合成头孢菌素类抗生素,对革兰氏阳性菌和阴性菌均有良好的抗菌活性,临床以每个乳区250mg用于治疗亚临床感染引起的乳房炎。头孢洛宁通过与敏感菌的青霉素结合蛋白相结合,从而阻断细菌细胞壁的合成,达到抑制细菌生长的目的。笔者开展头孢洛宁乳房注入剂(干乳期)在牛奶中的残留消除规律研究,以期为该药的合理使用和乳品安全生产提供指导。【方法】选取20头进入干乳期的健康奶牛,每个乳区分别注入一支头孢洛宁乳房注入剂。平均干乳期为50 d,奶牛产犊开始泌乳后的6、12、24、36、48、60、72、96和120 h,将每头奶牛每次采集的4个乳区的奶混合。采用Phenomenex Luna C18(150mm×2.1mm,3.5μm)。以乙腈-0.1%甲酸水溶液为流动相,梯度洗脱程序洗脱,流速0.25 mL/min,柱温为35℃,进样量为5.0 μL。头孢洛宁采用电喷雾离子源(ESI)离子化,正离子模式扫描,多反应监测模式(MRM),电喷雾电压(IS)为4kV,雾化气压力(GS1)为55psi,气帘气(CUR)为15 psi,辅助气流速(GS2)为35psi,离子源温度(TEM)为600 ℃;定量离子对为m/z→459.4/337.3。样品自然解冻,取5 g牛奶,置于50 mL离心管中,加入20 mL乙腈,涡旋混匀2min,在10℃条件下10 000 r/min离心10 min。将上清液用15 mL75%乙腈水溶液重复提取一次,合并两次的提取液,并加入10 mL乙腈饱和的正己烷,震荡1 min,弃掉正己烷,把提取液移至100 mL鸡心瓶中,在40℃用旋转浓缩仪旋转蒸发除去乙腈。向已除去乙腈的样品溶液中加入20 mL磷酸二氢钠缓冲溶液,然后用氢氧化钠溶液调节至pH8.5。将样品提取液以3 mL/min的流速通过HLB固相萃取柱,先用5 mL磷酸二氢钠缓冲溶液洗涤鸡心瓶并过柱,再用2 mL水洗柱。用2 mL乙腈洗脱,收集洗脱液于刻度样品管中,在40℃下用氮气浓缩仪吹干,用2 mL水溶解残渣,摇匀后,过0.22 μm滤膜,取样供LC-MS/MS检测。【结果】头孢洛宁在2—200 μg·kg-1的范围内呈良好的线性关系,相关系数在0.991—0.997之间。该方法检测限为0.5 μg·kg-1,定量限为2 μg·kg-1。头孢洛宁在2、20、40和200 μg·kg-1四个添加水平上的平均回收率为73.60%—103.52%,批内变异系数为1.85%—10.41%,批间变异系数为3.41%—8.97%。结果表明,该方法的回收率和变异系数满足残留检测定量分析要求。使用头孢洛宁干乳期乳房注入剂的初产奶牛,在产犊后96 h低于定量限。经产奶牛在产犊36 h低于定量限,24h均低于MRL。【结论】头孢洛宁乳房注入剂(干奶期)按推荐剂量4个乳区(250 mg/乳区)给药后,其产犊泌乳后牛奶中的弃奶期为24 h。
头孢洛宁;乳房注入剂(干乳期);奶牛;弃奶期
0 引言
【研究意义】奶牛乳房炎(bovine mastitis)是危害奶牛养殖业最重要的疾病之一,每年给全球奶牛业造成严重的经济损失[1-5]。引起奶牛乳房炎的病原体多样,该病的病因主要是毒性,创伤性,代谢紊乱以及致病菌感染引起的。传统上通过动物隔离以及改善环境卫生等来控制奶牛乳腺炎的发生[6-7]。但这些做法仅能减少外界环境带来的乳房炎。据报道,约70%奶牛乳房炎中可以分离出致病菌,细菌通过乳头和乳管进入乳房,从而引发感染,有临床症状的和无临床症状的分别称为临床乳房炎和亚临床乳房炎,葡萄球菌是其主要的致病菌[8]。控制牛乳腺炎仍然严重依赖于抗生素的治疗和预防。由于干奶期的奶牛免疫受到抑制,因此是感染奶牛乳房炎的高发期。尽管抗生素能改善奶牛的健康状况,提高牛奶产量,却增加了细菌耐药性的风险,同时牛奶中兽药的残留,加重食品安全问题[9-10]。临床上治疗乳房炎的药物种类繁多,主要是根据乳房炎的不同类型和时期来决定选择抗生素治疗、中草药治疗还是疫苗等措施,合理的用药才能够从根本上防止乳房炎的发生。青霉素类抗生素一直是我国首选治疗乳房炎的药物,随着药物的使用,耐药菌株的增多,乳房炎致病菌逐渐失去对该类药物的敏感性[11-13]。目前我国现有可供选择用于防治乳房炎的抗菌药物品种有限,因此研制新型乳房炎抗菌药物显得迫在眉睫。头孢洛宁(cephalonium)是第一代半合成头孢菌素类抗生素,对革兰氏阳性菌和阴性菌均有良好的抗菌活性。头孢洛宁属于头孢菌素类抗生素,其作用机理是抑制细菌细胞壁的合成,从而达到杀菌的目的,属于繁殖期杀菌药物[14-15]。临床主要用于预防和治疗奶牛亚临床乳房炎的发生。己经在国外上市的头孢洛宁乳房注入剂[16-17],给药剂量为每个乳区250 mg。【前人研究进展】干奶期是奶牛泌乳周期的关键时刻,也是防治奶牛乳房炎的最佳时期。目前在干乳期采用抗生素预防乳房炎仍是控制牛群乳房炎的一项重要措施。头孢洛宁目前只在欧盟、英国、日本和澳大利亚使用,中国还未进口或开发。【本研究切入点】目前尚无头孢洛宁在干乳期奶牛产犊后牛奶中残留消除规律的研究,笔者开展头孢洛宁乳房注入剂在乳汁中的残留消除研究,为该药防治乳房炎的安全性提供基础数据,以期保证消费者食用动物源性食品的安全,进而为其临床合理用药和制定临床给药方案提供依据。【拟解决的关键问题】鉴于我国现有可供选用防治奶牛乳房炎的抗菌药物数量有限,抗菌药物目前又是防治奶牛乳房炎的主要药物,因此头孢洛宁乳房注入剂在兽医临床上的使用,对我国奶牛乳房炎的控制有重要的意义。
1 材料与方法
1.1 材料
1.1.1 药品与试剂 头孢洛宁乳房注入剂(干乳期),规格3 g﹕250 mg,由河北阳光本草生物技术有限公司中试生产,批号:20140806。头孢洛宁精制品,含量为97.9%,批号:CAI1305001,由福建省福抗药业股份有限公司提供。乙腈和甲酸为色谱纯,正己烷、磷酸二氢钠、氢氧化钠均为国产分析纯,水为符合GB/T 6682规定的二级水。
1.1.2 仪器和设备 高效液相色谱串联质谱仪,包含美国Agilent公司1200型液相色谱仪(配备四元泵、自动进样器及柱温箱等);电喷雾-串联四级杆质谱仪(API 4000 MS/MS),美国应用生物系统公司,配备Analyst 4.1.5软件;MD200-1氮吹仪,上海沪西分析仪器厂;AE 160型电子分析天平,瑞士Mettler公司;Mach 1.6R冷冻型离心机,美国Thermo公司;XW-80A型旋涡混合仪,上海沪西分析仪器厂有限公司;Rotavapor R-210型旋转蒸发仪,瑞士BUCHI公司;B-260型恒温水浴锅,上海雅荣生化仪器设备有限公司;针式尼龙微孔滤膜(13 mm×0.22 µm),上海安普科学仪器有限公司;Poly-Sery HLB固相萃取柱(500 mg:6 mL),上海安谱科学仪器有限公司;Eppendorf Research型可调微量移液器,德国Eppendorf公司。
1.1.3 试验动物 试验时间和地点本试验于2015年1—8月间选取内蒙古某奶牛厂10头健康经产奶牛和10头初产奶牛(刚完成第一个哺乳期)。合格受试对象:(1)治疗给药前6次挤奶时每个乳区体细胞计数几何平均值低于200 000个细胞/mL;(2)奶牛产奶量超过10 L·d-1和体细胞计数稳定;(3)试验前30d内未给予任何抗感染和/或抗炎治疗;(4)无任何间发性疾病、无乳头损伤的奶牛。
1.1.4 给药 试验奶牛20头在刚进入干奶期(预产期前54 d)最后一次挤奶后,用消毒毛巾擦拭乳头,并用0.15%聚维酮碘溶液浸泡乳头30s左右,按推荐剂量250 mg/乳区的剂量,通过乳房注入方式给每头奶牛4个乳区给药一次。
1.1.5 样品采样 给药后50d左右,奶牛开产。在产犊开始泌乳后的6、12、24、36、48、60、72、96和120 h共9个时间点,清洗乳区,对奶头消毒。挤净每头奶牛4个乳区的奶于同一计量瓶内使成为混合奶(桶奶,挤奶时前两把奶不舍弃),从中取500 mL于聚丙烯瓶中,密封,做好标记,-20℃保存,待测。
1.2 牛奶样品中头孢洛宁的测定方法
参照国家标准GB/T 22989-2008【牛奶和奶粉中头孢匹林、头孢氨苄、头孢洛宁、头孢喹肟残留量的测定(液相色谱-串联质谱法)】处理样品[18]。
1.2.1 样品前处理 取出冷冻奶样,室温下解冻。称取5 g牛奶(精确到0.01 g)置于50 mL离心管中,加入20 mL乙腈,使用均质器均质1 min,提取液使用高速冷冻离心机在10℃条件下10 000 r/min离心10 min,将上清液转移至另一离心管中。用15 mL75%乙腈水溶液重复提取一次,合并两次的提取液,并加入10 mL乙腈饱和的正己烷,震荡1 min,弃掉正己烷,把提取液移至100 mL鸡心瓶中,在40℃用旋转浓缩仪旋转蒸发除去乙腈。
1.2.2 净化 向已除去乙腈的样品溶液中加入20 mL磷酸二氢钠缓冲溶液,然后用氢氧化钠溶液调节至pH=8.5。把样品提取液移至下接HLB固相萃取柱的贮液器中,以3 mL/min的流速通过固相萃取柱,先用5 mL磷酸二氢钠缓冲溶液洗涤鸡心瓶并过柱,再用2 mL水洗柱。用2 mL乙腈洗脱,收集洗脱液于刻度样品管中,在40℃下用氮气浓缩仪吹干,用2 mL水溶解残渣,摇匀后,过0.22 μm滤膜,液相色谱-串联质谱仪测定。
1.2.3 色谱条件 色谱柱:Luna C18(150 mm×2.1 mm,3.5 µm)美国Phenomenex公司;流动相A为乙腈,B为0.1%甲酸水;梯度洗脱;流速为0.25 mL/min:柱温为35℃;进样量为5 μL。
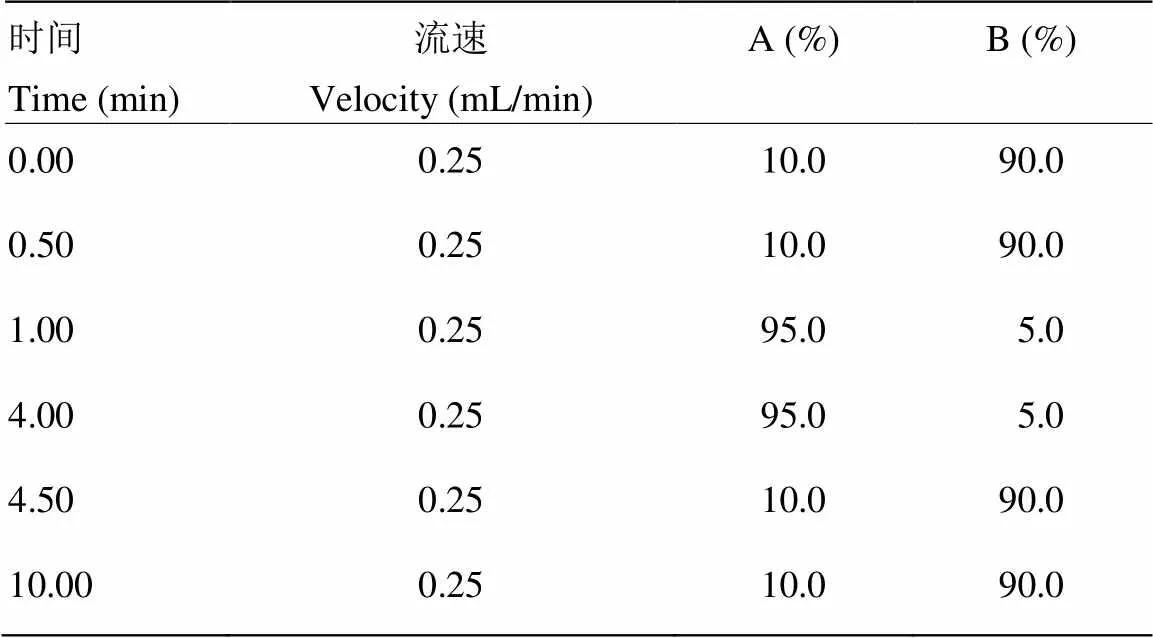
表1 流动相梯度洗脱程序
1.2.4 质谱条件 质谱扫描模式:多反应检测(multiple reaction monitor,MRM);电喷雾离子源正离子扫描(electrospray ionization,ESI+);电喷雾电压(ionspray,IS):4kV;雾化气压力(nebulizer gas1,GS1):55psi;辅助气压力(ion source gas2,GS2):35psi;气帘气压力(curtain gas,CUR):15psi;离子源温度(the source temperature,TEM):600℃。头孢洛宁用于定量和定性的离子分别为:m/z→459.4/337.3和m/z→459.4/152.3。
1.2.5 检测限(LOD)与定量限(LOQ) 以最低检出浓度计算,按照信噪比(S/N)≥3确定方法检测限(LOD),以S/N≥10确定方法定量限(LOQ)。
1.2.6 标准曲线与线性范围 准确称取5.00 g±0.05 g牛奶于50 mL离心管中,按照“1.2.1”方法处理样品,用所得空白基质提取液将头孢洛宁工作液稀释成2、5、10、20、50、100、200μg·kg-1的浓度,按照“1.2.3”和“1.2.4”确定的LC-MS/MS条件进行分析,将测得药物色谱峰面积(A)与所对应的药物浓度(C)做线性回归,求得标准曲线回归方程和相关系数(r)。
1.2.7 回收率与精密度 由于已经规定了牛奶中头孢洛宁的MRLs值,按照LOQ、MRL、2MRL、10MRL作为添加浓度进行批量回收率的计算。准确称取5.00 ±0.05 g空白牛奶样品,分别加入0.1、1、2、10mg·L-1的头孢洛宁标准工作液100 μL,制备成浓度为2、20、40、200μg·kg-1的空白牛奶加标样品,涡旋混匀,静置20 min。按照“1.2.1”方法处理样品,待LC-MS/MS条件进行分析检测。每个浓度做5个重复,共做3批。不同批次间隔一天或者数天,分别计算回收率和批内、批间变异系数。
1.3 数据处理和分析
每头奶牛每个时间点取两个平行样测定结果的平均值作为最终检测结果,WTM1.4软件计算头孢洛宁乳房注入剂(干奶期)在牛奶中的休药期。
2 结果
2.1 检测限与定量限
头孢洛宁在2—200 μg·kg-1的范围内呈良好的线性关系,相关系数在0.991—0.997之间。本方法检测限为0.5μg·kg-1,定量限为2μg·kg-1,表明方法的灵敏度高,可满足残留检测的要求(图1,2)。
2.2 回收率与变异系数
头孢洛宁在2、20、40和200μg·kg-14个添加水平上的平均回收率为73.60%—103.52%,批内变异系数为1.85%—10.41%,批间变异系数为3.41%—8.97%。结果表明,该方法的回收率和变异系数满足残留检测定量分析要求(表2)。
2.3 牛奶中头孢洛宁的浓度
依据上述建立的方法,对试验牛产犊后6、12、24、36、48、60、72、96和120 h的时间点采集的牛奶进行了检测,牛奶中头孢洛宁的测定结果见表3和表4。
由表3可知,初产奶牛在6 h,除1头奶牛外,其余均检出头孢洛宁,且有两份奶样中头孢洛宁的浓度分别达到了231 μg·kg-1和516 μg·kg-1;由表4可知,经产奶牛有5份奶样检出头孢洛宁,其有1头奶牛奶样中最大值为45.7 μg·kg-1。初产奶牛与经产奶牛在12 h分别有4头和1头牛检出头孢洛宁。至36 h,分别有2头和1头牛检出头孢洛宁,至48 h经产奶牛中仍有1头牛可检出头孢洛宁。表明头孢洛宁在经产奶牛与初产奶牛体内的残留消除存在差异性,这可能与奶牛的年龄及个体产奶量等因素相关。
2.4 牛奶中头孢洛宁的休药期
通过WTM1.4软件计算头孢洛宁乳房注入剂(干奶期)在牛奶中的休药期为24h(图3)。

图1 头孢洛宁检测限色谱图
图2 头孢洛宁定量限色谱图
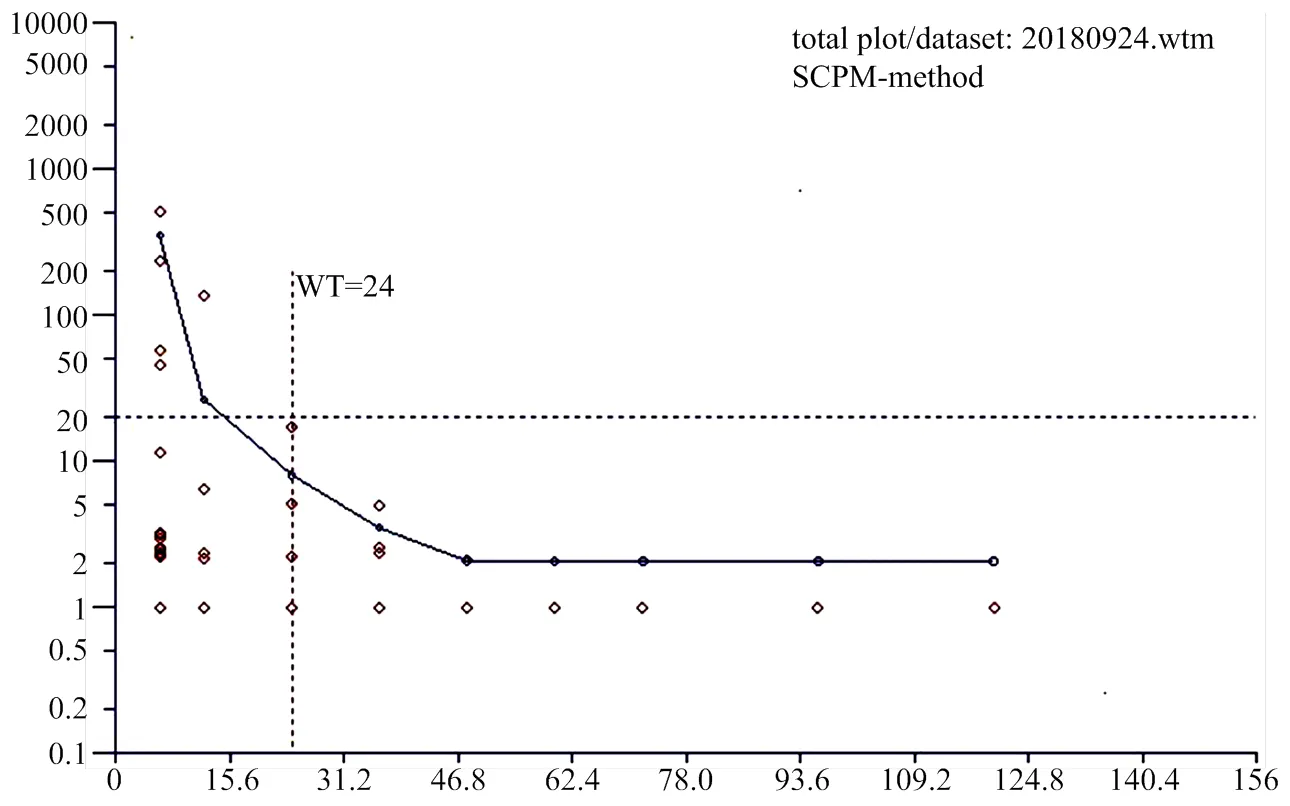
图3 头孢洛宁在牛奶中残留消除规律
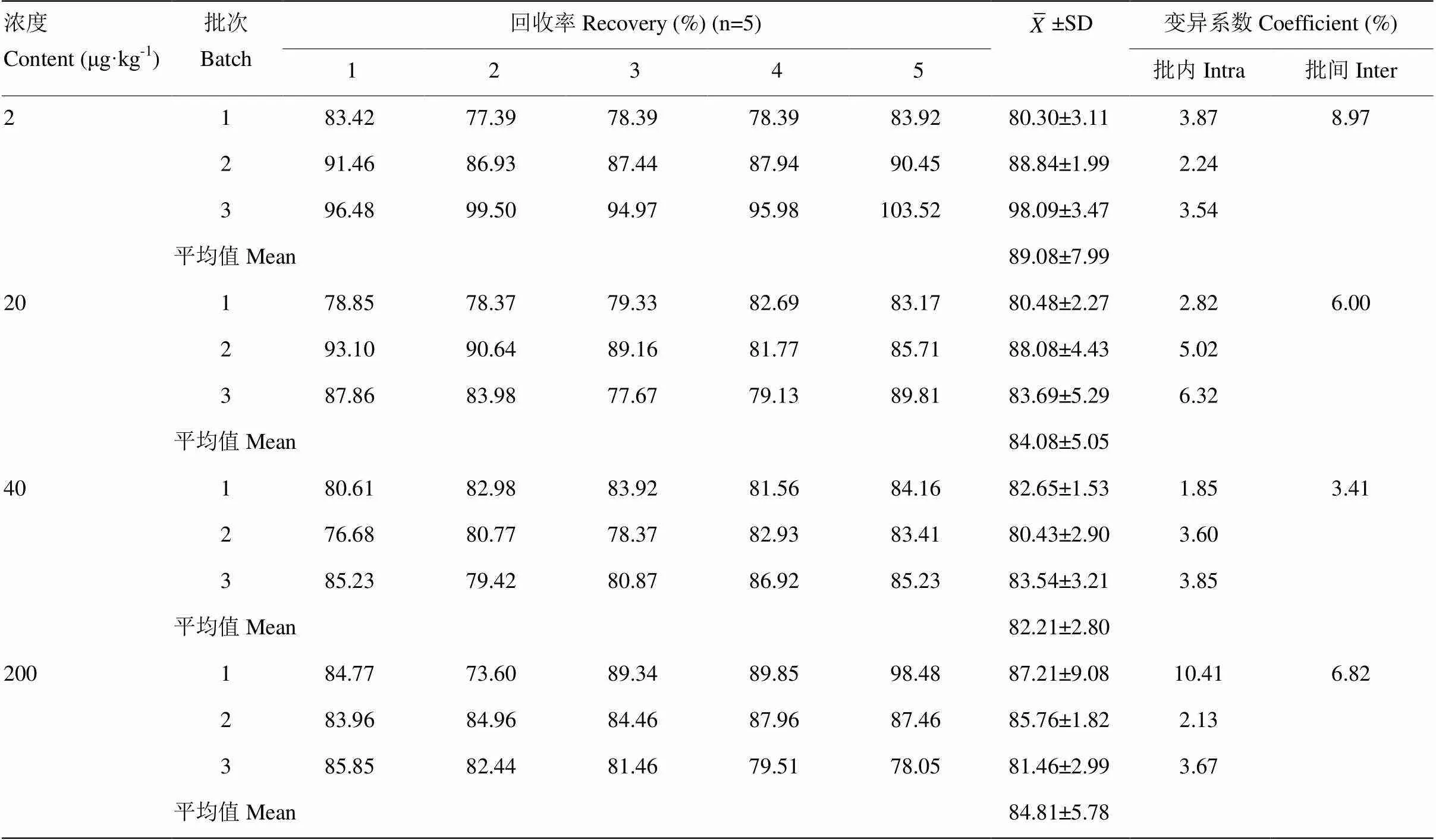
表2 头孢洛宁在牛奶中的回收率与变异系数

表3 牛奶中头孢洛宁的残留量(初产奶牛)
ND:表示检测不到,<LOQ:小于定量限。ND:not detect,<LOQ:Less than limit of quantification
3 讨论
奶牛乳房炎因为降低了奶的质量和产量,从而给奶牛业带来了巨大的经济损失,同时增加了药物的应用和动物死亡的风险[19-20]。目前我国治疗奶牛干乳期乳房炎主要途径是抗生素疗法,有青霉素类(如氨苄西林,氯唑西林等),随着该类抗生素的大量持久使用,耐药菌株逐渐增多,临床治疗效果也逐年下降[21-23]。头孢洛宁在欧美等国家早已广泛用于奶牛和奶山羊治疗,用于预防干乳期乳房炎、子宫内膜炎等疾病。头孢洛宁对酸和-内酰胺酶稳定,杀菌力强,抗菌谱广,对革兰氏阴性菌和革兰氏阳性菌均有效。尤其对引起奶牛乳房炎的病原菌有效[24]。有研究表明头孢洛宁对从欧洲分离的金黄色葡萄球菌,停乳链球菌、无乳链球菌,大肠杆菌,克雷伯氏菌等的MIC90在0.00178—2 μg·mL-1之间[25]。WENTE等研究头孢菌素类抗生素对奶牛乳房分离菌的最小抑菌浓度(minimal inhibitory concentrations,MIC),结果表明,分离菌对药物的敏感性依次为头孢喹肟、头孢洛宁和头孢匹林,但考虑到头孢喹肟是第四代头孢菌素类抗生素,临床推荐优先使用头孢洛宁[26]。李维静等研究了头孢洛宁对大肠杆菌、乳房链球菌、停乳链球菌、无乳链球菌、金黄色葡萄球菌的MIC90,分别为64、8、16、4、16 μg·mL-1[27]。PARKINSON等比较了头孢洛宁与氯唑西林、氨苄西林复方制剂在干奶期治疗乳房炎的临床治疗效果,结果表明头孢洛宁较其他给药组,具有显著的治疗效果[28]。OWENS等的研究同样表明头孢洛宁在奶牛乳房炎的治疗过程中具有好的治疗效果[29-30]。

表4 牛奶中头孢洛宁的残留量(经产奶牛)
ND:表示检测不到,<LOQ:小于定量限。
ND:not detect,<LOQ:Less than limit of quantification
欧盟及澳大利亚官方规定头孢洛宁最高残留限量(MRL)为20 μg·kg-1[17]。在已上市销售的头孢洛宁乳房注入剂产品中,先灵葆雅公司的产品Cepravin Dry Cow,干奶期持续时间至少为49d,并弃掉前8次所挤的牛奶;在英国上市的产品Kepravine Dry Cow,干奶期持续时间至少为54d,弃奶期为产犊后96 h。如果干奶期持续时间少于54d,弃奶期为给药后54d,再加96h,确保弃掉至少前7次所挤的牛奶。爱尔兰医药管理局规定干奶期奶牛乳房灌注Cepravin Dry Cow(3g:250mg)后,干奶期持续时间至少为54d,弃奶期为产犊后96h(4d)。
本试验中,78号和52号牛的干乳期分别为33和39d,弃奶期为1d;其它试验牛的干乳期都长于40d,弃奶期为0d。本次试验研究中,经产奶牛和初产奶牛在泌乳后6 h,有4头奶牛的奶牛中头孢洛宁的浓度大于20μg·kg-1。至12 h,仅有1头牛的奶样中头孢洛宁的浓度大于20μg·kg-1。通过WTM1.4软件计算头孢洛宁乳房注入剂(干奶期)在牛奶中的休药期为24h。为确保食品安全,防止由于个体间的差异,以及干乳期时间的不同。参考以上标准和产品,结合本次试验研究结果,将头孢洛宁乳房注入剂(干乳期)在干奶期产犊后牛奶中的休药期定为产犊后96 h。
4 结论
综上所述,在临床使用时,建议头孢洛宁乳房注入剂(干奶期)按推荐剂量4个乳区(250mg/乳区)给药后,其产犊泌乳后牛奶中的休药期为24 h。
[1] SCHUKKEN Y H, GÜNTHER J, FITZPATRICK J, FONTAINE M C, GOETZE L, HOLST O, LEIGH J, PETZL W, SCHUBERTH H J, SIPKA A, SMITH D G E, QUESNELL R, WATTS J, YANCEY R, ZERBE H, GURJAR A, ZADOKS R N, SEYFERT H M. Host- response patterns of intramammary infections in dairy cows.,2011, 144(3-4): 270-289.
[2] DE VLIEGHER S, FOX L K, PIEPERS S, MCDOUGALL S, BARKEMA H W. Invited review: Mastitis in dairy heifers: Nature of the disease, potential impact, prevention, and control.2012, 95 (3): 1025-1040.
[3] DEB R, KUMAR A, CHAKRABORTY S, VERMA A K, TIWARI R, DHAMA K, SINGH U, KUMAR S. Trends in diagnosis and control of bovine mastitis: A Review.2013, 16 (23): 1653-1661.
[4] LEE J W, O'BRIEN C N, GUIDRY A J, PAAPE M J, SHAFER- WEAVER K A, ZHAO X. Effect of a trivalent vaccine against staphylococcus aureus mastitis lymphocyte subpopulations, antibody production, and neutrophil phagocytosis.2005, 69 (1): 11-18.
[5] LEITNER G, LUBASHEVSKY E, TRAININ Z. Staphylococcus aureus vaccine against mastitis in dairy cows, composition and evaluation of its immunogenicity in a mouse model., 2003, 93 (3-4): 159-167.
[6] DUFOUR S, DOHOO I R. Monitoring dry period intramammary infection incidence and elimination rates using somatic cell count measurements., 2012, 95 (12): 7173-7185.
[7] Bradley A J. Bovine Mastitis: An Evolving Disease. The Veterinary Journal, 2002, 164 (2): 116-128.
[8] ROBERSON J R, FOX L K, HANCOCK D D, GAY J M, BESSER T E. Ecology of staphylococcus aureus isolated from various sites on dairy farms.1994, 77 (11): 3354-3364.
[9] SONG E, YU M, WANG Y, HU W, CHENG D, SWIHART M T, SONG Y. Multi-color quantum dot-based fluorescence immunoassay array for simultaneous visual detection of multiple antibiotic residues in milk.2015, 72: 320-325.
[10] WANG D, WANG Z, YAN Z, WU J, ALI T, LI J, LV Y, HAN B. Bovine mastitis staphylococcus aureus: antibiotic susceptibility profile, resistance genes and molecular typing of methicillin-resistant and methicillin-sensitive strains in China., 2015, 31: 9-16.
[11] 王娟, 黄秀梅, 曲志娜, 赵思俊, 盖文燕, 王玉东, 王君玮. 生鲜牛奶中金黄色葡萄球菌的分离及耐药性分析. 中国人兽共患病学报, 2014(12): 1214-1217.
WANG J, HUANG X M, QU Z N, ZHAO S J, GAI W Y, WANG Y D, WANG J W. Islation of staphylococcus aureus from raw milk and its drug resistance analysis., 2014(12): 1214-1217. (in Chinese)
[12] 吴天琪, 吴艳涛, 张小荣. 牛奶源金黄色葡萄球菌耐药基因分布特征的研究. 畜牧与兽医, 2015(11): 88-90.
Wu T Q, Wu Y T, Zhang X R. Study on distribution characteristics of staphylococcus aureus resistance gene in milk., 2015(11): 88-90. (in Chinese)
[13] 王娟, 黄秀梅, 刘书科, 李玉清, 赵思俊, 曲志娜, 王玉东, 王君玮.. 牛奶中金黄色葡萄球菌的耐药性及其肠毒素分型研究. 中国兽医杂志, 2013(03): 30-32.
WANG J, HUANG X M, LIU S K, LI Y Q, ZHAO S J, QU Z N, WANG Y D, WANG J W. Study on drug resistance of Staphylococcus aureus in milk and its enterotoxin typing., 2013(03): 30-32. (in Chinese)
[14] PAPADOPOULOU C, DIMITRIOU D, LEVIDIOTOU S, GESSOULI H, PANAGIOU A, GOLEGOU S, ANTONIADES G. Bacterial strains isolated from eggs and their resistance to currently used antibiotics: is there a health hazard for consumers?1997, 20 (1): 35-40.
[15] 陈杖榴. 兽医药理学. 北京: 中国农业出版社, 2017: 241-248.
Chen Z L.. Beijing: China Agriculture Press, 2017: 241-248. (in Chinese)
[16] EMEA. Cefalonium Summary Report(1). http: //www. ema. europa. eu/ docs/en/2009/11/WC5000 11710. pdf
[17] EMEA. CefaloniumSummaryReport (2). http: //www. ema. europa. eu/ docs/en/2009/11/WC5000 11741. pdf
[18] 中国国家标准化管理委员会. 牛奶和奶粉中头孢匹林、头孢氨苄、头孢洛宁、头孢喹肟残留量的测定(液相色谱-串联质谱法). 2008.
Standardization Administration of the People's Republic of China (SAC). Determination of cefapirin, cephalexin, cefalonium, cefquinome residues in milk and milk powder-LC-MS-MS method. 2008. (in Chinese)
[19] MELCHIOR M B, FINK-GREMMELS J, GAASTRA W. Comparative assessment of the antimicrobial susceptibility of staphylococcus aureus isolates from bovine mastitis in biofilm versus planktonic culture.2006, 53 (7): 326-332.
[20] FREITAS C H, MENDES J F, VILLARREAL P V, SANTOS P R, GONÇALVES C L. Identification and antimicrobial suceptibility profile of bacteria causing bovine mastitis from dairy farms in pelotas, Rio Grande Do Sul.2018.
[21] 王旭荣, 李宏胜, 李建喜, 王小辉, 孟嘉仁, 杨峰, 杨志强. 奶牛临床型乳房炎的细菌分离鉴定与耐药性分析. 中国畜牧兽医, 2012(07): 195-198.
WANG X R, LI H S, LI J X, WANG X H, MENG J R, YANG F, YANG Z Q. Isolation and antimicrobial susceptibility of pathogenic bacteria from case of clinical mastitis in Shanxi dairy herds., 2012(07): 195-198. (in Chinese)
[22] 金耀忠, 万世平, 姜法铭, 龚志亮, 曹杰, 瞿瑜萍, 叶承荣. 奶牛乳房炎流行情况调查及主要病原分离与药敏试验. 畜牧与兽医, 2011(05): 64-67.
JIN Y Z, WAN S P, JIANG F M, GONG Z L, CAO J, JU Y P, YE C R. Investigation of epidemic situation of mastitis in dairy cows, isolation of major pathogens and drug sensitivity test. Animal2011(05): 64-67. (in Chinese)
[23] 李宏胜, 郁杰, 罗金印, 李新圃, 徐继英, 王旭荣, 张礼华. 牛源性无乳链球菌血清型分布及抗生素耐药性研究. 中国畜牧兽医, 2012(01): 164-167.
LI H S, YU J, LUO J Y, LI X P, XU J Y, WANG X R, ZHANG L H. Serotype Distribution of bovine streptococcus agalactiae and its drug resistance to antibiotics in China.2012(01): 164-167. (in Chinese)
[24] 徐立萧, 岳永波, 吴鹏飞, 张志宾, 孙楠, 郧祺. 头孢洛宁的研究进展. 中国兽药杂志, 2017(06): 69-73.
XU L X, YUE Y B, WU P F, ZHANG Z B, SUN N, YUN Q. Research progress of cephalonium., 2017(06): 69-73. (in Chinese)
[25] Products C F V M. Cephalonium, Summary Report (2). 2002.
[26] WENTE N, ZOCHE-GOLOB V, BEHR M, KRÖMKER V. Susceptibility to cephalosporins of bacteria causing intramammary infections in dairy cows with a high somatic cell count in Germany.2016, 131: 146-151.
[27] 李维静. 头孢洛宁乳房注入剂防治干乳期奶牛乳腺炎的药效学及残留消除研究: [D] 扬州大学基础兽医学, 2014.
LI W J. Studies on the efficacy and residue elimination of cephalonium intramamary infusion for the treatment of bovine mastitis during the drying off period [D]. Yangzhou: Yangzhou University, 2014. (in Chinese)
[28] PARKINSON T J, VERMUNT J J, MERRALL M. Comparative efficacy of three dry-cow antibiotic formulations in spring-calving New Zealand dairy cows.2000, 48(5): 129-135.
[29] OWENS W E, NICKERSON S C, BODDIE R L, TOMITA G M, RAY C H. Prevalence of mastitis in dairy heifers and effectiveness of antibiotic therapy.2001, 84 (4): 814-817.
[30] WRAIGHT M D. A Comparative field trial of cephalonium and cloxacillin for dry cow therapy for mastitis in Australian dairy cows.2004, 82 (10): 624.
Residue Depletion Study and Withdrawal Period for Cefalonium Intramammary Infusion (Dry cow) in Bovine Milk
YAN ChaoQun1, LI ShuaiPeng2, ZHANG Shen1, XIE Shun1, WEI KaiYun1, HUANG XianHui1
(1Guangdong Provincial Key Laboratory of Veterinary Pharmaceutics Development and Safety Evaluation, College of Veterinary Medicine, South China Agricultural University, Guangzhou 510642;2Inner Mongolia Jinhe Animal Pharmaceutical CO., LTD, Tuoketuo 010200 Inner Mongolia)
【Objective】Cefalonium is a first generation semi-synthetic cephalosporin with broad spectrum of activity against both Gram-positive and Gram-negative bacteria. The cefalonium is used intramammarily during the dry period of cattle at a recommended dose of 250 mg per quarter to treat existing sub-clinical infections and to prevent new infections. The bactericidal activity of cefalonium is a result of its inhibitory action on bacterial cell wall synthesis due to binding to one or more penicillin binding proteins located under the cell wall susceptible bacteria. The resulting high internal osmotic pressure leads to rupture of the cytoplasmic membrane. The objective of this study was to establish a withdrawal period for cefalonium in milk, following intramammary infusion treatment.【Method】Twenty healthy dairy cows in the dry period were injected with a cefalonium injection into each breast area. The average dry period was 50 days, and they were mixed with the samples of 4 milk areas collected per cow at 6, 12, 24, 36, 48, 60, 72, 96 and 120 h after the start of milking. Residues of cefalonium were detectable in milk for up to 6, 12, 24, 36, 48, 60, 72, 96 and 120 h post-treatment, respectively. Phenomenex Luna C18 (150 mm×2 mm, 5 μm) was used. Acetonitrile-0.1% formic acid aqueous solution was used as the mobile phase. The gradient elution procedure was used. The flow rate was 0.25 mL·min-1, the column temperature was 35°C, and the injection volume was 5.0 μL. Phenomenex Luna C18 (150 mm×2.1 mm,3.5 μm) was used. Acetonitrile-0.1% formic acid aqueous solution was used as the mobile phase. The gradient elution procedure was used. The flow rate was 0.25 mL/min, the column temperature was 35°C, and the injection volume was 5.0 μL. MS conditions of detection method: ESI ion source, positive ion scan, multiple reaction monitoring, ion spray voltage: 4kV, the pressure of GS1: 55 psi, the pressure of CUR: 15 psi, the pressure of GS2: 35 psi, TEM: 600℃, the quantitative ion is: cefalonium m/z→459.4/337.3. The sample was thawed naturally, 5g of milk was accurately pipetted into a 50 mL centrifuge tube, 20 mL of acetonitrile was added, vortexed and shaken for 2min, and centrifuged at 10 000 r/min for 10 min. The supernatant was extracted once with 15 mL of 75% acetonitrile aqueous solution, and the extracts were combined twice, and 10 mL of acetonitrile saturated n-hexane was added, shaken for 1 min, the n-hexane was discarded, and the extract was transferred to a 100 mL heart bottle. Acetonitrile was removed by rotary evaporation at 40 ° C using a rotary concentrator. To the above sample solution, 20 mL of a sodium dihydrogen phosphate buffer solution was added, followed by adjustment to pH = 8.5 with a sodium hydroxide solution. The sample was passed through the HLB solid phase extraction column, then the heart bottle was washed with 5 mL of sodium dihydrogen phosphate buffer solution and passed through the column, and the column was washed with 2 mL of water. It was eluted with 2 mL of acetonitrile, dried at 40 °C with a nitrogen concentrator, dissolved in 2 mL of water, shaken, passed through a 0.22μm microporous membrane, LC-MS/MS detection analysis.【Result】Cefalonium showed a good linearity in the concentration range of 2-200 μg·kg-1, with a correlation coefficient of 0.991-0.997, a detection limit of 0.5 μg·kg-1, and a limit of quantification of 2 μg·kg-1. The relative recovery rate of this method at the four levels of addition of 2, 20, 40 and 200 μg·kg-1was 82.21%-89.08%. The coefficient of intra-assay coefficient of variation was 1.85%-10.41%, and the inter-assay coefficient of variation was 3.41%-8.97%. The experimental method has high sensitivity and simple operation, and can be used for the residue depletion studyof cefalonium in milk. Milk samples from these cows after calving were analyzed for antibiotic residues by a liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) procedure. Primiparous dairy cows that used cefalonium intramammary infusion (dry cow) were below the limit of quantitation at 96 hours after calving. Dairy cows were below the limit of quantitation at 36 hours after calving.【Conclusion】After the cefalonium intramammary infusion (dry cow) was administered at the recommended dose of 4 milk areas (250 mg/milk area), the withdrawal period in milk was 24 h.
cefalonium; intramammary infusion (dry cow); dairy cows; withdrawal periods
10.3864/j.issn.0578-1752.2019.02.015
2018-03-24;
2018-12-24
十三五重点研发项目(2016YFD0501306)
闫超群,E-mail:1368268753@qq.com。通信作者黄显会,E-mail:xhhuang@scau.edu.cn
(责任编辑 林鉴非)

