BRAF inhibitor: a novel therapy for ameloblastoma in mandible
Masanobu Abe, Liang Zong, Takahiro Abe, Hideyuki Takeshima, Jiafu Ji, Toshikazu Ushijima,Kazuto Hoshi
1Department of Oral & Maxillofacial Surgery, University of Tokyo Hospital, Tokyo 113-8655, Japan; 2Division for Health Service Promotion,University of Tokyo, Tokyo 113-8655, Japan; 3Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing),Center of Gastrointestinal Surgery, Peking University Cancer Hospital & Institute, Beijing 100142, China; 4Graduate School of Medicine,University of Tokyo, Tokyo 113-0033, Japan; 5Division of Epigenomics, National Cancer Center Research Institute, Tokyo 104-0045, Japan
Ameloblastoma is a benign but locally aggressive odontogenic neoplasm that accounts for 10% of all tumors arising in the mandible and maxilla (1). Eighty percent of ameloblastomas arise in the mandible, and they are usually found in young adults. It frequently recurs if not adequately resected. Therefore, the standard therapy for this tumor is complete bone resection with an adequate margin of safety:marginal or segmental osteotomy. However, aesthetic deformities, functional impairments and psychological impairments after radical surgery for large ameloblastoma,have been serious issues (1).
A representative case of huge ameloblastoma of a 68-year-old male is shown in Figure 1. Written informed consent was obtained from the patient. Computed tomography (CT) shows a well-demarcated, expansive and multiloculated neoplastic lesion within the posterior region of the left jaw invading and replacing the normal structure.This huge intraosseous lesion was pathologically diagnosed to be an ameloblastoma and was widely resected from the premolar region to the coronoid process, sparing the condyle. A titanium plate was used for the temporary reconstruction of the mandible; and its replacement with a fibula-flap was planned for the future. The surgical procedure was performed successfully, and no recurrence has been observed so far; however, the patient has been afflicted with functional impairment and aesthetic deformities.
To avoid such serious adverse outcomes, conservative therapies such as enucleation, curettage, peripheral osteotomy and other adjuvant therapy tend to be selected as a primary therapy, especially for young patients, taking into consideration the harmful effect on the growth of mandibles and esthetics. However, the high recurrence rate with conservative therapy, reported at 55%-90%, is a serious issue. Especially, large ameloblastomas (more than 6 cm) are known to be associated with early recurrence (2).Neither radiation therapy nor chemotherapy has evidence of effectiveness on the tumors (3). Therefore, finding a novel therapy is the one and only way to avoid extensive and/or repetitive surgeries for ameloblastoma.
Although little has been known about genetic anomalies in this tumor until recently, a highly recurrent somatic mutation was identified in the mitogen-activated protein kinase (MAPK) pathway: V600E mutations in the BRAF gene (BRAFV600E). Surprisingly, 57% of ameloblastomas were found to harbor BRAFV600Eand almost all ameloblastomas with the mutations were found in the mandible (96%). This finding strongly suggested the possibility of targeted therapy for patients with ameloblastoma (4-6).
After identification of the highly frequent BRAFV600Emutation, two case reports indicated the efficacy of BRAF inhibitor therapy for multiply recurrent large ameloblastomas with BRAFV600Emutations in the mandible(7,8) (Table 1). In one case report, both primary and metastatic recurrent ameloblastomas responded dramatically to therapy with dual BRAF/MEK inhibition(dabrafenib/trametinib) (7). In another report, therapy with a single BRAF inhibition (dabrafenib) demonstrated marked volume reduction of recurrent ameloblastoma; and an ongoing response was observed even after 12 months of therapy, despite a 50% reduction in the dose of dabrafenib compared with the dose for metastatic melanoma (8). In addition to the notable reduction of tumor volume, the BRAF inhibitor therapies improved the associated facial deformities (7,8). In melanoma, the clinical outcomes havebeen largely improved after the application of BRAF inhibitor therapy (9). Taking that result into consideration,BRAF inhibitor therapy is a promising for large ameloblastomas with BRAFV600Emutation, although large clinical trials are necessary to demonstrate the efficacy of the therapy for clinical application.

Table 1 Clinical studies of BRAF inhibition therapy for recurrent ameloblastoma
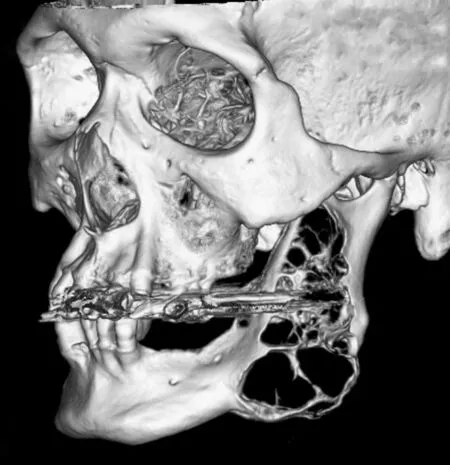
Figure 1 Computed tomography (CT) image of a 68-year-old male with huge ameloblastoma.
Recent developments in molecular medicine represent the effectiveness of personalized targeted therapy in ameloblastoma. However, for complete cures of large ameloblastoma, adjuvant or neoadjuvant therapies are considered feasible. Long-standing issues in the treatment of ameloblastoma might be settled by the novel therapies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
 Chinese Journal of Cancer Research2018年6期
Chinese Journal of Cancer Research2018年6期
- Chinese Journal of Cancer Research的其它文章
- Tumor pyruvate kinase M2: A promising molecular target of gastrointestinal cancer
- Novel circular RNA expression profile of uveal melanoma revealed by microarray
- Limited energy parametrial resection/dissection during modified laparoscopic nerve-sparing radical hysterectomy
- Identification of liver metastasis-associated genes in human colon carcinoma by mRNA profiling
- Construction and external validation of a nomogram that predicts lymph node metastasis in early gastric cancer patients using preoperative parameters
- Voltage-gated K+ channels promote BT-474 breast cancer cell migration
