Ecotoxicological Assessment of Virgin Plastic Pellet Leachates in Freshwater Matrices
S.Schiavo,M.Oliviero,V.Romano,S.Dumontet,S.Manzo
1C.R ENEA,Portici Naples,Italy
2Department of Science and Technology,Parthenope University of Naples,Italy
Keywords PE PP PS Toxicity test battery Integration index
Abstract The environmental contamination caused by the widespread diffusion of plastic material all over the world is a topic of high concern.Virgin plastic pellets of different polymers are often used in particle toxicity studies as referencematerials.Inthis studywe exposedorganismsofdifferenttrophic levels,spanning form prokaryotes to eukaryotes,to leachate of polypropylene(PP)and polyethylene(PE),and polystyrene(PS)pellets in acute and chronic ecotoxicological tests.A toxicity test battery integrated index(TBI)was used to rank the relative toxicity of studied polymers and to define their possible ecotoxicological risk in freshwater environment.Daphnia magna showed the highest susceptibility in the chronic exposure tests(around 50%of effect)while Aliivibrio fischeri(around 25%of effect)in the acute one.No relevant toxic effects were observed on Sorghum saccharatum,Lepidium sativum and Sinapis alba seeds,while significant toxicity for Vicia faba along 21 days of exposure was reported.TBI allowed us to rank the toxicity risk associated to the studied materials as follows:PP>PS>PE.PP toxicity could be related to the presence of solvents(methanol,oil,cyclohexane)employed for its production,whereas PS toxicity was probably due to the depolymerization,occurring in water,followed by styrene release,while the mild toxic effects of PE and its temporarybio stimulation could be attributable to the thermoregulatoryadditives present in the polyethylene resins.Our results highlighted that also the virgin plastic pellets could be responsible of toxic effects that should not be neglected.
1 Introduction
The global consumption of plastic materials is nowadays steadily increasing,with more than 240 million tons of plastic produced annually.Geyer et al.(2017)estimated that the cumulative amount of plastic waste,from 1950 to 2017,totals around 6 billion of metric tons.The same authors estimate that this value will increase up to 25 billion of metric tons by 2050.The low recycling rates of plastic products,together with their persistence in the environment,ends up in accumulation of plastic debris and plastic microparticles in both terrestrial and marine systems(Barnes et al.,2009).
The plastic polymers are usually not regarded as hazardous materials,because their large molecular size and their insolubility in water,that make them not bio-available and biochemically inert(da Costa,2018).However,several low molecular weight and non-polymeric molecules present in plastic products are known as hazardous.They are either weakly bound or not bound at all to the polymeric macromolecules and may be released from the plastic product(Crompton,2007;OECD,2004).These include residual monomers,oligomers,low molecularweight fragments ofcatalyst remnants,polymerization solvents,and a widerange ofadditives(Crompton,2007).Release from plastic products of hazardous substances as phthalates,brominated flame retardants,bisphenol A,formaldehyde,acetaldehyde,4-nonylphenol,and many volatile organic compounds has been already reported by several authors(Tønning et al.,2010;Kim et al.,2006;Brede et al.,2003;Mutsuga et al.,2006;Fernandes et al.,2008;Henneuse-Boxus and Pacary,2003).
The study of the impact of plastic residues on different environmental matrices needs a multidisciplinary approach integrating chemical analysis(Wagner et al.,2014;Syberg et al.,2015)and ecotoxicological tests.Generally,the toxicity study on plastic polymers found in the environment also include the exposure of test organisms to leachates of virgin plastic pellets,supposed to be free from any additives and/or residual monomers,in order to separate the effects derived for virgin plastic itself from those due to chemicals used during product manufacturing and/or sorbed from the environment.An important work in this field was authored by Lithner et al.(2009),who studied the toxicity of leachates of 32 different plastic products founding acute toxicity in Daphnia magna exerted by leachates of plasticized PVC and polyurethane.Similarly,Wagner and Oehlmann(2009)cultivated mud-snails in polyethylene terephthalate(PET)mineral water bottles founding evident endocrine disrupting effects,if compared to those cultivated in borosilicate Erlenmeyer flasks.
However,the absence of any leachable compounds in virgin plastic is still a question under debate.Scientific literature dealing with toxicity tests on plastic leachatesis relatively scant,despite theseare recognised as dangerous pollutant of high ecotoxicological concern(Hermabessiere et al.,2017).
In this line,this study is aimed at studying the acute and chronic adverse effects of leachates,coming from different virgin plastic polymers,on test organisms belonging to several species and pertaining to different trophic levels.The test organisms used here were:a)the bacteria Aliivibrio fischeri(acute toxicity),b)the plants Sorghum saccharatum,Lepidium sativum,and Sinapis alba(sub-chronic toxicity)and Vicia faba(chronic toxicity)c)the crustacean Daphnia magna(acute and chronic toxicity).Moreover,in order to identify the possible ecotoxicological risk for the freshwater biota stemming of the investigated plastic leachates,the toxicity test battery integrated index(TBI)(Manzo et al.,2008)was used.
2 Material and methods
2.1 Plastic samples
Toxicity tests have been performed using theleachates ofthree different polymers:polypropylene(PP),polyethylene(PE),and polystyrene(PS)pellet.
2.2 Samples preparation and leaching
The test is conducted according to Italian and European Standard procedure(UNI EN 12457-2:2004)by using pure water(18MΩ resistivity)as leaching solution.Each sample(PE,PP,PS)was shaken for 24h at room temperature,with a liquid-to-solid ratio of 10(L/S=10).At the end of the leaching process,the solid was removed through decantation followed by a filtration on qualitative filter papers Whatman Grade 1(11μm porosity)to remove plastic fragments,and the water phase was tested for its toxicity.
2.3 Acute toxicity
•Aliivibrio fischeri
The Microtox®tests were performed according to the supplier’s protocol(Modern Water,New Castle,DE).Lyophilized bacteria were rehydrated with the reconstitution solution supplied by the manufacturer prior to perform the test.Luminescence inhibition of polymers leachates(PE,PP,PS)was assessed for 5,15 and 30 min of exposure according to the 81.9%Basic Test Protocol(screening test)(Azur Environment Ltd,1998).The sample toxicity was evaluated measuring the decrease of the intensity of light produced by the luminescent bacteria.A control group was also set up without leachates.The negative control was the Microtox®diluent(NaCl 2%).The luminescence decrease was evaluated after 5,15 and 30 min of exposure by using a Microbics Model 500 Toxicity Analyzer,according to the manufacturer’s instructions(Modern Water,New Caste,DE).The results were expressed as luminescence inhibition percentage with respect to the control using the Abbot’s formula and as EC50 calculated using ICp method(USEPA,1993)
•Daphnia magna
The D.magna immobilization test was performed according to OECD(2004).Four 2.5 folds serial dilutions were prepared in order to reach the leachate concentration of 100%,75%,50%and 25%.Ten individuals,aged less than 24 h,were transferred in Petri dishes and exposed to 10 mLof each leachate dilutions at 20 ±2◦C.After 24 and 48 h the immobilization and mortality of D.magna individuals were recorded.Tests were performed in triplicate.Results were reported as percentage of the effect respect to the control(Abbott,1925).
2.4 Subchronic toxicity on plants
Germination and root elongation tests were carried out on S.alba,S.saccharatum and L.sativum according to Italian of ficial protocol(UNICHIM 10780/2003).Ten seeds were placed in Petri dishes,lined with filtered paper imbibed with non-diluted PE,PP,PS leachates.Five replicates for each polymer leachates were prepared.Deionized water was used as negative control.The Petri dishes were incubated in darkness at 25 ±2◦C in closed plastic bags in order to avoid evaporation.After 72 h,the number of germinated seeds(n)and the root length(L)were measured and the germination index(GI)and the root elongation inhibition(REi)were calculated as follows:
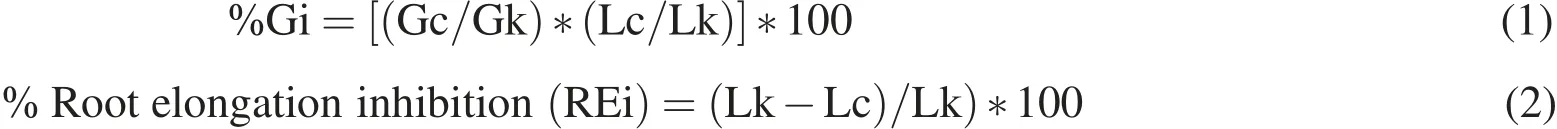
where:Gc=mean of germinated seeds in samples;Gk=mean of germinated seeds in the control;Lc=mean of root length in samples;Lk=mean the root length in the control.
2.5 Chronic toxicity
•Daphnia magna
The 21-day life experiments were performed with D.magna,according to OECD 202(OECD,2008),on 2 dilutions(50%and 75%)of each leachate.All test solutions were prepared and stabilized at 20◦C one day before the onset of the experiment.Twenty neonates(<24h old)were exposed in 50 ml of the sample suspensions in glass vessels.Daphnids were fed twice a week with Raphidocelis subcapitata and Saccaromyces cerevisiae(1.5 10 elevated to 5 cells mL-1)in conjunction with the renewal of the test medium.All experiments were performed under a controlled light cycle(16 h of light:8 h of dark).Survival and number of neonates were recorded daily.A negative(standard water)and positive control(K2Cr2O7)were performed simultaneously.Results were expressed in terms of mortality and reproduction inhibition(Ri).
•Vicia faba
The chronic test with V.faba was carried out using a modified Monteiro et al.(2009)protocol.Brie fly,germinated V.faba seeds were cultivated in a hydroponic solution(Hoagland and Amon,1950)supplemented with polymers leachates in a concentration of 50%.For each polymer 5 replicates(3 plantules for each replicate)were provided.The experiment was performed under controlled condition:light cycle,16 h of light:8 h of dark),temperature of 25◦C during the day and 16◦during the night.After 21 days,the following parameters were evaluated:fresh weight,fresh weight of aerial parts,dry weight of aerial parts,fresh weight roots,dry weight roots,leaves number,roots length,length of aerial parts.
2.6 Data integration
For each polymer leachate,the results of the different tests were integrated using the Toxicity test Battery integrated Index(TBI)(Manzo et al,2008).For each polymer leachate,the effects on the chosen endpoints were expressed as percentage and classified according to Ispra(2011).
To calculate the TBI,the percentage of the effect(%E)on each endpoint was corrected to obtain the Score test Endpoint(SEi)using the following formula:

where:SCF(Statistical Correction Factor)=Student t-test differences between samples and control.The values of 0,1,2,3 and 4 were attributed to SCF,corresponding to no effect(p>0.05),biostimulation(p<0.05),high biostimulation(p<0.01),toxicity(p<0.05),and high toxicity(p<0.01),respectively;Matrix(M)was set as 2 for samples leachates;Severity(S)was set as 2 for bioluminescence,3 for root elongation,germination and plants growth,4 for reproduction and 5 for mortality.
SEi is expressed in a 0–100 scale relative to test battery utilized as follows:

where:%Em=maximum effect percentage observed corresponding to the maximum MS obtained,and SEmaxis the maximum Score test Endpoint calculated.
The Toxicity test Battery integrated Index(TBI)is calculated according to the following formula

where:N=number of endpoints.
The ecotoxicological risk is defined as follows:not significant(TBI≤ 5%),medium(5< TBI≤ 20%),high(20<TBI≤50%),very high(TBI>50%).
3 Results
3.1 Plants
The results of the phytoxicity tests are reported in table 1.PE and PS polymer leachates showed no toxic effects on tested plants.PP leachate resulted non-toxic for L.sativum and S.alba,but exerted a negative effect on germination and root elongation of S.saccharatum.The IG%values highlighted a biostimulating effect on S.saccharatum and S.alba in the case of PE leachate.A slight biostimulation on root elongation was obtained also in the case of PS leachate for all the tested plants.On the contrary,the chronic test carried out with V.faba(Fig.1)showed severe negative effects on different endpoints.A significant toxicity was recorded as visible damages,such as necrosis and chlorosis,during the test.Among the 3 tested leachates,PS showed the highest adverse effects with values surpassing the 50%for all considered endpoints.In particular,the more pronounced negative effect was recorded upon the root elongation,the most sensitive endpoint among those considered here.
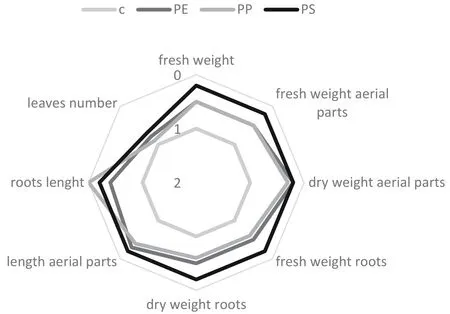
Fig.1 Evaluation of different endpoint for V.faba chronically exposed to PE,PP and PS leachates.
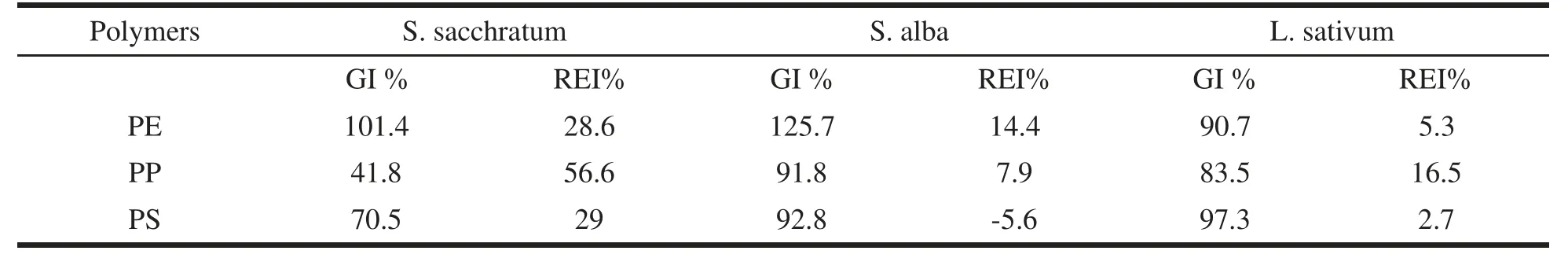
Table 1 Germination Index(GI)and Root Elongation Inhibition(REI)for S.saccharatum,S.alba L.sativum exposed to PE,PP and PS leachates.
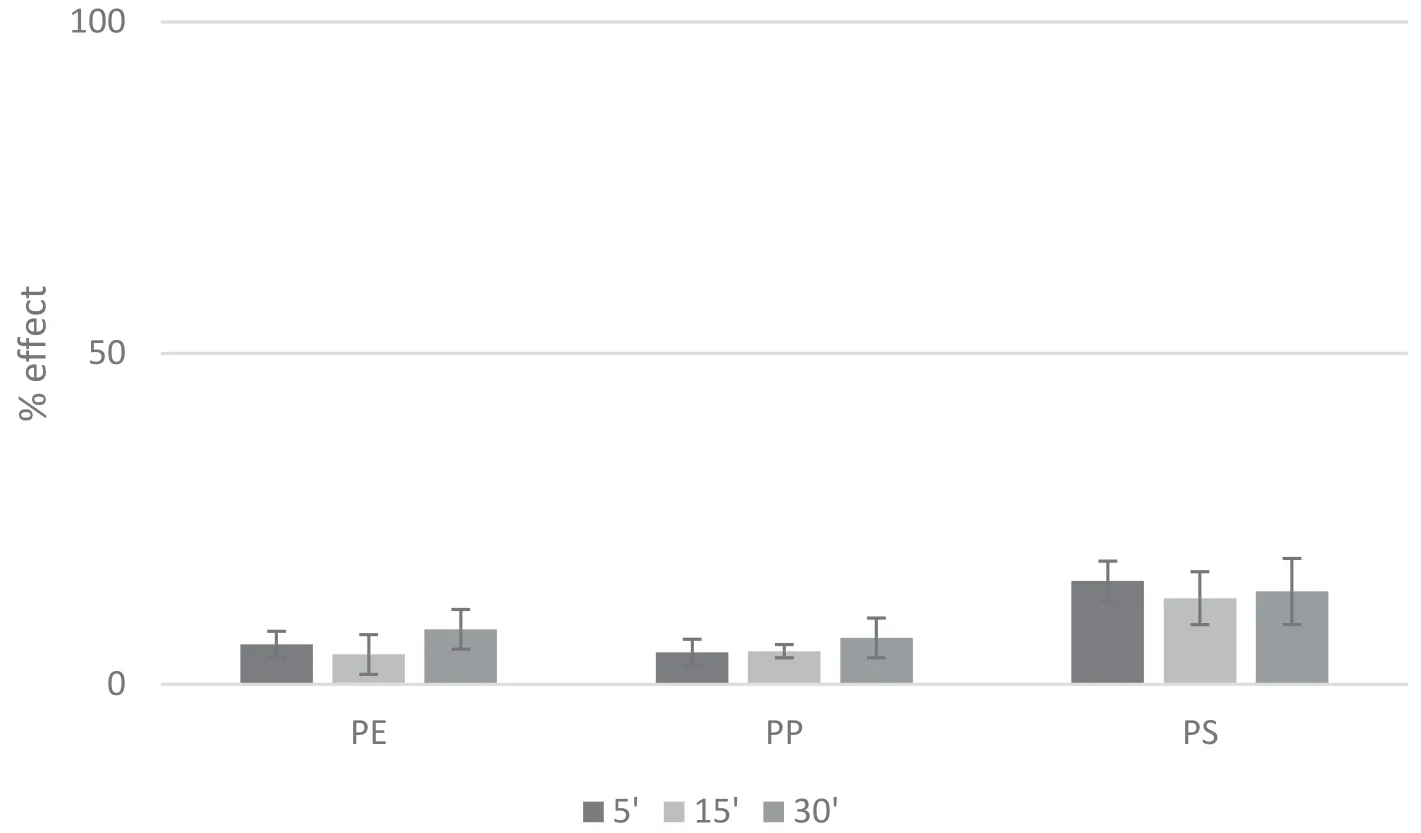
Fig.2 Inhibition of bioluminescence(%effect)for A. fischeri exposed to PE,PP,PS(5,15 and 30 min).
3.2 Bacteria
Toxic effects registered on A. fischeri exposed to plastic leachates are reported in figure 2.To the best of our knowledge,no data are available in literature on the effects of exposition of A. fischeri to plastic leachates.These leachates inhibited the bio-luminescence of A. fischeri below the threshold of 25%.Among the 3 leachates,PS exerted the highest effects.Similar results were recorded at 5,15 and 30 min of exposure.Despite the low effect recorded,the inhibition of bioluminescence seems to be the most sensitive test among the acute assays carried out in this work.
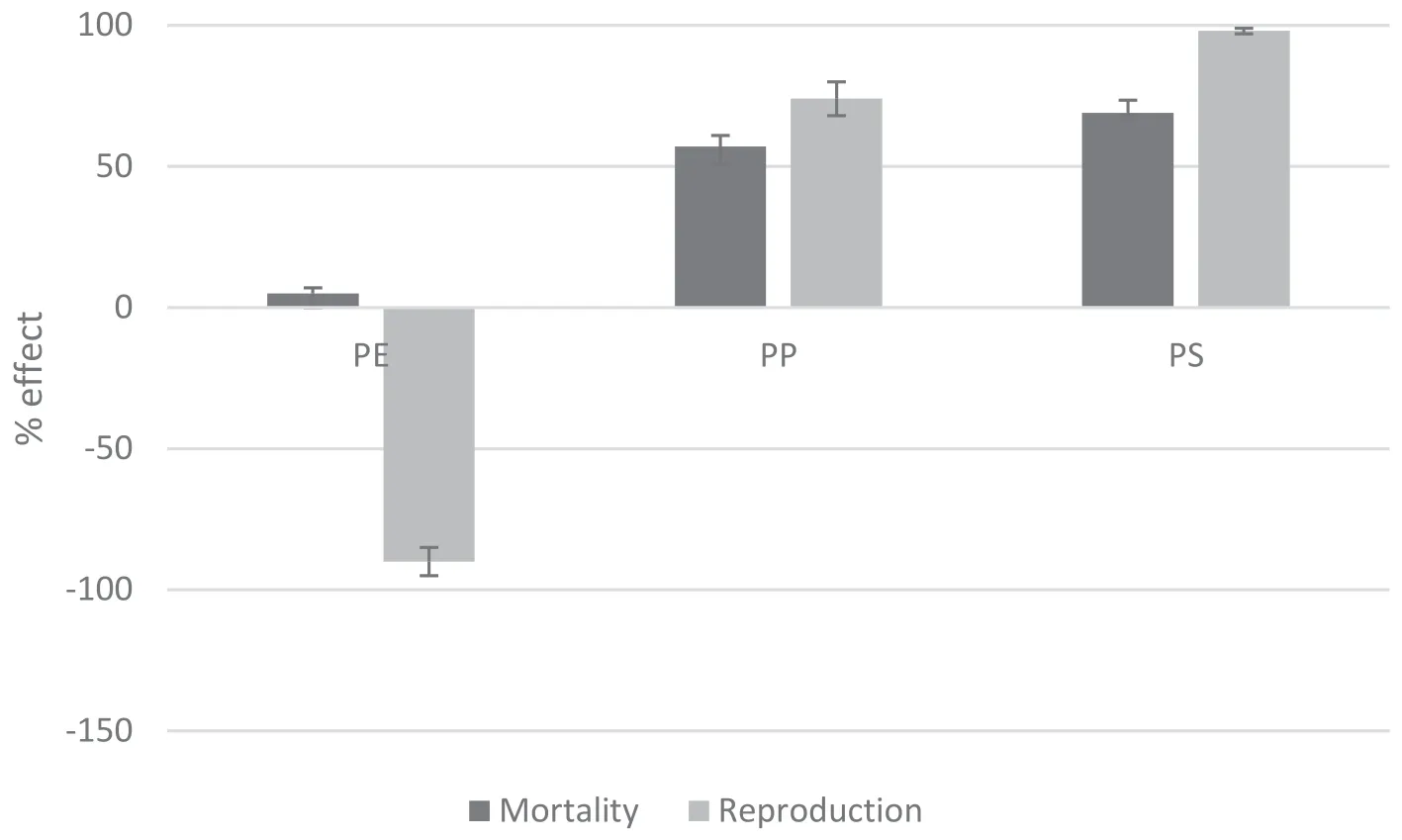
Fig.3 Mortality and reproduction(%effect)for D.magna exposed to PE,PP,PS at the end of exposure time(21thday).
3.3 Cladocera
Although the D.magna 48 h acute exposure test showed no mortality,adverse effects became evident during the 21 days of exposure to leachates.In Figure 3,the effects of PE,PS and PP leachates in terms of surviving and reproduction rate(number of nauplii/number of individual)were reported.D.magna survival was significantly affected by PP and PS leachates,as the recorded rates of mortality were around 69%for PS and slightly below 50%for PP.The first dead individuals were recorded after 4 days for PP,7 days for PS and 11 days for PE.The negative effect increased with time for PP and PS,whereas PE leachate showed a very low mortality rate(around 7%)(Fig.4a).
The reproduction of D.magna(11th day)was affected by the exposure to PP leachate,while no effect was noticeable up to 2 weeks for PS and PE.Starting from the 3rd week,an increasing inhibition trend was more evident for PS and PE exposure.PS reached almost the 100%of the effect(Fig.4b).For PE,instead,an enhancement of reproduction rate was observed starting from the 14th day.This increased along the exposure time with a final 98%increment of reproduction rate respect the control(Fig.4).
3.4 Data integration
The data analysed through the TBI approach allowed to rank the toxicity of leachates as follow PP>PS>PE.The PP leachate showed a high ecotoxicological risk(TBI=12.4%),according to the battery of test organism used here,whereas PS showed a moderate risk(TBI=8.4%)and PE a negligible one(TBI=4%).
4 Discussion
The toxic effect of the leachate of virgin plastic pellets observed here could be due to residues of chemicals used in the polymer production and non-intentionally added substances(impurities,polymerisation by products,breakdown products),catalysts,solvents,and additives easily leaching from virgin plastic materials.Leachates often exert higher toxic effects than virgin plastic themselves,suggesting that some compounds became more bioavailable during leaching(Teuten et al.,2009).In addition,Browne et al.(2013)and Nobre et al.(2015)observed that additives used in the production of different plastics are more toxic than pollutants adsorbed on plastic polymers.
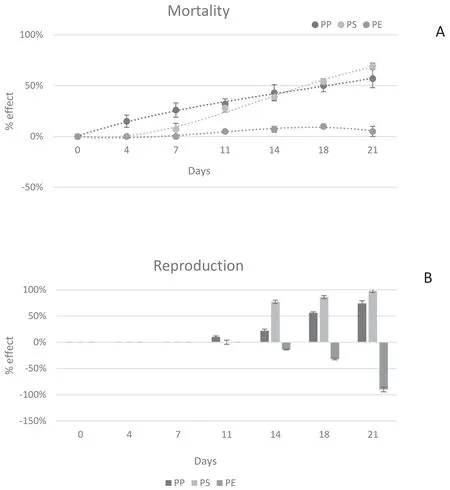
Fig.4 Mortality(A)and reproduction(B)(%effect)for D.magna exposed to PE,PP,PS at different time of exposure.
In this study,the TBIhighest toxicity was evidenced for PPvirgin plastic and could be related to the presence of solvents(methanol,oil,cyclohexane)used in polypropylene production(Harding et al.2007).The moderate toxicity exerted by PS was probably due to its depolymerization,occurring in water,followed by styrene release,whose ecotoxicity has been well documented(Gibbs et al.,1997;Thaysen et al.,2018).According to Murphy(2001),PE mild toxicity effects and its bio stimulation effect on D.magna reproduction rate could be due to the effect of some additives present in the polyethylene resins with known estrogenic potential(Yang et al.,2011).As expected,single biological responses to the complex chemical mixtures present in leachates varied with the polymer concerned and the species specific susceptibility of the exposed model organism.In our experiments,we evidenced relevant differences and sometimes contrasting effects among the exposed organisms.Actually,anthropogenic stressors can differ markedly in their effects on species diversity(Petrin et al.,2008).Taking into account that the variability of the responses of tested organisms is a common frame in ecotoxicological studies,the use a data integration algorithm was of great help in classifying the risk posed by such chemicals to the investigated ecosystem.
In the recent past,the “worst-case”assumption was considered adequate to assess the ecotoxicological risk stemming from the presence of molecule with xenobiotic characteristics in the environment(Calow and Forbes,2003).In the last decades,it became evident that this approach does not satisfactorily assess the environmental risk connected to the exposure to chemicals(Grenni et al.,2018).The development of indices,able to combine the responses to different tests into a toxicity hazard score(Hartwell,1997),allows to translate the test results to the effects exerted by xenobiotic compounds on complex natural systems in which many individuals and species live and thrive.
5 Conclusion
Virgin plastic pellets of different polymers are often used in particle toxicity studies as reference materials.However,a release of bioavailable additives and unknown substances could occur during the exposure in aquatic matrices,provoking adverse effects upon the test organisms especially when they are chronically exposed.We assessed that PP,PS and PE virgin plastic pellets leachates exerted several and diverse toxic effects upon the battery of selected test organisms:bacteria(A. fischeri),plants(V.faba,S.saccharatum,S.alba,L.sativum)and cladocera(D.magna).In particular,D.magna showed the highest susceptibility in the chronic exposure and the A. fischeri in the acute one.
Therefore,the selected tests,in particular the chronic assays,proved to be a suitable tool for assessing the toxicity of different plastic polymers.In addition,the TBI approach,adopted here to integrate the toxicity data,was able to classify the toxicity of the investigated leachates as follows:PP>PS>PE.
Overall,considering the assessed toxicity related with the virgin polymers,the direct loss of virgin plastic pellets into the environment during manufacturing and transport should be also monitored.
The known heterogeneity of plastic properties was mirrored in our results,which showed a highly variable and material-dependent leachates toxicity to the organisms tested here.However,due to industry policies of privacy we have no additional information about the type and the concentration of plastic additives used in the virgin granules studied here.All this makes a more accurate discussion difficult.
 Journal of Environmental Accounting and Management2018年4期
Journal of Environmental Accounting and Management2018年4期
- Journal of Environmental Accounting and Management的其它文章
- Light Fertilization Affects Growth and Photosynthesis in Mung Bean(Vigna radiata)Plants
- Capture Rate of Selected Heavy Metals In Q.Ilex L.Leaves Collected At Two Sites With Different Land Uses
- Impact of Biochar Amendment on Soil Quality and Crop Yield in a Greenhouse Environment
- Regional Redistribution Effects of Renewable Energy Subsidies
- Phytotoxic Extracts as Possible Additive in Subsurface Irrigation Drip for Organic Agriculture
- Uptake of Micro and Macronutrients in Relation to Increasing Mn Concentrations in Cistus salvifolius L.Grown in Hydroponic Cultures
