Effects of feeding time on complement component C7 expression in Pelteobagrus vachellii subject to bacterial challenge*
SHAO Ting (邵婷) , QIN Chuanjie (覃川杰) , , DUAN Huiguo (段辉国),YUAN Dengyue (袁登越), WEN Zhengyong (文正勇), WANG Jun (王钧) ,GE Fanglan (葛芳兰)
1 College of Life Science, Sichuan Normal University, Chengdu 610101, China
2 College of Life Science, Neijiang Normal University, Key Laboratory of Sichuan Province for Fishes Conservation and Utilization in the Upper Reaches of the Yangtze River, Neijiang 641000, China
Abstract Shifting the feeding time for fish from daytime to nighttime could alter their digestive behavior,disturb their metabolism, and may affect immune-related genes. This study aimed to clone complement component C7 and analyze the different expression of C7 mRNA in fish fed during either the day or at night and then challenged with Aeromonas hydrophila infection. The Pv- C7 cDNA of Pelteobagrus vachellii contained 2 647 bp with an open reading frame encoding a protein of 818 amino acids. Multiple sequences analysis indicated that Pv- C7 included eight domains, which was similar to results for other species.Quantitative PCR analysis showed that Pv- C7 was mainly expressed in the liver, spleen, intestine and head kidney tissues of healthy P. vachellii. Quantitative PCR analysis showed that C7 mRNA transcript in the liver, spleen and head kidney also increased significantly when the fish were fed at nighttime (20:00). In addition, the expression of Pv- C7 mRNA significantly increased with A. hydrophila challenge in the liver(48–96 h), spleen and head kidney (12–96 h) tissues of P. vachellii. Pv- C7 mRNA expression of the fish fed at nighttime showed significant higher than that in the fish fed at day time at 12–48 h in head kidney and 12–24 h in spleen. This study indicates that altering the feeding time from daytime to nighttime could increase Pv- C7 mRNA expression, and feeding time may affect the immune response involving C7.
Keyword: Pelteobagrus vachellii; complement component C7; shifting feeding time; clock
1 INTRODUCTION
The complement system has been found in both vertebrates and a wide range of invertebrate species(Fujito et al., 2010; Wang et al., 2013; Liu et al.,2016). Complement was a system of plasma proteins,which were central to many defense mechanisms, and included more than 30 distinct plasma and membraneassociated proteins (Morgan et al., 2005; Wang et al.,2015). It was fundamental for innate immunity and a key modulator of specific immunity. The complement system mediates various immune effector functions,including the energization of inflammatory responses,the regulation of specific immune responses, and the killing and elimination of pathogens (Sun et al.,2013). These studies indicated that complement genes could play an essential role to clear pathogens.
Complement component, C7 was an important plasma protein, which involved in the cytolytic phase of complement activation, through a series of catalytic reactions with other terminal complement components. C7 played an essential role in the assembly of the cytolytically active membrane attack complex (MAC) within target cell membranes (Bossi et al., 2009); this was generated by the sequential addition of single molecules of C5b, C6–8, and variable numbers of C9 molecules as terminal complement components (TCCs). The invaded bacteria were cleared by the final MAC assembly with disrupting their membranes (Muller-Eberhard,1986; Katagiri et al., 1999). Recently, the C7 gene had been identified in fish, including large yellow croakerLarimichthys crocea, miiuy croakerMiichthys miiuy, grass carpCtenopharyngodon idella, and rock breamOplegnathus fasciatus(Shen et al., 2012;Niroshana Wickramaarachchi et al., 2013; Wang et al., 2015; Guo et al., 2016). Due to stimulation by pathogenic bacteria or lipopolysaccharides (LPS),significant increase in C7 mRNA expression was observed in the above fish; this indicated that C7 participated in the innate immune responses of teleost fish to pathogenic infections (Shen et al., 2012;Niroshana Wickramaarachchi et al., 2013; Wang et al., 2015; Guo et al., 2016).
In animals, many studies have shown that shifting feeding time could disturb the synchrony of behavioral and physiological rhythms, which may lead to manifestations of the metabolic syndrome. Mukherji et al. (2015) reported that shifting the feeding time of mice to their rest phase increased the free fatty acid and glucagon levels, and created further metabolic alterations. In addition, altering mealtime affected daily cycles in the metabolic (glucose) and endocrine(cortisol and thyroid hormones) systems, digestive enzymes in gilthead seabreamSparus aurata(Montoya et al., 2010a). Working during the night had similar effects to shifting feeding times, likely due to disruption of the circadian rhythm, which could affect many physiological activities and immune function(Logan and Sarkar, 2012). Clock mutation can also suppress daily rhythms of circulating leukocytes and a greats of immune-related genes (Oishi et al., 2006).Weekly phase-shifts (6 h/week for 4 weeks) resulted in a significant difference of immune responses to high-dose (12.5 mg/kg) lipopolysaccharide (LPS)challenges in mice (Phillips et al., 2015). The disruption of circadian rhythms, from changes in feeding times or daily activities, may result in chronic illnesses, such as metabolic syndrome and cancer(Kalsbeek et al., 2011). However, there was little knowledge about the effects of shifting feeding time on immune molecules such as C7 in fish.
Darkbarbel catfish(Pelteobagrus vachellii) is an important commercial carnivorous aquaculture fish species in China (Li, 2010). Due to its high market value, the demand for this species has grown considerably in recent years, with total production reaching 417 347 tons in 2016 (Fisheries and Fisheries Administration Bureau of the Ministry of Agriculture,2017). Previous studies indicated that juveniles of a similar species,Pelteobagrus fulvidraco, showed nocturnal feeding behavior (Yang et al., 2006). In our earlier study, theCLOCKgene expression in the brain, liver and intestine ofP.vachelliiwas found to be under the control of physiological rhythms, and the acrophases ofCLOCKmRNA expression in the brain,liver and intestine were at Zeitgeber time (ZT), of 21:35, 23:00 and 23:23, respectively (Qin and Shao,2015). So, feeding at night may be better forP.vachellii, due to the synchronization with its feeding rhythms. However, farmedP.vachelliiare generally fed twice per day, during daylight.Therefore, this study aimed to clone the C7 gene and analyze its expression in fish fed either during the day or night.
2 MATERIAL AND METHOD
2.1 Fish and experimental treatments
A total of 500 fish (initial weight 12.32±4.71 g)were obtained from the local fish farm (Meishan,Sichuan, China), and acclimatized at 25±2°C for 20 days in a flow-through system. Then, 360 healthyP.vachelliiwere randomly assigned to triplicate tanks of four treatment groups (30 fish/tank; 300 L/tank)(Fig.1). Two groups were fed once a day in the daytime (08:00; daytime group), and two other groups were fed at night time (20:00; nighttime group), for 60 days with an automatic timer-feeder (EHEIM,model 3581, Germany). The diet was formulated to meet or exceed the known nutrient requirements of darkbarbel catfish; the 3-mm floating pellets (Neijiang Zhengda Inc., Sichuan, China) contained approximately 40% protein, 3.5% fat, 15% ash, 2%lysine and 12.5% moisture. The dissolved oxygen levels in each tank were approximately at saturation,due to continuous aeration. The fish were exposed to natural a light-dark cycle, with approximately 12-h light/12-h dark (from 7:00 to 7:00), and a natural water temperature (25±2°C). The ammonia and nitrate concentrations were measured once a week,and were always <0.05 mg/L.
At the end of the feeding trial, four fish from each tank (twelve fish for each group) were anesthetized with MS-222 at 20:00. They were dissected and separately collect the liver, spleen, head kidney tissues and intestine, which were immediately stored in liquid nitrogen. In addition, for tissues expression analysis, ten tissue samples (brain, muscle, liver, head kidney, skin, spleen, heart, gill, intestine and fins)were separately collected from nine acclimatized healthy fish and immediately stored in liquid nitrogen.

Fig.1 Schematic diagram of the experimental design
2.2 Bacterial challenge assay
To examine the effect of feeding time on thePv-C7expression inP.vachellii, the fish remaining from the feeding trial (Section 2.1) were given an intraperitoneal injection with 100 μL live Gram-negativeAeromonas hydrophila(isolated from sick darkbarbel catfish and identified with 16s Rrna method) in saline water(8.5×109CFU /mL per fish) (Qin et al., 2017). Thus,there were two groups in the bacterial challenge: fish fed at the daytime (08:00), and those fed at night(20:00). The other groups were the negative control groups. There were two groups in the negative control:fish fed at the daytime (08:00), and fish fed at night(20:00). The two negative groups were injected with 100 μL sterile saline water per fish. Details of the four treatments were shown in Fig.1. Injections were carried out under anesthesia using MS-22 at 10 mg/L.Nine fish were randomly sampled at 0, 3, 12, 24, 48 and 96 h post-injection. The liver, head kidney, and spleen tissues were collected from each fish for total RNA isolation and further analysis.
2.3 RNA extraction and cloning of the C7 cDNA
In order to identify thePv-C7genes, C7 partial sequences from theliver transcriptome ofP.vachelliiwere selected for further amplification (unpublished data) using local BLAST software (NCBI, USA).Following the protocol of RNAiso Plus kit (TaKaRa,Dalian, China), total RNA was extracted from the liver ofP.vachellii. The full length C7 cDNA ofP.vachelliiwas gained with the reverse transcription PCR (RT-PCR) and rapid amplification of cDNA ends(RACE) methods. For 3′-RACE and 5′-RACE, the first strand cDNA synthesis was carried out using SMARTScribe Reverse Transcriptase (Clontech,USA) with 3′-RACE CDS Primer and 5′-CDS primer A, respectively.
The SMART RACE cDNA Amplification Kit(Clontech, USA) was used to obtain the full length of thePv-C7gene with the manufacturer’s instructions.For 5′-RACE, the primer set consisted of 5′-RACE GSP1 (5′-TGCTCATCTAAACCATCCTCC-3′) and the universal primer mix (UPM). For 3′-RACE, the primer set consisted of 3′-RACE GSP2(5′-CTCACCCTTGAAGCAGACG-3′) and the UPM. The PCR reaction conditions defined in the Advantage TM 2 PAC kit (Clontech, USA) were carried out.
2.4 Cloning, sequencing and analyses
The purification of the PCR fragments and clone of cDNA fragments were carried out with electrophoresis on 1.5% agarose gel pGEM-T Easy vector, following the manufacturer’s instructions (Promega Corporation,Madison, WI, USA). Recombinant bacteria were selected by blue/white method and identified by PCR.
The insert cDNA fragments in Plasmids were purified with miniprep kit (Promega), and the DNA sequencing was completed by Sangong (Sangon Biotech (Shanghai) Co., Ltd.) used. The Expert Protein Analysis System (http://us.expasy.org/) was use to analyze the C7 deduced amino acid sequence ofP.vachellii. The BLAST (NCBI; http://ncbi.nlm.nih.gov/blast/) was used to perform similarity searches. Multiple alignment of thePv-C7was carried out with Clustal W Multiple Alignment.
2.5 Expression of the Pv- C7 gene in tissues
The expression ofP.vachelliiC7 mRNA in tissues was detected by real-time RT-PCR analysis. Total RNA was also isolated with RNAiso Plus kit (Takara, Dalian,China). PrimeScript RT Reagent Kit with gDNA Eraser(TaKaRa, Dalian, China) was used to synthesize the first strand cDNA. The gene-specific primers QC7-S(5′-AATGAACTGAAGCGGGACA-3′) and QC7-A(5′-GCAGGATTGAGCGGTAAGC-3′) were designed to amplify the 172 bpPv-C7transcript. The primers of β-actin F (5′-CACTGTGCCCATCTACGAG-3′) and β-actin R (5′-CCATCTCCTGCTCGAAGTC-3′) were amplified the 200 bp fragment as a reference gene(Zheng et al., 2010). The PCR profiles were followed the instructions in the SYBR Premix Ex Taq (TaKaRa),and were performed on a Roche Nano Real-Time PCR System (Roche, USA). The 2-ΔΔCtmethod was used to calculate the gene mRNA relative expression levels(Livak and Schmittgen, 2001).
2.6 Statistical analysis
The differences in gene expression within tissues distribution and tissues from fish subject to the bacterial challenge assay were analyzed by one-way analysis of variance (ANOVA) and multiple comparison (Duncan) tests using SPSS software(SPSS v. 18.0). The difference expression ofPv-C7mRNA at the same time point withinA.hydrophilainfection after shifting feeding time experiment were analyzed by independent-samplet-tests (SPSS v. 18.0), andP<0.05 was considered to be statistically significant.
3 RESULT
3.1 C7 cDNA sequence of P. vachellii
A unigene was identified from the transcriptome of theP.vachelliiliver tissue (BioProject ID:PRJNA362523); the fragments showed high identity with the C7 mRNA ofC.idella(JN655169.1),Marsupenaeus japonicus(AB108542.1) andHomarus gammarus(AJ697860.1) in the GenBank database.The C7 cDNA ofP.vachelliiconsistedof 2 647 bp,including a 5′ UTR of 52 bp, an open reading frame(ORF) of 2 454 bp, and a 3′ UTR of 141 bp. A consensus polyadenylation signal (AATAAA)sequence was observed in the 3′ UTR. The ORF encoded a protein consisting of 818 amino acid residues. The nucleotide and the deduced amino acid sequences ofP.vachelliiPv-C7cDNA were submitted to the GenBank (Accession No. KX196317).
Pairwise sequences analysis indicated thatPv-C7had 68%, 55%, and 47% amino acid identity with the C7 proteins ofC.idella(AEQ53931.1),Oplegnathusfasciatus(AFZ93893.1), andSusscrofa(NP_999447.1), respectively. Multiple alignments showed that thePv-C7contained principally functional domains of the other C7 amino acid sequences, including two complement control protein modules (CCP; amino acids 555–609, 613–665), a low density lipoprotein receptor class A domain(LDLa; 83–115), one membrane-attack complex/perforin domain (MACPF; 216–432), two thrombospondin type 1 repeats (TSP1; 26–76, 487–533), and two factor I membrane attack complex(FIMAC; 755–816, 682–742) domains (Fig.2).
3.2 Tissue expression patterns of Pv- C7
The mRNA expression ofPv-C7in different tissues was detected inP.vachelliiusing the q-PCR assay, as shown in Fig.3. The highest expression levels ofPv-C7mRNA were observed in the liver tissue. ThePv-C7mRNA was also detected in spleen, intestine, head kidney, gill and skin ofP.vachellii, but not in other tissues analyzed.
3.3 Effects of feeding time and bacterial challenge on C7 expression
The RT-qPCR analysis showed that altering feeding time from 08:00 to 20:00 significantly increased the expression ofCLOCKmRNA in liver and intestine(P<0.05). It also increased the expression ofCLOCKmRNA in spleen and head kidney, not appearing significant difference (P>0.05) (Fig.4a). Moreover,when shifting feeding to nighttime, thePv-C7mRNA significantly increased in the liver, spleen, intestine and head kidney tissues (P<0.05) (Fig.4b).
In the two bacterial challenge groups,Pv-C7mRNA in liver was significantly up-regulated from 48 to 96 h afterA.hydrophilachallenge (P<0.05)(Fig.5a), and C7 mRNA in head kidney and spleen tissues was found at obviously higher levels from 12-to 96-h post challenge, with highest expression levels at 48 h (P<0.05) (Fig.5b, c). Moreover, with the bacterial challenge,Pv-C7mRNA expression from 48 to 96 hin the liver tissues of fish fed in the daytime appeared lower than that fed in the nighttime,although the differences were not significant(P>0.05) (Fig.5a). However,Pv-C7mRNA expression from 12 to 48 h in head kidney tissues of fish fed in the daytime was significantly lower than that fed in the nighttime (P<0.05) (Fig.5b), and the same result appeared in spleen tissues from 12 to 24 h (P<0.05) (Fig.5c).
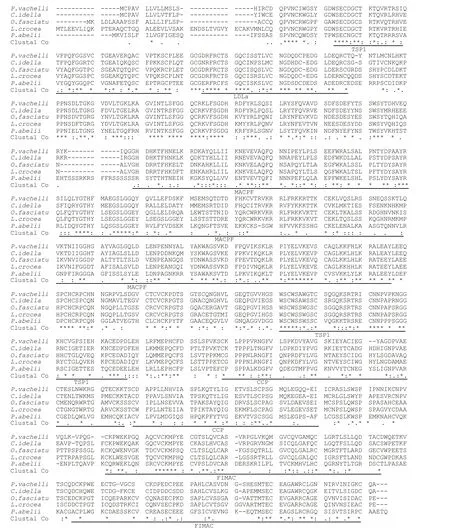
Fig.2 Multiple alignments of the C7 protein

Fig.3 Tissue expression profiles of Pv- C7 mRNA in Pelteobagrus vachellii
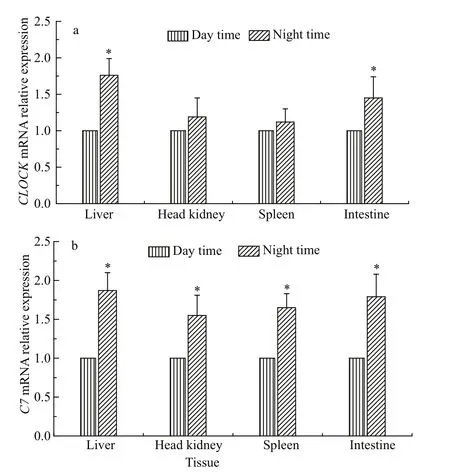
Fig.4 Expression profiles of CLOCK (a) and Pv- C7 (b)mRNA in Pelteobagrus vachellii fed either only at day time or at night time
4 DISCUSSION
The immune system is under direct circadian control by systemic cues and molecular clocks within immune cells (Arjona and Sarkar, 2006; Logan and Sarkar, 2012; Phillips et al., 2015). A number of studies have indicated that circadian misalignment,which resulted from environmental desynchronization,shifting work or shifting feed, led to metabolic syndrome and diseases (Knutsson, 2003; Gao et al.,2009; Logan and Sarkar, 2012). Although complement components have been considered to play an essential role in immune system, and the investigation of complement components in fish has highlighted in molecular structure and expression with infection(Guo et al., 2016; Wang et al., 2015; Shen et al.,2012), the knowledge of their molecular expression was very limited especially in fish with an alternative feeding time. Therefore, in this study, the full-length sequence of C7 was identified inP.vachellii, and C7 mRNA expressions were firstly analyzed subject to bacterial challenge with an alternative feeding time.
Similar to human C7 andC.idellaC7 (González et al., 2003; Shen et al., 2012), the SMART domain analysis showed that thePv-C7mature protein mainly consisted of eight domains (Fig.2). Following the multiple alignment, these structural motifs were also observed in all the detected C7 homologues ofC.idella,M.miiuy,L.crocea, andO.Fasciatus,including the C7 deduced amino acid sequences(Shen et al., 2012; Niroshana Wickramaarachchi et al., 2013; Wang et al., 2015; Guo et al., 2016). The others motifs ofPv-C7showed extensive identity to the corresponding region in the other C7s. The C7 molecule indicated high conservation and integral function across species in the assembly of the MAC on the surface of complement-opsonized cells (Bossi et al., 2009).

Fig.5 Expression profiles of Pv- C7 mRNA in liver (a), head kidney (b) and spleen (c) tissue of Pelteobagrus vachellii fed either only at daytime or at nighttime, after challenge with A. hydrophila
In accordance with results obtained fromC.idellaandO.fasciatus, the highest expression level of C7 mRNA was found in the liver tissue (Fig.3). Previous reports found similarly high C7 expression levels in liver tissues ofC.idella,M.miiuyand mammals(González et al., 2003). From these studies, it seemed almost certain that the liver was the main production site of C7 (Witzel-Schlomp et al., 2001). The results suggested thatPv-C7may act similarly to the other complement components that were generally of hepatic origin. ThePv-C7mRNA was also detected in the spleen, intestine, head kidney, skin and gill ofP.vachellii, but not in heart, brain and fin tissues.Similarly, C7 expression was found in all detected tissues except the heart inL.crocea, and it was ubiquitously expressed in all ten tested tissues inM.miiuyandO.fasciatus, although the levels of expression among the tissues were variable (Niroshana Wickramaarachchi et al., 2013; Wang et al., 2015;Guo et al., 2016). There was no C7 expression in the blood, brain, muscle, gill or fin tissues ofC.idella(Shen et al., 2012). The different expression pattern in tissue resulted from specificity in different species,which need further investigation.
Del Pozo et al. (2012) reported that feeding rhythm(diurnal vs. nocturnal) strongly influencedCLOCKgene mRNA expression in the liver (feeding-entrained clock) of European seabassDicentrarchus labrax(Del Pozo et al., 2012). Similarly, with a shift of feeding time to night, theCLOCKmRNA showed significantly increase in the liver and intestine ofP.vachellii(P<0.05). This increase also appeared in spleen and head kidney, without significant difference(P>0.05) (Fig.4a). This result indicated an altering feeding time could impact clock rhythms of peripheral tissues inP.vachellii. In addition, theCLOCKgenes may also modulate the response of innate and adaptive immune systems (Logan and Sarkar, 2012), by regulating the level of cytokines and other factors,through direct control of the NF-κB signaling pathways (Scheidereit, 2006; Bozek et al., 2009).Interestingly, the putative transcription factor binding sites for NF-κB were found in the promoters of other TCC genes inC.idella(C9 and C7) (Li et al., 2007;Niroshana Wickramaarachchi et al., 2013), which suggested that theCLOCKgene could regulate TCC genes by NF-κB, including C7. This study proved that the significant increase of the expression of C7 mRNA appeared in the liver, spleen, intestine and head kidney tissues when altering feeding time from 08:00 to 20:00 (Fig.4b). This result indicated that feeding time did affect thePv-C7mRNA expression.Similarly, the serum alkaline phosphatase (ALP),lysozyme (LYZ), peroxidase (PER) and protease(PRO) exhibited significant rhythmicity under 12L/12D cycles in Nile tilapia (Oreochromis niloticus)(Lazado et al., 2016). Additionally, in serum of shifted mice with treatment of four consecutive weekly 6-h phase advances of the light/dark schedule, several key immune factors were up-regulated, including IL-6,macrophage inflammatory protein- 2 (MIP-2), and leukemia inhibitory factor (LIF) (Castanon-Cervantes et al., 2010). Although the exact interactions and functions were still unknown, Arjona and Sakar(2006) hypothesized that, to promote the efficiency of the immune response, the coordination of immune factors was under the direct regulation of the molecular clock (Arjona and Sarkar, 2006). These studies indicated that altering the feeding time firstly altered the circadian expression ofCLOCKin peripheral tissues, and then altered the circadian rhythm of the C7 expression, which was regulated by theCLOCKgene; but this hypothesis needed further investigation.
After challenging withA.hydrophila,an upregulation of mRNA was observed in the liver, spleen and head kidney tissue, inPv-C7comparison to the control group (Fig.5). Similarly, Wang et al. (2015)indicated that expression levels of C7-1 in miiuy croaker were higher after exposing toVibrio anguillarumand LPS (Wang et al., 2015). Significant changes in C7 transcript expression (>20-fold) were observed followingA.hydrophilainfection inC.idella(Shen et al., 2012). Rapid and drastic responses toVibrio alginolyticuschallenges were observed for C7 inL.crocea(Guo et al., 2016).Moreover, perturbations of circadian rhythms by either external stressors, or internal stressors (e.g.,psychological perturbations), may negatively affect health by harming immune function (Logan and Sarkar, 2012). For example, interleukin-17 (IL-17) in plasma was significantly increased than that of controls (Phillips et al., 2015). In serum of shifted mice with treatment of four consecutive weekly 6-h phase advances of the light/dark schedule, IL-6,leukemia inhibitory factor (LIF) showed upregulation, and the circadian desynchronization resulted in uncoordinated inflammatory responses to LPS challenge, which led to increased mortality(Castanon-Cervantes et al., 2010). In present study,afterA.hydrophilachallenge,Pv-C7mRNA expression of fish fed at daytime was lower than that at nighttime in spleen and head kidney tissues from 12 to 48 h (P<0.05) (Fig.5b, c), and in liver from 48 to 96 h (P>0.05) (Fig.5a). These differences of C7 mRNA expression in spleen and head kidney may result from the perturbations of clock rhythms of immune cell by shifting feeding time. The light-dark and feeding cycles are the most important factors that entrain biological rhythms in fish species (Montoya et al., 2010b). Feeding time could coordinate the biological rhythms in peripheral tissues, such as in the liver (del Pozo et al., 2012). Rhythms cues were transmitted to immune tissues by neural and endocrine signals, and then mediated immune responses (Logan and Sarkar, 2012). Therefore, if feeding time synchronized with daily rhythms of behavior and physiology in animals, it may increase the ability to resist pathogens. However, this hypothesis needs deep investigation. Similarly, Liu et al. (2006) found period 2 mutant mice can be relatively resistant to LPS-induced endotoxic shock, in part due to decrease in the levels of the proinflammatory cytokines, IFN-γ and IL-1β. However, at 96 h, there was no significant difference in C7 mRNA expression among the liver,spleen and head kidney tissues of the fish in this study(P>0.05) (Fig.5); maybe the circadian rhythm of the fish was mainly controlled by the light-entrained clock in the peripheral tissues, not the feedingentrained clock (Del Pozo et al., 2012), and light signal promoted phase coherence of peripheral clocks in the immune system, and also governed daily variations in immune function (Logan and Sarkar,2012).
In conclusion,Pv-C7was successfully cloned inP.vachellii.Pv-C7gene expression might be upregulated with feeding at nighttime in four tissues.Pv-C7mRNA expression of the fish fed at nighttime showed significantly higher than that at daytime in head kidney and in spleen withAeromonas hydrophilainfection. This study contributed to give proper consideration for further accurate feeding schedules in this species.
 Journal of Oceanology and Limnology2018年6期
Journal of Oceanology and Limnology2018年6期
- Journal of Oceanology and Limnology的其它文章
- Neuroanatomy and morphological diversity of brain cells from adult crayfish Cherax quadricarinatus*
- Cryopreservation of strip spawned sperm using programmable freezing technique in the blue mussel Mytilus galloprovincialis*
- Pf- D mrt4, a potential factor in sexual development in the pearl oyster Pinctada f ucata*
- Specific genetic variation in two non-motile substrains of the model cyanobacterium Synechocystis sp. PCC 6803*
- Functional characterization of a Δ6 fatty acid desaturase gene and its 5′-upstream region cloned from the arachidonic acidrich microalga Myrmecia incisa Reisigl (Chlorophyta)*
- The expression characteristics of vitellogenin (VTG)in response to B(a)p exposure in polychaete Perinereis aibuhitensis*
