Adsorption and migration of Li-ion in layered SnSe2:a first principle study*
FANG Lincan, HAO Kuanrong, YAN Qingbo†, ZHENG Qingrong
(1 College of Materials Science and Opto-Electronic Technology, University of Chinese Academy of Sciences, Beijing 100049, China;2 School of Physics, University of Chinese Academy of Sciences, Beijing 100049, China)
Abstract The properties of Li-ion adsorption and migration in layered SnSe2 are systematically investigated using the first principle calculations. It is found that the Li atoms are adsorbed strongly on substrate SnSe2, and the binding energy (>3 eV) is significantly higher than those on graphene, phosphorene, MoS2, and some other two-dimensional (2D) layered materials. Bader charge analysis reveals that almost the whole charge of 2s electron of the Li atom transfers to substrate SnSe2 and Li exists in the cationic state. The Li-ion migration energy barrier for monolayer SnSe2 is 0.197 eV, which is significantly lower than those for graphene, MoS2, and other 2D materials. The average open-circuit voltage of 3.05 V is predicted in the monolayer SnSe2-based Li-ion battery. The Li intercalation also leads to a transition from semiconductor to metallic state and gives rise to a good electrical conductivity. These findings provide insights into the Li-ion adsorption properties and migration mechanism in layered transition-metal dichalcogenide.
Keywords SnSe2; Li-ion adsorption; Li-ion migration; first principle calculation
The development of rechargeable batteries, particularly rechargeable lithium-ion battery (LIB), greatly promotes the application of mobile electronic devices and environmentally friendly vehicles[1-5], which is subverting the human’s life style and the way of energy application, initiating a new chapter of the new energy area. In order to further improve the performance of LIB, it is urgent to develop advanced electrode materials that provide rapid charging/ discharging rate, cyclic stability, satisfactory capacity, and safety[6-12].
In the past several decades, graphite has been used as traditional anode material, but its energy capacity (372 mAh·g-1) and rate of Li are still far away from what are expected to meet the requirements of many applications[13-14]. Recent years, more and more attentions were focused on two-dimensional (2D) layered materials with the characteristics of high specific surface area,large interlayer spacing, and other peculiar properties[15-17]. For example, the well-studied graphene exhibits the unique capacity (774 mAh·g-1) because of its high charge carrier mobility, large surface area, and a broad electrochemical window[18-22]. Up to date, graphene has been utilized as both cathode and anode materials with great success[23-27]. MoS2has been explored to exhibit a high reversible lithium storage capacity and superior rate capability[28-33]. Black phosphorus was shown to have a series of good characteristics as Li-battery electrode[34-36]. With the decreasing of dimensionality, the 2D phosphorene has been validated as ideal anode material with a low energy barrier (0.08 eV) of Li and higher average charging voltage (2.9 V) than other 2D materials[37-39]. In addition, the group IV-VI compounds MX (M=Ge, Sn; X=S or Se), which display puckered structures similar to black phosphorus and form buckled honeycomb lattices, has received much attention as electrode materials of LIB[40-44]. Based on the interlayer spacing from 3.1 Å to 3.26 Å, their structural anisotropy directly leads to anisotropy of the migration direction of lithium ion. Sn-based materials, like SnS2and SnO2composites, provide ideal space for Li atom intercalation and exhibit high reversible capability and good cycling performances when they are used as anode materials[45-48]. However, as far as we know, the properties and performance of SnSe2as electrode material have not been studied, while SnSe2presents prominent characteristic of large interlayer spacing up to 4.175 Å and hence provides more possibility for the adsorption and diffusion of the Li ions. In this work, the Li-ion adsorption properties and migration mechanism in monolayer/bilayer/bulk SnSe2materials have been systematically investigated.
Our study shows that the binding energy is significantly higher than that on graphene, phosphorene, MoS2, and some other two-dimensional (2D) layered materials, indicating a strong interaction between the Li atom and SnSe2substrate. Bader charge analysis reveals that Li exists in cationic state with its 2s electron being completely transferred to SnSe2. The extremely low energy barrier (0.197 eV) on the monolayer SnSe2guarantees rapid diffusion of the Li atom. Due to the unique anionic framework structure of bulk SnSe2and formation of the Oh-Th-Ohmigration channel for the Li ion, the energy barrier arises to 0.483 eV. Moreover, a remarkably large average voltage of 3.05 V is predicted, which is even lerger than on phosphorene (2.9 V). The intercalation of Li leads to a transition from semiconductor to metallic state, which gives rise to a good electrical conductivity. These findings provide insights into the Li-ion adsorption properties and migration mechanism in layered transition-metal dichalcogenides (TMDS).
1 Computational methods
All the calculations were performed using Vienna ab initio simulation package (VASP) based on density functional theory[49]. The interactions between ion cores and valence electrons were described using the projector augmented wave (PAW) potentials[50]. The Perdew-Burke-Ernzerhof (PBE) generalized gradient approximation (GGA) was carried out for the electron exchange-correlation interactions[51-52]. The cutoff of plane-wave kinetic energy was set to be 550 eV for all the calculations. Atomic relaxation was performed with the convergence of total energy less than 10-5eV and all the forces on each atom smaller than 0.0 1 eV/Å. The climbing image nudged elastic band method (CI-NEB) was used to search the minimum energy path (MEP) of Li migration[53]. For the Li adsorption and diffusion studies, we selected supercell containing 4×4 primitive cells for eliminating the interaction between Li atoms. For the monolayer/bilayer SnSe2, we set a vacuum space of 20 Å between adjacent layers to avoid mirror interaction. K-point meshes of 5×5×1 for monolayer/bilayer SnSe2and 5×5×5 for bulk SnSe2were employed in all calculations, and denser K-point meshes of 12×12×1 and 12×12×10 were adopted for monolayer and bulk band structures. Bader charge analysis[54]method was used to estimate the charge transfer between Li and substrate SnSe2. In addition, van der Waals correction was taken into account using the D2 method of Grimme[55]in the bilayer and bulk calculations.
2 Results and discussion
2.1 Adsorption of Li on monolayer SnSe2 surface and in bilayer/bulk SnSe2
Bulk SnSe2possesses the typical layered TMDS structure, in which per SnSe2layer has the sandwich-like structure with the Sn layer sandwiched between two Se layers, as shown in Fig. 1.
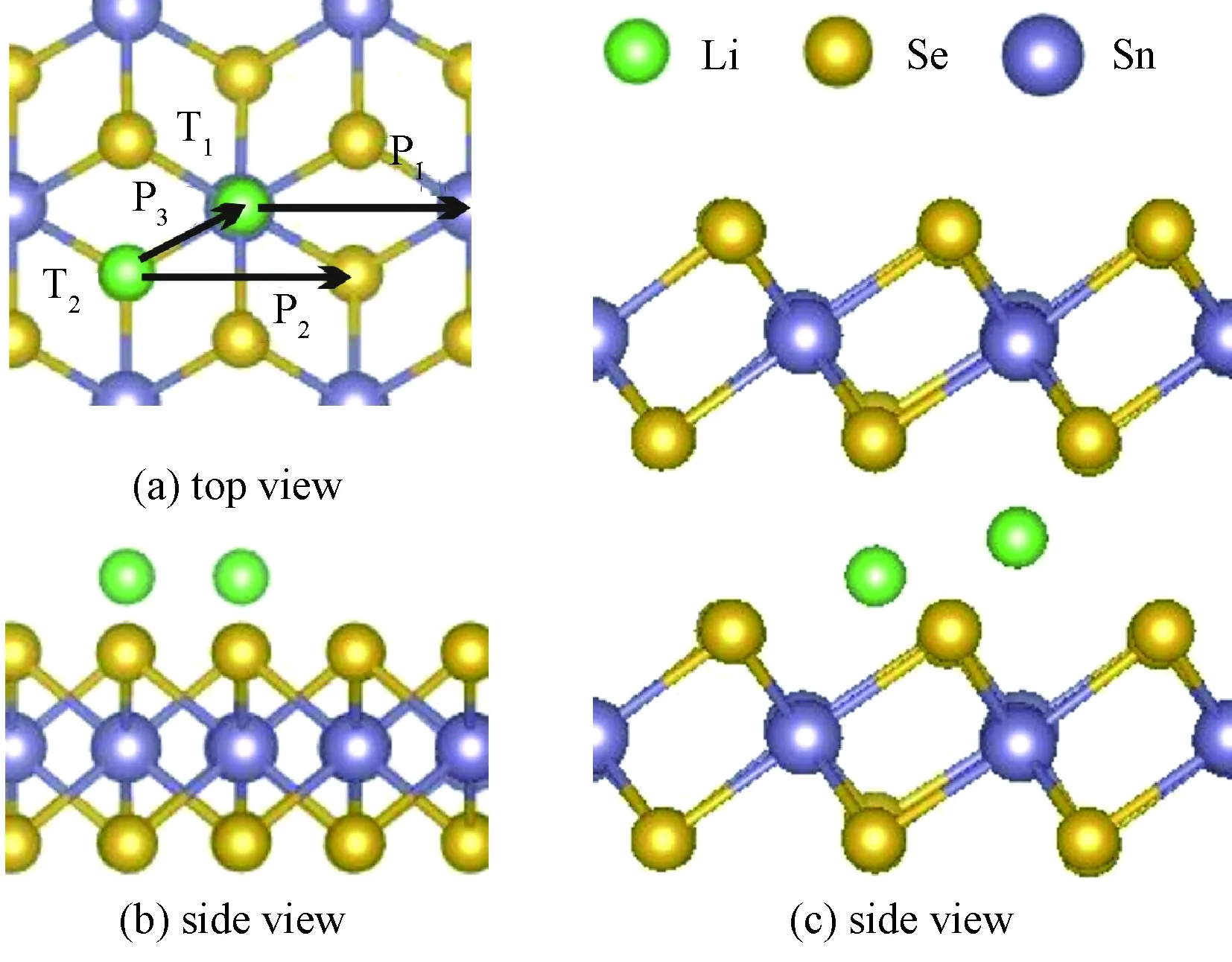
Fig.1 Top (a) and side (b) views for Li adsorbed on monolayer, and Li in bulk (c)
We investigated the adsorption of one Li atom on the surface of monolayer SnSe2and in the bilayer/bulk SnSe2. In Fig.1(a) and 1(b) shown are the two possible adsorption sites T1and T2on SnSe2monolayer. The T1site is directly above the Sn atom that is located in the second atomic layer and the T2site is directly above the Se atom that is located in the third atomic layer. As for bilayer/bulk SnSe2, there are also two possible adsorption sites: the tetrahedral (Th) site formed by three Se atoms from lower triple layers and one Se atom from upper layer and the octahedral (Oh) site at which Li binds three Se atoms from the triple layers as shown in Fig. 1(c). The binding energy (Eb) was defined
Eb=ELi-SnSe2-ESnSe2-ELi,
(1)
whereELi-SnSe2,ESnSe2, andELiare the total energies of Li-adsorbed SnSe2, SnSe2, and a Li atom, respectively. A negative value ofEbsuggests an exothermic reaction, and a more absolute value ofEbindicates a stronger interaction between Li and SnSe2. The values ofEbfor Li are -3.01 eV (T1) and -2.98 eV (T2) on monolayer SnSe2, -3.52 eV (Th) and -3.56 eV (Oh) in bilayer SnSe2, and -3.70 eV (Th) and -3.91 eV (Oh) in bulk SnSe2, which are significantly higher than those on graphene, phosphorene, MoS2, and some other two-dimensional (2D) layered materials.
The spatial distributions of the charge difference between Li and SnSe2for monolayer/bulk SnSe2are illustrated in Fig. 2. The large charge deciency at Li and the charge excess around nearby the Se atom indicate strong electron transfer from Li to SnSe2. Bader charge analysis reveals that Li transferred almost the whole charge of 2s electron to SnSe2(see Table 1) and thus exists in the cationic state, which gives an explanation for the strong interaction between Li and SnSe2.
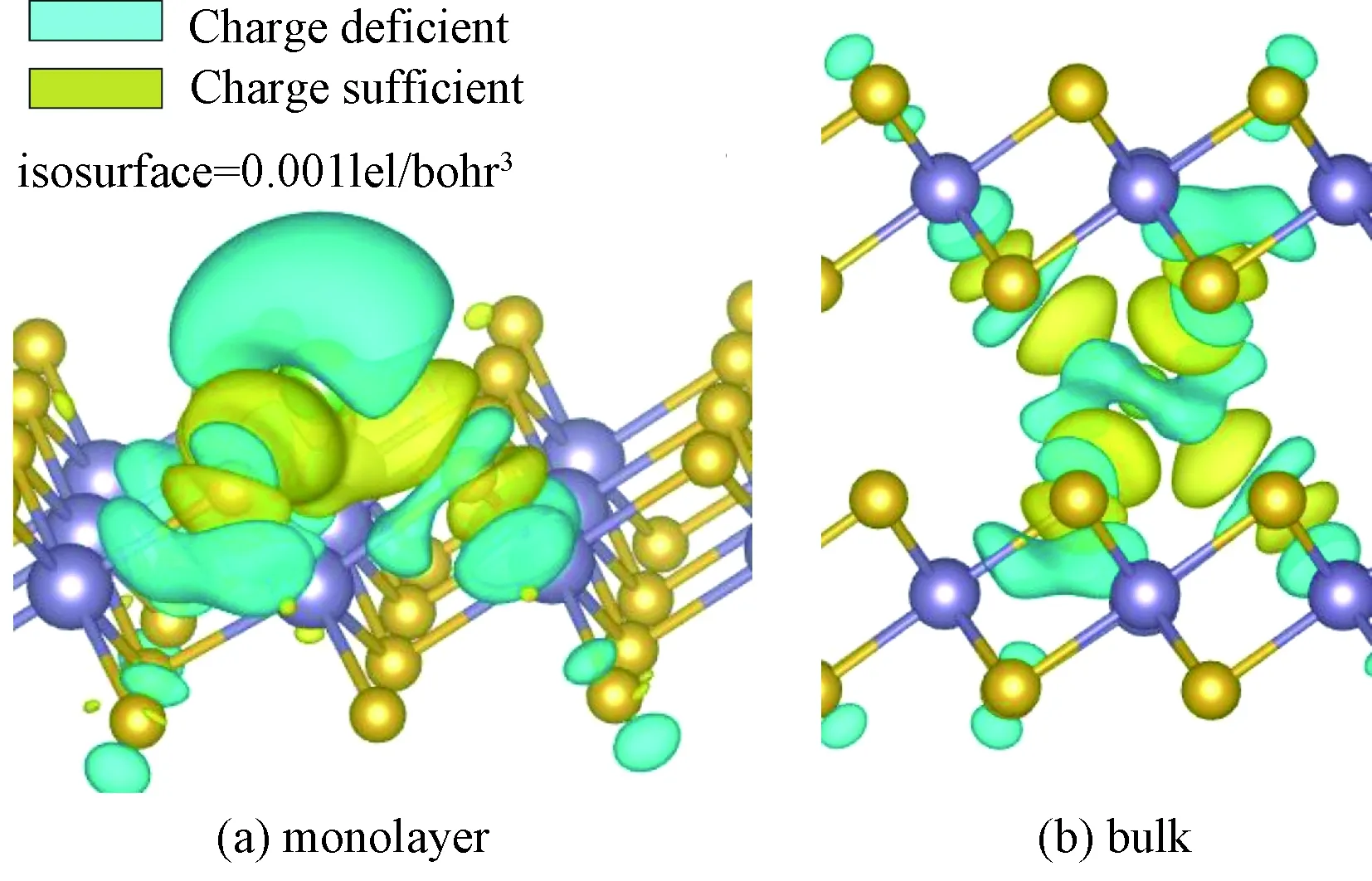
Fig.2 Charge density difference between Li and substrate SnSe2

Table 1 Transferred charges of Li at T1 (q1) and T2 (q2) sites on monolayer and at Th (q1) and Oh (q2) sites in bulk SnSe2
2.2 Diffusion processes of the Li-ion on monolayer and bulk SnSe2
The performances on charging/discharging and circuit rate in Li ion battery are highly dependent on the diffusivity of Li atom in the electrode material.
As shown in Fig. 1(a), three possible diffusion pathways (P1: Sn-Sn; P2: Se-Se; P3: Se-Sn) are considered to explore the optimistic migration path on monolayer SnSe2surface. As shown in Fig. 3(a), the Li ion migrates with a remarkably low barrier of only 0.197 eV along path P1from one T1site to another T1site, passing through a T2site (Fig.3(c) and (d)). Compared with the other 2D materials, such as MoS2, VS2, and graphene, the Li diffusion is essentially isotropic in the plane, and the migration barriers are 0.25 eV on MoS2[28], 0.22 eV on VS2[56], and 0.327 eV on graphene[23], showing that the monolayer SnSe2is more favorite for Li ion migration. For bulk SnSe2, it is found that Li ions prefer the Oh-Th-Ohmigration path[57], which connects two neighboring Ohsites by passing through a face-sharing Thsite, as shown in Fig. 3(b), 3(e), and 3(f). The corresponding energy barrier arises to 0.483 eV, implying that the diffusion of the Li ions on surface of SnSe2are much faster than in bulk SnSe2.

Fig.3 Energy profiles and schematic representations for Li diffusion in monolayer (a,c,d) and bulk SnSe (b,e,f)
2.3 Average open circuit voltage for monolayer/bulk SnSe2-based LIB
The open-circuit-voltage (OCV) is widely used to characterize the charging/discharging performance of Li ion battery. Thus, to further understand the performance of SnSe2-based Li ion battery, the open-circuit-voltage has been derived. In theory, the open circuit voltage curve can be obtained by calculating the average voltages over different Li concentrations. The charging/discharging process of SnSe2-based anode can be assumed as
Lix1A+(x2-x1)Li++(x2-x1)e-↔Lix2A.
(2)
Therefore, the average voltage of LixSnSe2in thex1≤x≤x2range can be evaluated using equation
(3)
whereELix1SnSe2,ELix2SnSe2, andELiare the energies of Lix1SnSe2, Lix2SnSe2, and metallic Li, respectively. In this work, a series of LixSnSe2(x=0.04, 0.083, 0.125, 0.25, 0.5) are considered by adopting the 1×1, 2×2, 2×4, 3×4, and 5×5 supercells with one Li atom absorbed on monolayer SnSe2surface. The voltage profile and geometrical configuration are shown in Fig. 4, and there is a slight drop from 3.2 V to 2.84 V. The calculated average voltage by averaging numerically the voltage profile is 3.05 V in the 0≤x≤ 0.25 range, which is obviously higher than the values of 2.9 V for phosphorene[38], 1.5 V for graphite and TiO2[25], and 0.93 V for VS2[56], indicating that the monolayer SnSe2provides a higher charging voltage. Furthermore, for the intercalation of Li in bulk-SnSe2, a series of Li concentrations were considered by using 1×1, 2×2, 2×4, 3×4, and 5×5 supercells with one Li atom intercalated in bulk-SnSe2. The obtained average OCV is about 3.7 V, which is even higher than the value for monolayer SnSe2. Besides, the OCV does not drop a lot when the Li concentration increases from 0.04 to 0.25.
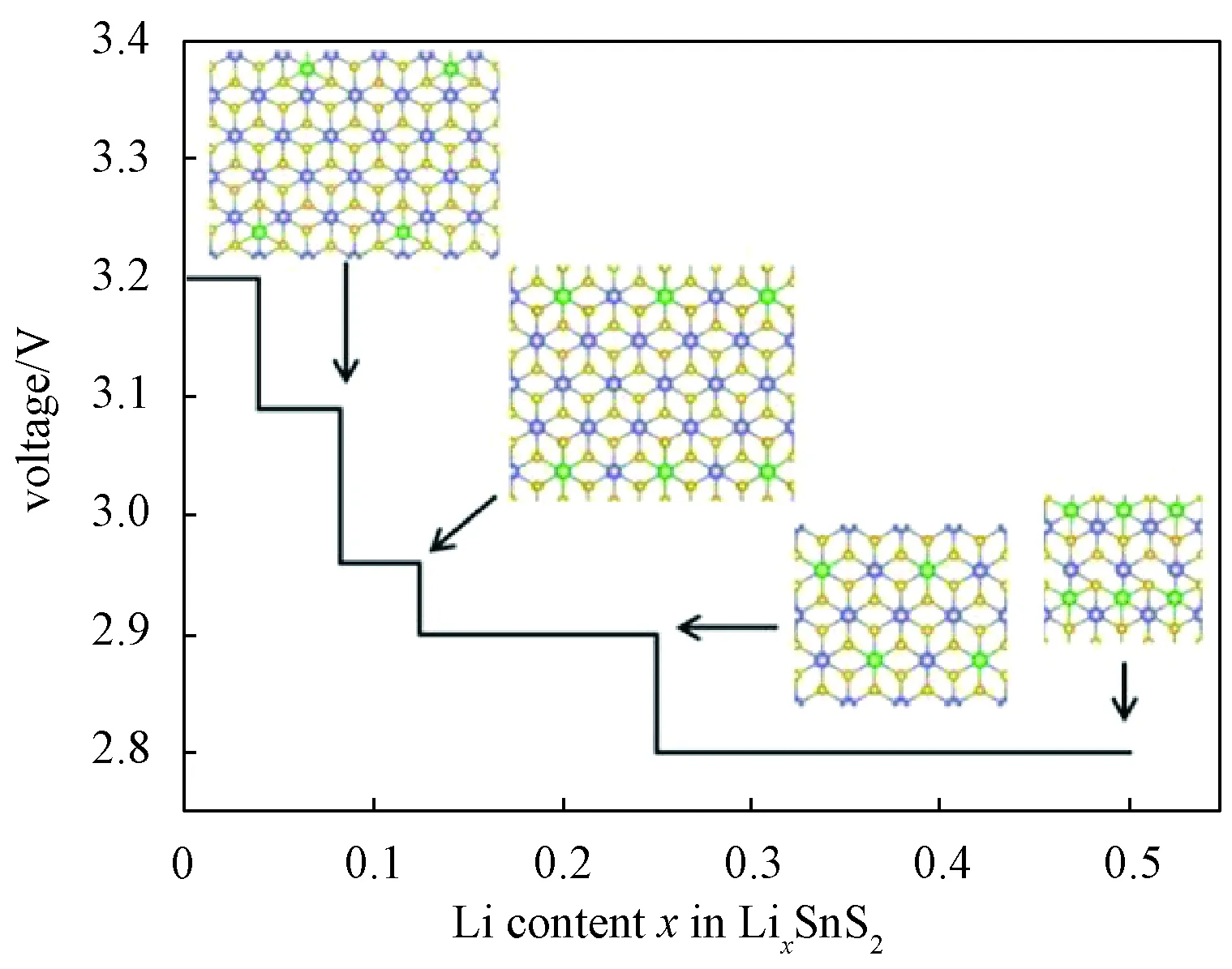
Fig.4 Calculated voltage profile with respect to Li content from 0 to 0.5
2.4 The electronic properties of Li-intercalated monolayer/bulk SnSe2
As we know, the Li ion battery has been one of promising battery systems, used to power a great deal of devices in many applications, such as low-current cells applied in portable electronics and memory backup and high-current cells used in military applications. Thus, it is essential for us to investigate the electronic properties of electrode materials.
As revealed by Bader charge analysis, the Li atoms transfer almost the whole charge of 2selectron to substrate SnSe2, which affects the electronic structure of the whole system distinctly, such as shift of Fermi energy level compared to the original band structures. The electronic band structures of SnSe2with different Li concentrations have been calculated, and the results are shown in Fig.5. Obviously, Fermi energy levels shift up with increasing of Li concentration, and the whole system transits from original semiconducting state to metallic state during the process of Li intercalation, giving rise to a good electrical conductivity.
3 Conclusion
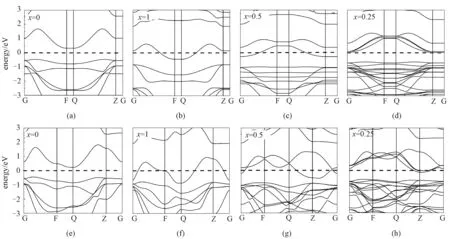
Dashed lines denote the Fermi level.Fig.5 Electronic band structures of the LixSnSe2 monolayer (a,b,c,d) and bulk (e,f,g,h)
In conclusion, based on first-principle calculations, we performed a systematically study on the adsorption properties and migration mechanism of the Li atoms in monolayer/bilayer/bulk SnSe2systems. Our study shows that the Li atom is able to form stable adsorption with SnSe2with a higher binding energy than with some common 2D layered materials. Upon adsorption, the Li atom transfers its 2s electron to substrate SnSe2and exists in the cationic state. The adsorption complex becomes metallic with Li interaction, giving rise to a good electrical conductivity, which is essential for an electrode. The energy barrier is only 0.197 eV for Li in monolayer SnSe2, significantly lower than those on other 2D materials, such as MoS2, graphene, SnS2, VS2, etc. Due to the unique anionic framework of bulk SnSe2, the Oh-Th-Ohmigration channel turns out to be preferred. Furthermore, the average voltage for monolayer SnSe2has been estimated to be 3.05 V, which is obviously suitable for high charging voltage applications. On the basis of our present findings, monolayer SnSe2is expected to be one of candidates for electrode materials. These findings provide insights into the Li-ion adsorption properties and migration mechanism in layered TMDS.
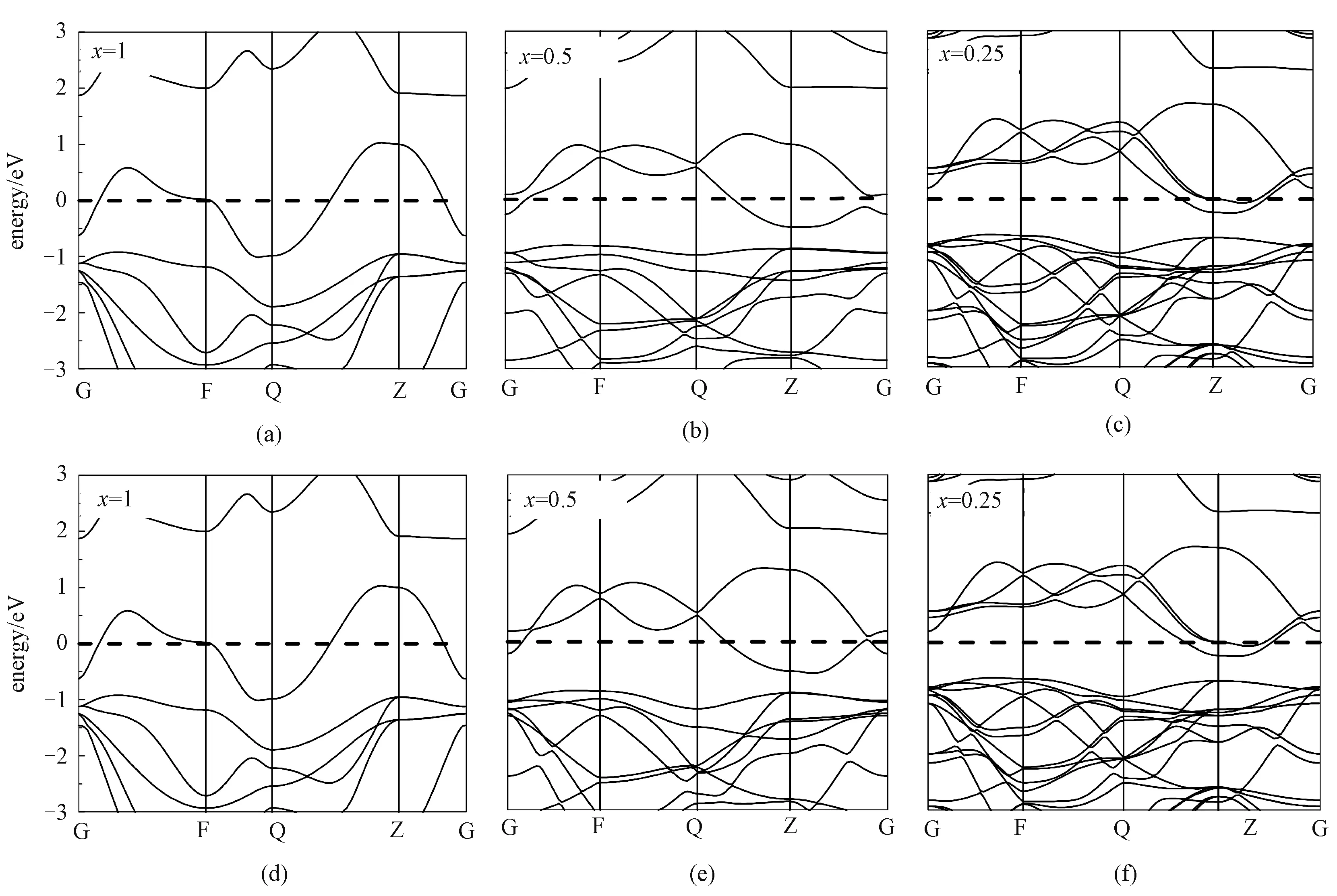
The dashed lines denote the Fermi levels.
Fig.S1 Electronic band structures of the bulk LixSnSe2without vdW corrections (a,b,c) and with vdW corrections (d,e,f)

