Endohedrally Helium-doped CL-20: A DFT Treatment
Lemi Türker
(Department of Chemistry Üniversiteler,Middle East Technical University, Ankara Turkey 062310)
Abstract:In order to investigate whether endohedral He-doping is possible or not in CL-20, a density functional treatment has been carried out at the levels of B3LYP/6-31++G(d,p) and B3PW91/6-31++G(d,p). Some physicochemical and quantum chemical properties of the helium-doped CL-20 (He@CL-20) are compared with the respective values of the parent explosive CL-20. The helium doping caused swelling of CL-20 cage but no bond rupture occurred. Doped helium acquired some positive charge.
Keywords:CL-20;explosives; Helium;Endohedral doping; density functional
Introduction
2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtizane, commonly called CL-20 or HNIW, was synthesized in 1987 by Nelsen[1-2]. CL-20 is a novel, high-density (2.04g/cm3) cyclic nitramine which can be used as an energetic component in explosive formulations and propellants. Its standard molar enthalpy of formation is of the order of about 419 kJ/mol[2-4]. It has several polymorphs[3]. The epsilon-form is the one having the highest density and greatest thermal stability among its polymorphs[4-5]. Its gas phase dissociation yields high concentration of NO2and few ring fragments with nitro groups on them. The ring fragments of [CxHyNz]+were detected[6-7]. Thermally induced damage in CL-20 was reported by Tian et al.,[8]and also by Zeman and his coworkers[9]. Its shock sensitivity in a polyisobutylene matrix was investigated[10].
Although helium has diverse utility in science and technology, chemically it is the most unreactive element, so it was commonly believed that helium compounds do not exist at all[11]. However, Zhang et al., managed to prepare NgBeCO3type compounds (where Ng: Ar, Kr, Xe) in a low-temperature neon matrix and made calculations for NgBeCO3and NgBeO (Ng = He, Ne, Ar, Kr, Xe) using ab initio methods and density functional theory which show that the Ng-BeCO3bonds are slightly longer and weaker than the Ng-BeO bonds[12]. Helium′s first ionization energy of 24.57eV is the highest of any element[13]. However, helium can be doped into fullerenes by heating fullerenes in several atmospheres of helium[14]. The effects of interactions between He-and clusters of fullerenes in helium-nanodroplets are described[15]. Helium plasma ionization mass spectrometry has been developed to detect nitro explosives[16].
Usually, inert gases, such as helium are used as the displacement medium in density calculations such as determination of densities of explosives. The gas displacement methods are much more accurate and reproducible than the traditional Archimedes water displacement method[17]. The helium pycnometer measures the true volume and density of solid powders and is widely employed for explosives[18].
On the other hand, endohedral doping is quite common in fullerenes/nanotubes[19-23]. Endohedral fullerenes represent a novel family of carbon nanostructures, which are characterized by a robust fullerene cage with atoms, ions, or clusters trapped in its interior. Not only their synthesis, but also their theoretical aspects have been on the focus of attention[24-26]. Although there exist some explosive compositions including metals like Al, Mg etc., however, doping in caged-explosives is yet unknown (up to the best knowledge of the author).
In the present study, endohedrally helium-doped CL-20 molecule is considered within the constraints of density functional theory (DFT).
1 Method of calculation
Geometry optimizations of all the structures leading to energy minima were initially achieved by using MM2 method followed by semi-empirical PM3 self-consistent fields molecular orbital (SCF MO) method[27-28]at the restricted level[29-30]. Subsequent optimizations were achieved at Hartree-Fock level using various basis sets hierarchically. Then, geometry optimizations were managed within the framework of density functional theory[31-32], finally at the levels of either RB3LYP /6-311++G(d,p)[29]or RB3PW91 /6-311++G(d,p). The exchange term of B3LYP consists of hybrid Hartree-Fock and local spin density (LSD) exchange functions with Becke′s gradient correlation to LSD exchange[32-33]. Note that the correlation term of B3LYP consists of the Vosko, Wilk, Nusair (VWN3) local correlation functional[34]and Lee, Yang, Parr (LYP) correlation correction functional[35]. The vibrational analyses were also done. The total electronic energies are corrected for the zero point vibrational energy (ZPE). The stationary points to energy minima were proved in all the cases by calculation of the second derivatives of energy with respect to the atom coordinates. The normal mode analysis for each structure yielded no imaginary frequencies for the 3N-6 vibrational degrees of freedom, where N is the number of atoms in the system. This indicates that the structure of each molecule corresponds to at least a local minimum on the potential energy surface. All these calculations were done by using the Spartan 06 package program[36].
2 Results and discussion
One of the caged energetic materials of nitramine type is CL-20 (see Fig.1). It possesses six nitramine groups. In its structure, a small cavity is embraced by symmetrically oriented two pentagonal and two heptagonal rings. Possibly a small atom, like hydrogen or helium could be inserted into it. Such kind of doped-atoms are common in the family of fullerenes and nanotubes[19-26].

Fig.1 Optimized structures of CL-20 and He@CL-20 from different angle of views
2.1 Structures
Fig.1 shows the optimized structures of endohedrally helium-doped CL-20 (He@CL-20) and the parent compound CL-20 from different angles of view. As seen from the figure, within the constraints of density functional theory at the levels of B3LYP/6-31++G(d,p) and B3PW91/6-31++G(d,p), CL-20 holds endohedrally doped helium atom without cleavage of any bonds. However, the direction of dipole moment vector dramatically differs in the doped structure (Fig. 1). Nevertheless the magnitudes of them are comparable (Table 1). The other properties in the table slightly increases in favor of the doped structure. Note that the same basis set with different functionals have been used for the data presented in Table 1.
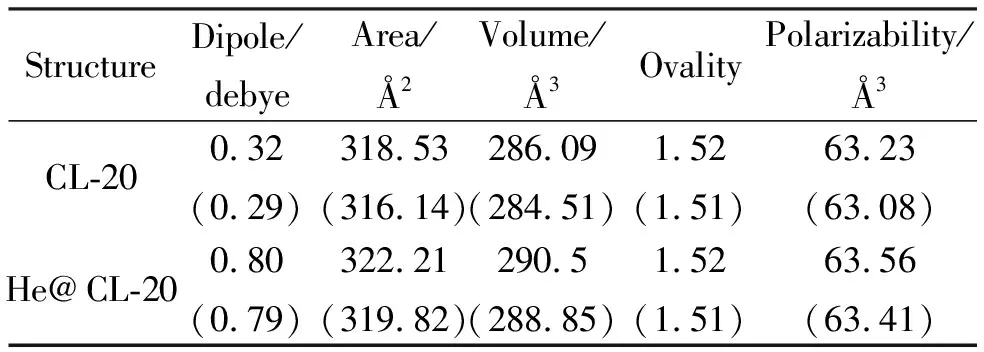
Table 1 Some properties of the structures considered
Note: B3LYP/6-31++G(d,p) and B3PW91/ 6-31++G(d,p) (in parenthesis) level of calculations. All belong to C1 point group. Both have 16 conformers.
Fig.2 shows the common numbering of atoms in both of the structures and some distances/bond lengths in comparison with each other. The data reveal that helium doping in CL-20 causes extension of some bonds or distances, totally resulting in somewhat swelling of the doped structure. Although, overall volume increase seems to be small (Table 1), the interior cavity should increase albeit the fact that no bond cleavage occurs.

Fig.2 The numbering of CL-20 and helium-doped CL-20 and some distances (B3LYP/6-31++G(d,p)). Values in parenthesis stand for CL-20
Fig.3 shows the bond lengths in the structures considered. The doping elongates not only C1-C2 bond of the roof but also some bonds of the pentagonal rings elongate.
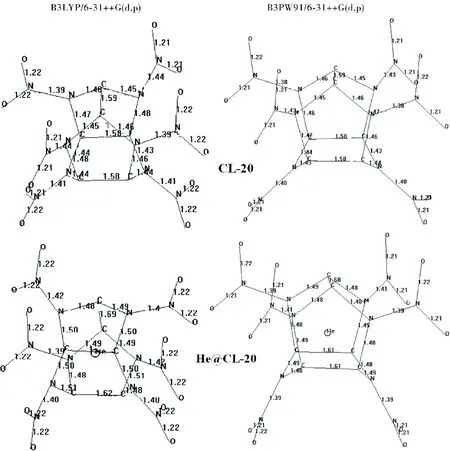
Fig.3 Bond lengths of the structures considered (Hydrogens not shown)
2.2 IR Spectra
Fig.4 displays the calculated (B3LYP/6-31++G(d,p) IR spectra of the structures considered (a similar spectra were obtained by employing B3PW91/6-31++G(d,p)). In the case of CL-20, the peaks at about 1690-1649cm-1are asymmetric N-O stretching of the nitro groups coupled with N-NO2bendings. The strongest of them occurs at 1690cm-1. In the doped structure the respective peaks occur at 1649-1680cm-1. The wagings of C-H bonds in both of the structures occur at about 1360-1364cm-1and bendings of them are at about 1324cm-1. At 1357cm-1the helium atom also vibrates in the cage. Below 1200cm-1the spectra are quite different because some vibrations of the He atom also occur there.
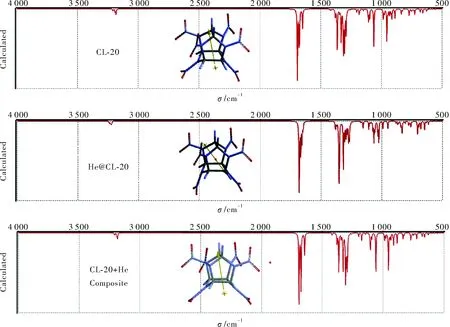
Fig.4 IR spectra of the structures considered (B3LYP/6-31++G(d,p))
2.3 Charges
Fig.5 shows the electrostatic charges calculated based on ESP method at the level of B3LYP/6-31++G(d,p). The ESP charges are obtained by the program based on a numerical method that generates charges that reproduce the electrostatic potential field from the entire wavefunction[36]. Note that the helium atom acquires some positive charge meaning that the potential field present in the cavity of CL-20 is able to remove some electron population from the helium atom. At the B3PW91/6-31++G(d,p) level of calculations the He atom acquires positive charge as well but less in magnitude.
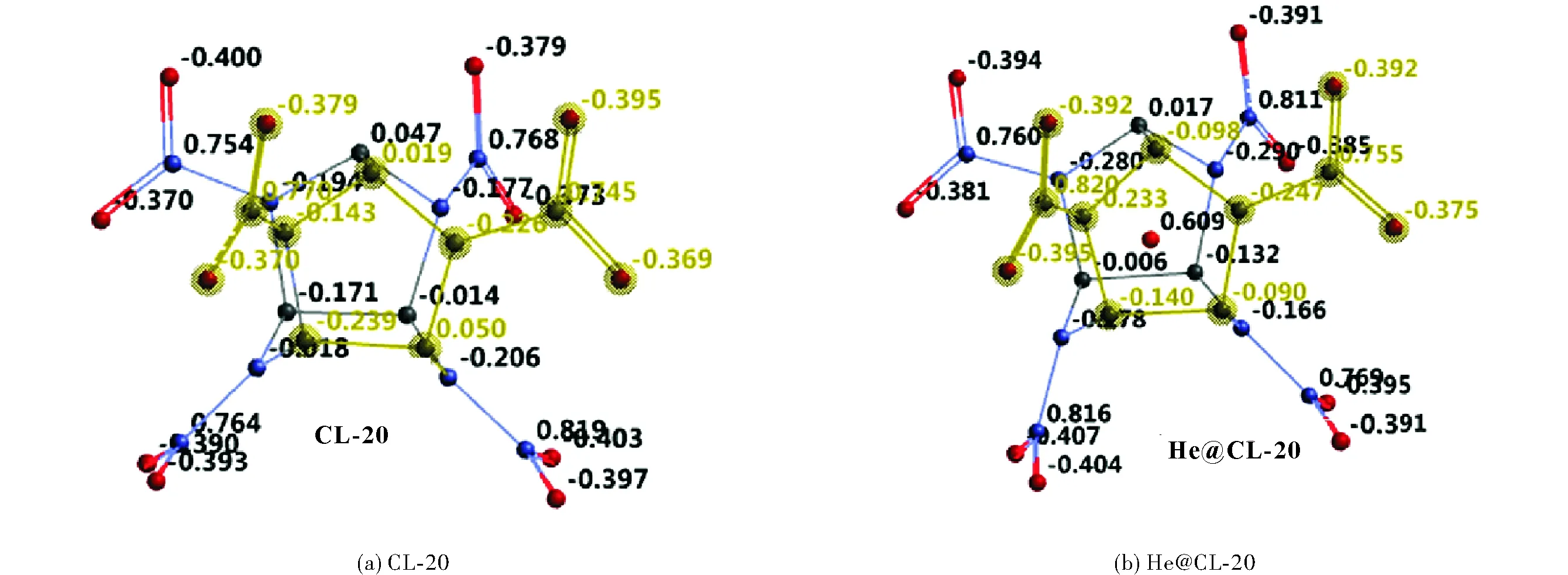
Fig.5 Electrostatic (ESP) charges on CL-20 and He@CL-20 structures (hydrogens not shown, B3LYP/6-31++G(d,p))
2.4 Energies
Table 2 shows the total electronic energies (E), zero point vibrational energy (ZPE) and the corrected energy (Ec) for the structures considered. Note that the table also includes CL-20+He composite system. Although the doped structure is less stable than the composite one, nevertheless bonds remain intact.

Table 2 Various energies of the structures considered
Note: Energies in B3LYP/6-31++G(d,p) and B3PW91/ 6-31++G(d,p) (in parenthesis) level of calculations.
2.5 Molecular orbital energies
Fig.6 displays some of the molecular orbital energy levels of the structures considered. Although, the frontier molecular orbital (HOMO and LUMO) energies for the two structures are comparable, the inner-lying occupied molecular orbital energies differ up to a certain extent.
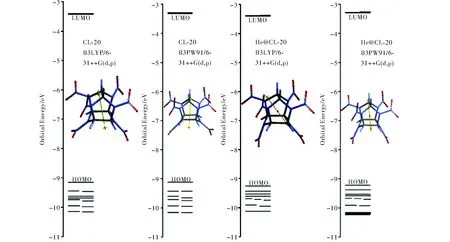
Fig.6 Some of the molecular orbital energy levels of the structures considered
2.6 Frontier molecular orbitals and energies
Table 3 shows the frontier molecular orbital, HOMO and LUMO, (FMO) energies as well as the interfrontier molecular orbital energy gaps (Δε, LUMO-HOMO energy difference) of the structures considered. Note that the table also includes the composite system, CL-20 + He. The data reveal that the doping process lowers the HOMO but raises the LUMO energy level. This is a complex process and perplexing at first sight. Although, the helium atom donates some electron population to the cage (see Fig. 5) therefore by analogy to electron donating substituents[37],it is expected to raise both the HOMO and LUMO energy levels, but it is not the case because the doped structure is swollen and the electronic behavior of it totally changed compared to CL-20. On the other hand, positively charged helium atom has lost its natural stability and attracts some electron population of the cage. The overall effect results the tabulated Δε values. The extended FMO gap should cause hypsochromic effect[38]. The composite system has higher HOMO but lower LUMO energies as compared to the doped structure.
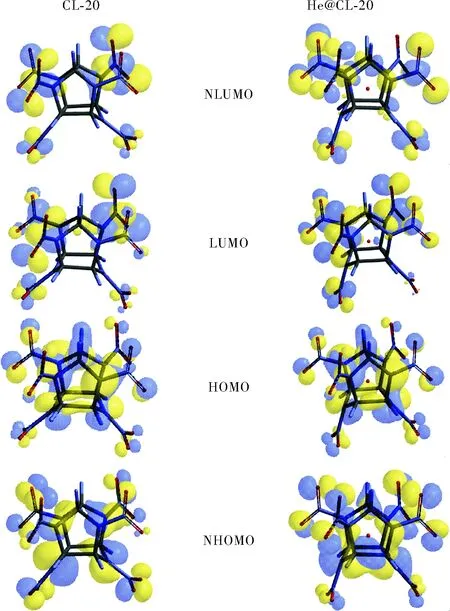
Fig.7 NHOMO, HOMO, LUMO and NLUMO patterns of the structures considered (B3LYP/6-31++G(d,p))
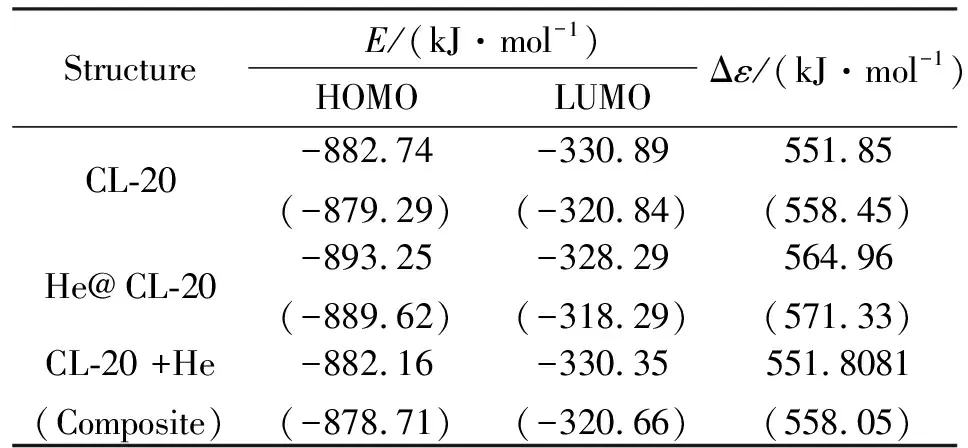
E/(kJ·mol-1)StructureHOMOLUMOΔε/(kJ·mol-1)CL-20-882.74(-879.29)-330.89(-320.84)551.85(558.45)He@CL-20-893.25(-889.62)-328.29(-318.29)564.96(571.33)CL-20 +He(Composite)-882.16(-878.71)-330.35(-320.66)551.8081(558.05)
Note:B3LYP/6-31++G(d,p) and B3PW91/ 6-31++G(d,p) (in parenthesis) level of calculations. Energies in kJ/mol.
Fig.7 shows the patterns of some molecular orbitals of the structures comparatively. Although, the HOMOs and LUMOs of the structures have some resemblance in appearance, the next orbitals (NHOMO and NLUMO) are greatly different in pattern. Note that the doping has greater influence on the energy levels of the inner orbitals (see Fig.6).
Fig.8 shows the electrostatic potential maps of the structures. In both of them the interior cavity has positive field mainly located on the base ring (Piperazine ring). Note that both levels of calculations yield resembling maps.

Fig.8 Electrostatic potential maps of the structures considered (at B3LYP/6-31++G(d,p)) and B3PW91/ 6-31++G(d,p) (lower ones) levels)
3 Conclusions
(1) The helium-doped CL-20 structure is stable.
(2) No bond cleavage occurs in doping process but the structure swells.
(3)Doping mainly affects the inner-lying molecular orbitals.
(4) FMO energy gap increases after doping and hypsochromic shift is expected.

