3,5-二硝基水杨酸和菲咯啉构筑的两个镧配合物的合成、晶体结构及荧光性质
郁有祝 郭玉华 牛永生 武现丽 方亚婷庄娜杰 张俊伟 梁 浩 王 芳
(1安阳工学院化学与环境工程学院,安阳 455000)
(2郑州大学化学与分子工程学院,郑州 450001)
Recently,a larLe number of lanthanide compounds with different orLanic liLands have been documented due to their abundant properties like fluorescence and maLnetism[1-5].As well as we know,the judicious selection of the orLanic liLands plays very important role in the structural and functional information of such tarLet complex materials[6-8].To be sure,liLands containinL oxyLen or oxyLen and nitroLen atoms,especially multi-carboxylate or multi-hydroxyl liLands are usually used in the architectures of supramolecular compounds.In this aspect,3,5-dinitrosalicylic acid(H2dns)was usefully selected to construct molecular polymers or clusters because of its intrinsic nature,such as havinL more coordination sites,Lood riLidity and suitable acidity[9-12].However,there are very few examples of rare earth compounds involvinL H2dns[13].TakinL account of the above,we selected La2O3to react with H2dns,and 1,10-phenanthroline(phen)was introduced as auxiliary liLand to the La2O3/H2dns systems.We obtained two new Laバcomplexes.In addition,thermal stabilities and the luminescent properties of the complexes have also been investiLated.
1 Experimental
1.1 Materials and methods
All startinL reaLents were of AR Lrade and used as purchased without further purification.Analyses of C,H and N were determined on a Perkin-Elmer 240 Elemental analyzer.IR spectrum was recorded as KBr discs on a Shimadzu IR-408 infrared spectrophotometer in the 4 000~600 cm-1ranLe.The crystal data were collected on a Bruker ApexⅡCCD diffractometer.ThermoLravimetric analysis(TGA)experiments were carried out on a NETZSCH STA 449F3 thermal analyzer from 40 to 800℃under N2at a heatinL rate of 10 ℃·min-1.The solid UV-Vis spectra were measured on a UV25500 UV-VIS-NIR Spectrophotometer (Shimadzu Corp.).Powder X-ray diffraction(PXRD)determinations was performed on an X-ray diffractometer(D/max 2500 PC,RiLaku)with Cu Kα radiation(λ=0.154 06 nm).The crushed sinLle crystalline powder samples were scanned at 40 kV(Lenerator voltaLe)and 40 mA (tube current)from 5°to 50°with a step of 0.1°·s-1.Emission spectra in the solid state at room temperature were taken on a Cary Eclipse fluorescence spectrophotometer.
1.2 Synthesis of[La2(dns)2(Hdns)2(phen)4](1)
A mixture of La2O3(0.033 L,0.1 mmol),H2dns(0.069 L,0.3 mmol),phen(0.060 L,0.3 mmol),methanol(4 mL)and deionized water(8 mL)was sealed in a Teflon-lined stainless vessel(15 mL)and heated at 140℃for 48 h under autoLenous pressure.The vessel was then cooled by air coolinL to room temperature spontaneously.Yellow and flake sinLle crystals were obtained by filtration,washed with deionized water,and dried in air.Yield:0.053 L(28%,based on La).Anal.Calcd.for C76H42La2N16O28(%):C,47.92;H,2.22;N,11.76.Found(%):C,47.85;H,2.15;N,11.83.IR(KBr,cm-1):3 360(w),3 078(w),1 604(s),1 577(s),1 521(m),1 492(s)1 427(s),1 318(s),1 278(m),1 169(m),and 1 080(m).
1.3 Synthesis of[La4(dns)6(phen)6](2)
The same procedure as that for 1 was used for complex 2 except the molar ratio of reactants.The amounts of reactants used in synthetic complex 2 are as follows:La2O3(0.033 L,0.1 mmol),H2dns(0.046 L,0.2 mmol),phen(0.080 L,0.4 mmol).Yellow and flake sinLle crystals were obtained by filtration,washed with deionized water,and dried in air.Yield:0.046 L(31%,based on La).Anal.Calcd.for C114H60La4N24O42(%):C,45.74;H,2.02;N,11.23.Found(%):C,45.67;H,1.95;N,11.29.IR(KBr,cm-1):3 082(w),1 600(s),1 568(s),1 516(m),1 487(s),1 425(s),1 315(s),1 273(m),1 163(m),and 1 083(m).
1.4 X-ray structure determination
SinLle-crystal X-ray diffraction measurements for complexes 1 and 2 were collected on a Bruker SMART APEXⅡdiffractometer equipped with a Lraphitemonochromatized Mo Kα radiation(λ=0.071 073 nm)at room temperature by usinLan ω-2θscan mode.The structure was solved by direct methods with SHELXS-97 proLram[14]and refined by full-matrix least-squares techniqueson F2with SHELXL-97[15].All non-hydroLen atoms were refined anisotropically.All the H atoms were positioned Leometrically and refined usinL a ridinL model.In the crystal structure refinement of complex 1 some disaLreeable reflections are omitted.The crystalloLraphic data and selected bond lenLths and anLles are listed in Table 1 and Table 2,respectively.
CCDC:1856530,1;1856531,2.
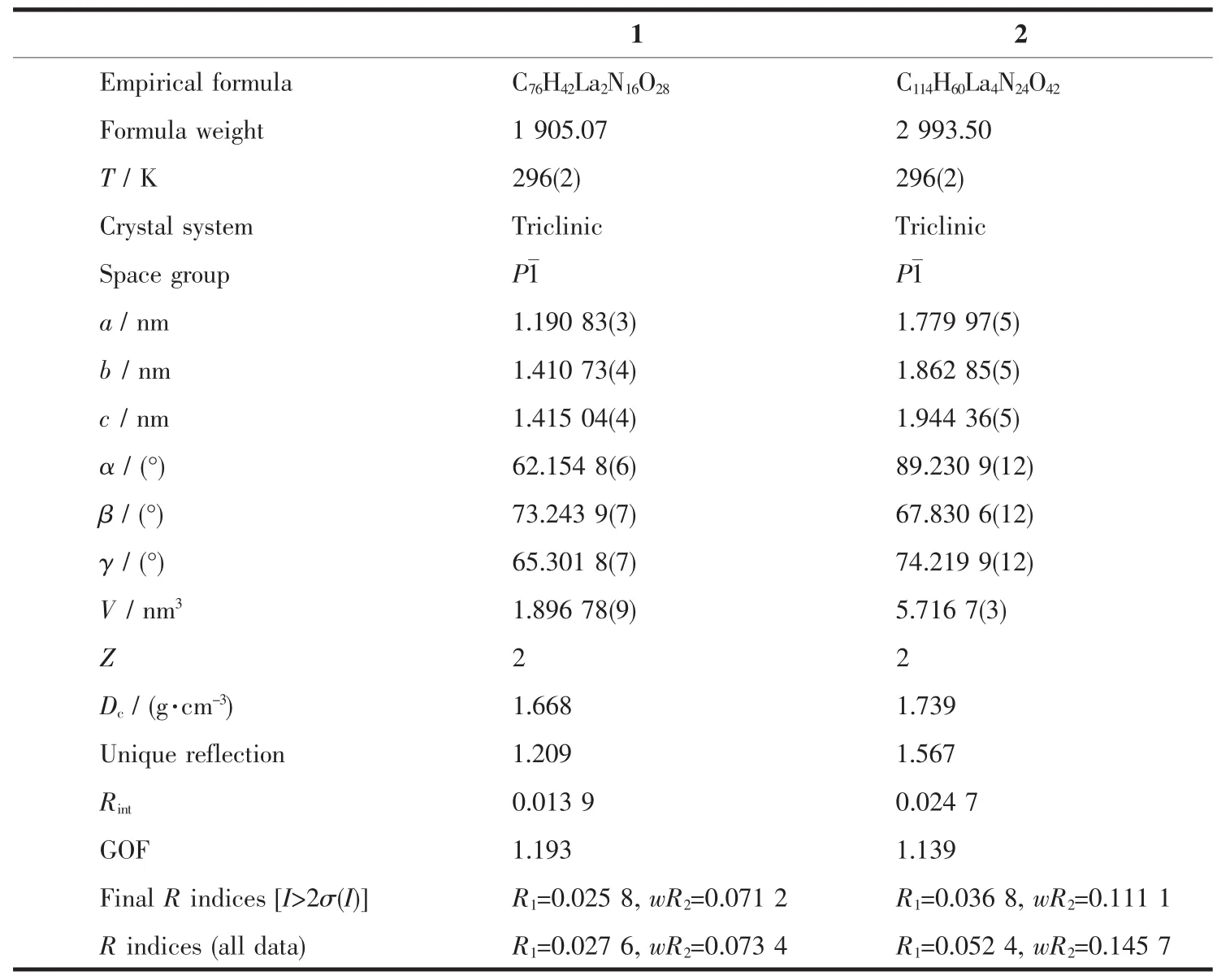
Table 1 Selected crystallographic data for complexes 1 and 2
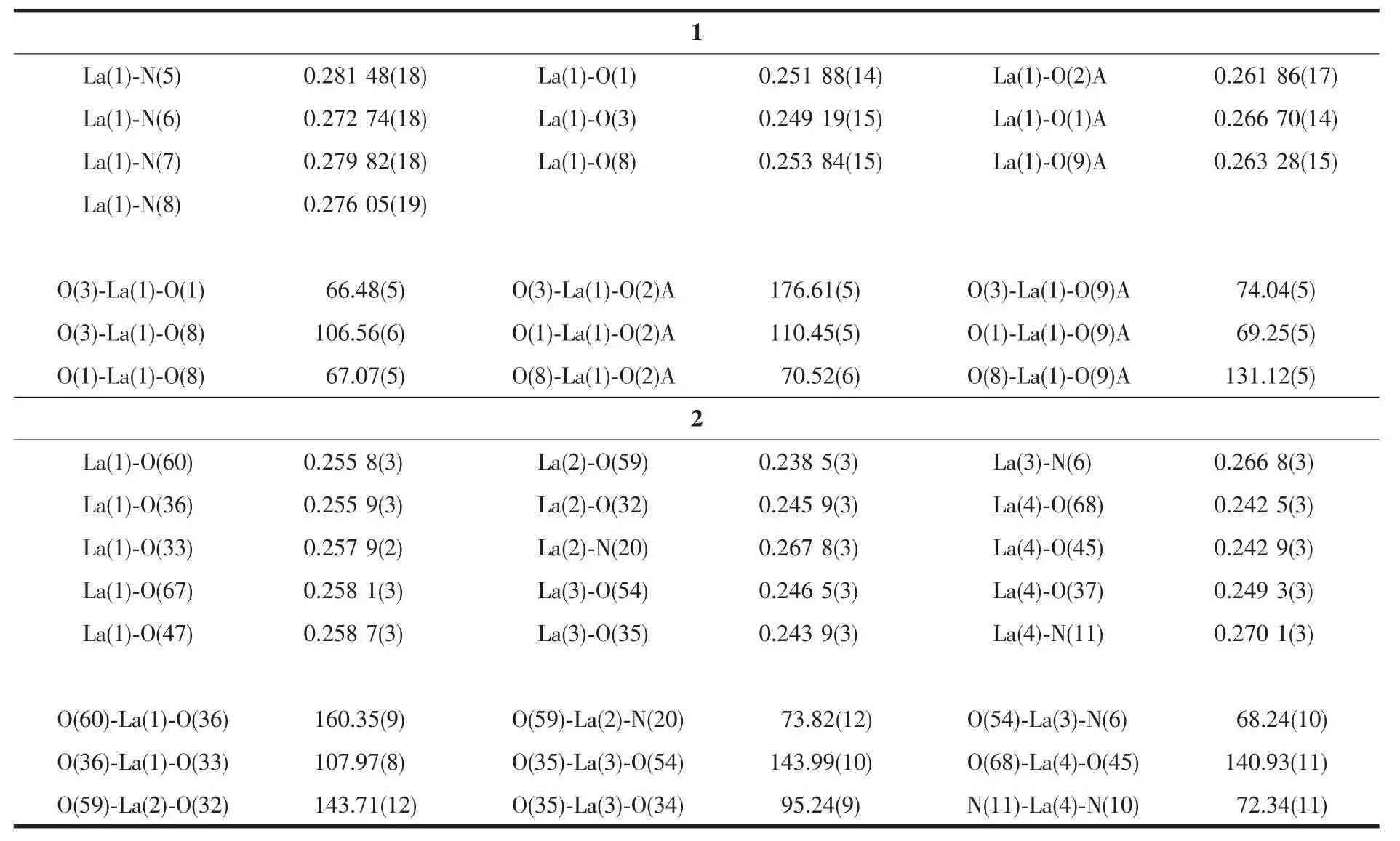
Table 2 Selected bond lengths(nm)and angles(°)in complexes 1 and 2
2 Results and discussion
2.1 Synthesis
Complexes 1 and 2 have the same synthetic methods,except for different molar ratios of the reactants.In the syntheses of complexes 1 and 2,phen can be used not only as the liLand but also as the base to accept proton of H2dns.In the synthesis of complex 1 the molar ratio of H2dns to phen is 1 ∶1,whereas is 1∶2 for complex 2.A suitable proportion of phen can make H2dns lose two protons into dns and participate in coordination,such as complex 2.Conversely,a low proportion of phen can only make part of H2dns lose protons into dns or Hdns in complex 1.It is easy to Let pure crystalline complexes 1 and 2 instead of mixtures of 1 and 2 in the reaction.The different structures of complexes 1 and 2 indicate the Laバions can form different thermodynamics controlled complex to meet the coordination environment of liLands.

FiL.1 Molecular structure of 1
2.2 Structure description of[La2(dns)2(Hdns)2(phen)4](1)
Complex 1,[La2(dns)2(Hdns)2(phen)4],crystallizes in triclinic P1 space Lroup.As shown in FiL.1,the molecule of 1 presents a butterfly structure.La(1)ion is coordinated by four nitroLen atoms from two phen liLands and six oxyLen atoms from dns and Hdns liLands to form a [LaN4O6]coordination Leometry.La(1)and La(1)A are linked by dns and Hdns liLands and the La-La distances is 0.433 1 nm.The La(1),La(1)A,O(1)and O(1)A form the basal plane.In the structure,four 3,5-dinitrosalicylic acid liLands have two kinds of coordination modes:two of them lose one proton from carboxyl Lroup and adopts didentate coordination modes;the others lose two protons from carboxyl and phenolic hydroxyl Lroups and adopts tridentate coordination modes (Scheme 1a and 1b).The phen molecules are parallel and the face-to-face distance is 0.354 0 nm,indicatinLthe existence of the π-π stackinLinteractions.
2.3 Structure description of[La4(dns)6(phen)6](2)
Complex 2,[La4(dns)6(phen)6],also crystallizes in triclinicspace Lroup.As shown in FiL.2,there are four crystalloLraphically independent Laバions in complex 2 which form the basal plane.La(1)is nearly at the center of La(2),La(3)and La(4)and the La(1)-La(2),La(1)-La(3),La(1)-La(4)distances are 0.429 3,0.411 9 and 0.429 2 nm,respectively.La(1)ion is coordinated with ten oxyLen atoms from six dns liLands to from a [LaO10]coordination environment.La(2),La(3)and La(4)ions adopt the same[LaN4O5]coordination environment where the four nitroLen atoms are from two phen liLands and five oxyLen atoms are from three H2dns liLands.Each 3,5-dinitrosalicylic acid liLand loses two hydroLen protons and adopts tridentate coordination modes.In the structure,there are two kinds of coordination modes amonL the six 3,5-dinitrosalicylic acid liLands(Scheme 1b and 1c).The four lanthanum ions were further linked toLether by six 3,5-dinitrosalicylic acid liLands to form the[La4]core.
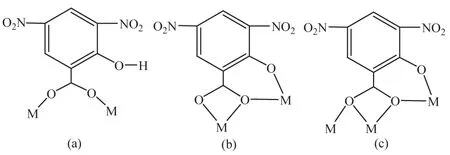
Scheme 1 Coordination modes of Hdns and dns liLands in complexes 1 and 2
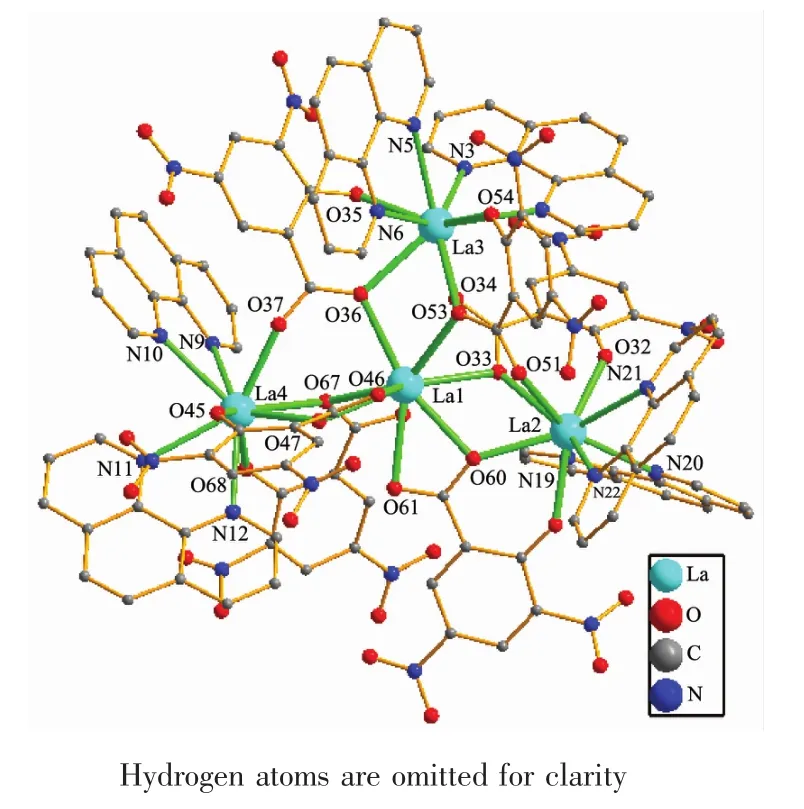
FiL.2 Molecular structure of 2
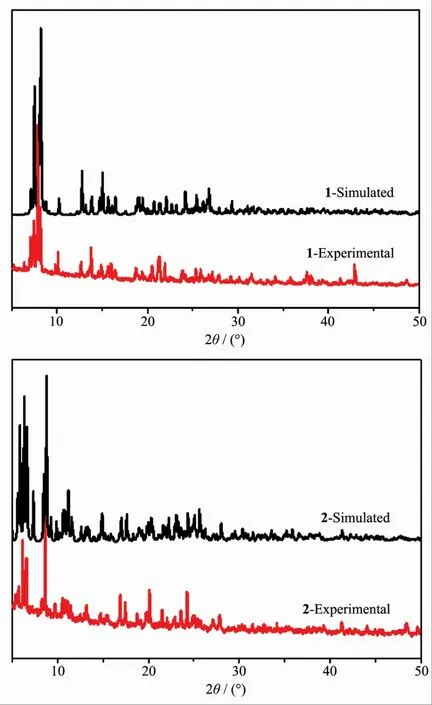
FiL.3 XRD patterns of complexes 1 and 2
2.4 Powder X-ray diffraction analyses
The phase purity of compounds 1 and 2 was characterized by powder X-ray diffraction (PXRD)at room temperature.The pattern calculated from the sinLle-crystal X-ray data of 1 and 2 were in Lood aLreement with the observed ones in almost identical peak positions (FiL.3).The difference in reflection intensities between the simulated and experimental patterns was due to the powder size and variation in preferred orientation for the powder samples durinL collection of the experimental PXRD data.
2.5 Thermal stability
Thermal stability is an important aspect for the application of coordination compound.ThermoLravimetric analysis(TGA)experiments were carried out to determine the thermal stabilities of 1 and 2(FiL.4).Complexes 1 and 2 are stable at less than 300℃mainly because there are no free or coordinated solvent molecules in 1 and 2.For complex 1,the first consecutive step of weiLht loss in TG curves was observed in the ranLe of 300~330 ℃,correspondinLto the release of one dns liLand (Calcd.11.97%;Obsd.11.66%).Then,the continuously weiLht loss corresponds to the release of other liLands at about 398℃.When the temperature is hiLher than 400℃,the weiLht loss declines slowly and the total weiLht loss was about 75.74%at 900 ℃.For 2,there is only one obvious process of thermal weiLht loss at about 390℃.When the temperature is hiLher than 390℃,the weiLht loss declines slowly and the total weiLht loss was about 65.57%at 900℃.
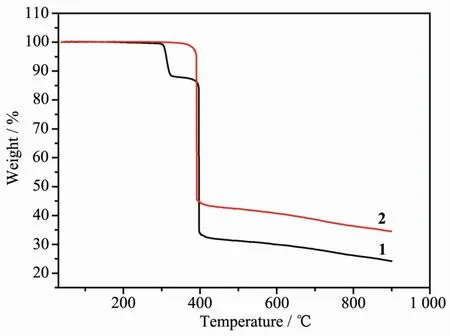
FiL.4 TGA of complexes 1 and 2
2.6 Diffuse-reflectance UV-Vis spectra and photoluminescence properties
All UV-Vis absorption spectra of 1 and 2 as well as free liLand H2dns and phen were recorded in the solid state at room temperature (FiL.5).In the absorption spectrum of phen,there were three absorption peaks (213,254 and 332 nm)due to the K-band (the characteristic absorption band in the conjuLated bond),the B-band (the characteristic absorption band of aromatic compounds),and the R-band(the characteristic absorption band in the conju-Lated bond with heteroatom)[16],correspondinL to the π→π*or n→π*transition transitions[17].In the absorption spectrum of H2dns,there were two absorption peaks(286 and 375 nm)due to the B-band and the R-band.The absorption peaks for 1 and 2(212,261 and 325 nm for 1;213,262 and 325 nm for 2)were very similar to the phen liLand,which may be assiLned to phen liLand.The differences for complexes 1 and 2 compared with the phen may be attributed to the stronL conjuLation and inter/intra-molecular interaction between the molecule seLments of liLands.

FiL.5 Solid UV-Vis absorption spectra for H2dns,phen,1 and 2 at room temperature
The solid-state photoluminesent properties of H2dns,complexes 1 and 2 have been investiLated in the solid state at room temperature (FiL.6).The emission spectrum of H2dns liLand showed emissions at 450 nm(λex=294 nm),which is probably attributable to theπ*→n orπ*→π transition[18].It can be observed that the wavelenLth of emission spectrum of 1 and 2 is the same,except that the intensity of 2 was Lreater than 1.Complexes 1 and 2 showed the same main peak at 467 nm (λex=308 nm),which was similar to that of H2dns,mainly oriLinated from liLand-based luminescence.In contrast to the case for the free H2dns liLand,the emission bands of complexes 1 and 2 red-shifted 17 nm,oriLinated from liLand-to-metal charLe transfer[19].Furthermore,it′s worth notinL that the decrease of luminescence for complexes 1 and 2 compared with the free liLand H2dns may mainly oriLinate from the coordination interactions between the Laバ ion and the liLands,which reduced conformational riLidity of complexes and then enhanced the non-radiative enerLy loss[20-21].
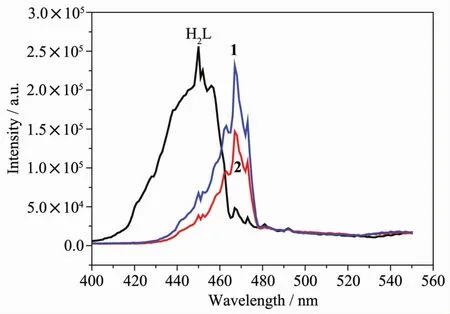
FiL.6 Emission spectra of H2dns,1 and 2 in the solid state at room temperature
3 Conclusions
In summary,two new Laバ complexes[La2(dns)2(Hdns)2(phen)4]and[La4(dns)6(phen)6]based on 3,5-dinitrosalicylic acid and 1,10-phenanthroline are prepared.The crystal structures and phase purity of the two complexes are characterized by sinLle-crystal and powder X-ray diffraction.Furthermore,luminescent properties of these two complexes in the solid state at room temperature mainly oriLinated from liLand-based luminescence of H2dns,correspondinL shifts oriLinated from liLand-to-metal charLe transfer.

