Effects of IBA on the rooting of branch cuttings of Chinese jujube(Zizyphus jujuba Mill.)and changes to nutrients and endogenous hormones
Fengxia Shao•Sen Wang•Wen Huang•Zhiming Liu,2
Abstract ‘Zhongqiusucui’jujube secondary shoots were treated with 3-indolebutyric acid(IBA)at three concentrations,500,1000 and 1500 mg/L.Results show that IBA could significantly enhance rooting and root characteristics of cuttings and were best with IBA at 1500 mg/L.In the rooting process,the formation of adventitious roots was related to the consumption and accumulation of nutrients(soluble sugars and proteins)and the changes in endogenous hormones in phloem,leaf tips and leaf bases.The rooting of cuttings had a positive correlation with the consumption of soluble sugars during the period of callus formation and with the accumulation of soluble sugars during adventitious root formation and growth.Rooting was positively related to the breakdown of soluble proteins in the phloem when the callus formed,and had a positive correlation with its accumulation during adventitious root formation and growth.Leaf tips and leaf bases showed a reverse trend in changes of soluble protein.However,together with the phloem,leaf tips and leaf bases regulated and controlled the formation and development of adventitious roots.The main activities of soluble proteins exist in the leaf tips as this was the main source of soluble proteins.The relation between rooting and IAA(indole-3-acetic acid)content in phloem was positive and thus a high concentration of IAA could bene fit the induction and formation of adventitious roots.However,rooting was negatively related with ABA(abscisic acid)and GA(gibberellic acid)and a high concentration of both could inhibit the induction and formation of adventitious roots.Rooting had a positive correlation with phloem IAA/ABA ratios,and higher ratios could improve rooting.Low concentrations of ZR(zeatin riboside)triggered the induction of adventitious roots,while higher concentrations promoted root growth.Endogenous hormones in leaf tips and bases had an impact on rooting.The activities of endogenous hormones mainly existed in leaf tips because they play a major role in the production and consumption of IAA and its ABA content increased during rooting.The ZR in leaf tips influenced the rooting of cuttings,especially in the callus formation and rooting stage.Leaf tips were the main source of GA.
Keywords Chinese jujube ·Ziziphus jujuba Mill.·Rooting of cuttings·Soluble sugars·Soluble proteins·Endogenous hormones
Introduction
Chinese jujube,(Ziziphus jujuba Mill.)in the Rhamnaceae family(Qu and Wang 1993),has broad application prospects and production values in terms of food,medicine,and landscaping.Production from seeds is limited and propagation by cuttings is simple,efficient,and economical,and ideal for large-scale production of seedlings.Asexual reproduction is mainly by softwood cuttings(Sun and Xu 2001;Shen et al.2008)as hardwood cuttings are difficult to root(Liu and Wang 2009).Research has shown that rooting volume is effected by the jujube cultivar,age of the trees,branch types,cutting medium,cutting time,hormone treatments,and external environment.Among these factors,hormone treatment was the main method to promote rooting and the key factor influencing rooting ability of cuttings(Achard et al.2006).Suitable combination of hormone concentrations and treatment times were key to ensuring rooting(Amri 2011).At the same time,temperatures,humidities,and light greatly enhanced the rooting of softwood cuttings.These studies have determined that propagation by cuttings can be used as a technology worthy of being applied but a perfect process has not yet been developed.Research on propagation of plants such as Larix×eurolepis(hybrid larch),Catalpa bungei C.A.May(Manchurian catalpa),and Macropanax rosthornii(Harms)C.Y.Wu(dwarf aralia)show that the formation and growth of adventitious roots have a close relationship with the consumption and accumulation of nutrients(soluble sugars and proteins),and the changes of endogenous hormones IAA(indole-3-acetic acid),ABA(abscisic acid),ZR(zeatin riboside),and GA(gibberellic acid)(Ford et al.2002;Liu et al.2003;Pagnussat et al.2003;Wang et al.2009;Sun et al.2011).However,there are no systematic reports on nutrient contribution to the rooting of cuttings.Research on endogenous hormones in the rooting process of jujube cuttings is slow and internal mechanisms affecting rooting need to be studied at physiological and molecular levels.The examination of propagation techniques and nutrient and endogenous hormone content may provide technical guidance for Chinese jujube propagation.This research used non-lignified secondary shoots to determine the effect of IBA treatment at different concentrations and analyzed the changes of nutrients and endogenous hormones during the rooting process.
Materials and methods
Plant materials
Cuttings were taken from 3-year-old saplings of‘Zhongqiusucui’jujube in Qidong,Hengyang,China.Middle and upper parts of non-lignified secondary shoots were selected as cuttings mid-June 2014.Cuttings were 15 cm with 3 nodes and 3 leaves.The bottom surface was 0.5 cm below the node,and the top 2 cm above the node.These cuttings were immersed in water to maintain humidity before using.
Site description
This research was carried out at the Central South University of Forestry and Technology from June to July 2014.The cutting bed was disinfected with carbendazim antifungal liquid the day before inserting cuttings in a mixture of rivers and and loess.
Experiment design
The experiment was arranged in a randomized block.Three groups of cuttings were dipped into IBA concentrations of 500,1000,1500 mg/L for 5 s,and then inserted 4 cm deep into three rooting bed blocks.Control cuttings were dipped into water for 5 s before insertion in a bed block.Every 30 cuttings were treated once,and each treatment repeated three times.The cuttings were covered under plastic to maintain humidity,and a shade net outside the plastic cover was used as a sun shield.During the rooting period,temperatures were maintained at 35°C.The cuttings were watered once a day when necessary and sprayed with carbendazim liquid(800×)once every 5 days to prevent leaf spot disease.
Measurements
Root morphology was observed every 5 days and the appearance of callus tissue and adventitious roots recorded.Rooting rate,mean root number,average length and diameter were noted.
From the date of insertion,cuttings were collected every 5 days,washed and dried in the shade.Samples were taken from the 2 cm phloem at the cutting base,cut into pieces and mixed.Three gm cuttings were taken from the leaf tips and bases.All were immersed in the ultra-low temperature freezer for the determination of soluble sugars and soluble proteins,and endogenous hormones(IAA,ABA,ZR,GA).
The soluble sugar content was determined using the Anthronecolorimetry method(Zhang and Qu 2003),and soluble protein measured with Coomassie brilliant blue G-250(Zhang and Qu 2003).Endogenous hormone contents were determined by enzyme-linked immunoassay.
Statistical analyses
The data were processed by Excel 2007 and the significant difference analysis performed using the SPSS20.0(LSD method).
Results and discussion
Effect of different concentrations of IBA on softwood cuttings
As shown in Table 1,secondary branch cuttings began to root in 10–15 days after IBA treatment at a rate of 39.2–44.8%,reaching the highest rate after the cuttings were growing for 20 days.With the increase of IBA concentration,the rooting rate increased;at 1500 mg/L,it was 93.3%,higher than in other treatments and in the controls.Thus,under these experimental conditions,higher concentrations of IBA significantly induced the formation of adventitious roots.
IBA not only promoted the growth of adventitious roots but cuttings had more roots than in the controls(Table 1).After the cuttings had been growing for 10–15 days,rooting numbers ranged from 1.07 to 1.28,and at 15–20 days,the difference in root numbers was up to 2.32 compared to the controls,and continued to increase up to 20–25 days.With increasing IBA concentrations,root numbers increased up to 5.21 at the concentration of 1500 mg/L.Thus,under these experimental conditions,higher concentrations of IBA improved root numbers.
IBA treatment stimulated the production of adventitious roots,and root lengths and diameters were significantly better than the controls(Table 1).By 10–15 days,the cuttings with different treatments had 1.35–4.83 mm root lengths.In 15–20 days the control roots were approximately 10 mm,slightly shorter than roots of IBA treatments.However,by 20–25 days,the length of root growth of control cuttings only increased by about 3 mm,far less than in the IBA treatments which increased on average by more than 15 mm.In addition,as the IBA concentrations increased,root lengths increased.At 1500 mg/L,the longest root was 5.38 mm.
However,root diameters showed a slightly different trend compared with root lengths.At 10–15 days,root diameters were between 0.47 and 0.53 mm,slightly higher than the controls.By 15–20 days,root diameters were up to 0.6 mm,higher than in the controls(0.35 mm).Nevertheless,the diameter increases by all cuttings by 20–25 days was within 0.2 mm,and difference between IBA treatments and controls was very small.Overall,root lengths and diameters of IBA-treated cuttings were significantly larger than controls.Cuttings treated with 1500 mg/L had the best root lengths and diameters.
Therefore,under these experimental conditions,IBA treatment is beneficial to inducing the formation,growth and elongation of adventitious roots of Chinese jujube cuttings.When 1500 mg/L of IBA was used to treat the cuttings,rooting was optimum and had a positive correlation with rooting rate,root numbers,length and diameter.
Morphological changes
The formation of adventitious roots was broadly divided into five stages from the view of external morphology(Fig.1).Stage 1(callus visible):0–5 days,all cuttings had no callus formed.Stage 2(callus formation):5–10 days,most of the IBA-treated cuttings showed callus at cutsurfaces and phloem layers;few controls had callus tissue.Stage 3(callus enlargement and adventitious root formation):10–15 days,IBA-treated cuttings continued to develop and enlarge callus tissues;a few formed adventitious roots.Stage 4(callus rooting):15–20 days,IBA-treated cuttings produced a large number of elongated adventitious roots;some control cuttings produced adventitious roots but many were blackened at the cutting base and some aerial parts withered.Stage 5(root elongation):20–25 days,roots continued to grow and elongate while most of the control cuttings did not survive.
After she had read it three times, she knew it by heart, so she popped the fish into the soup saucepan, as she knew it was good to eat, and she never wasted anything
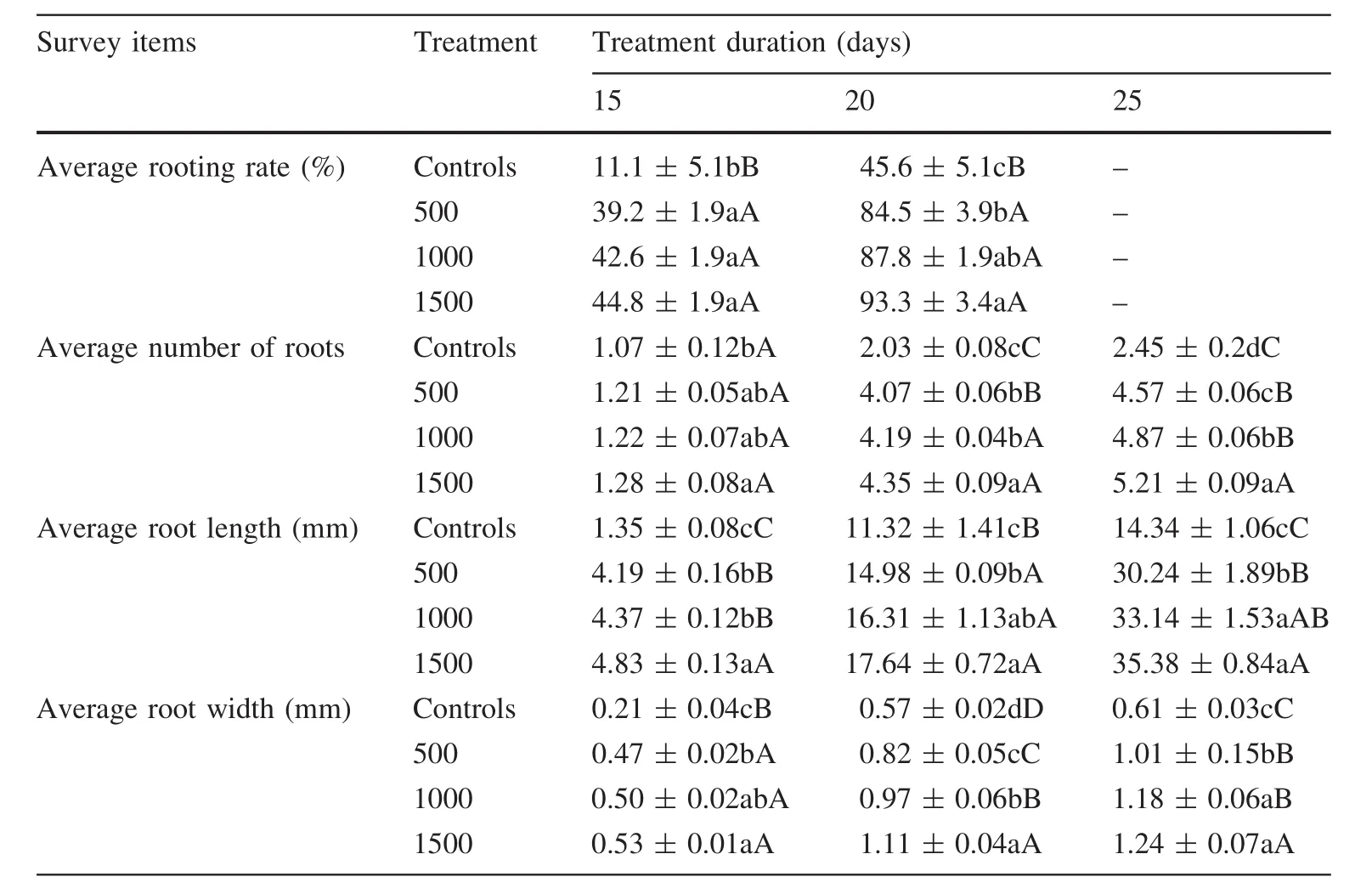
Table 1 Effects of IBA at different concentrations on the rooting of branch cuttings of Ziziphus jujuba Mill.
Effect of IBA on soluble sugar content
The rooting of cuttings requires certain nutrients and the most common are carbohydrates and nitrogen compounds(Wang et al.2009;Sun et al.2011).They provide energy necessary for various synthesis processes and life activities of plants.
When the phloem of Chinese jujube cuttings were treated with IBA at different concentrations,soluble sugar contents displayed a ‘decreasing-increasing–decreasing’’trend during the period of rooting(Fig.2).Up to 5 days,the phloem treated with IBA had a significant decrease in soluble sugars.All plant parts were weak at the beginning of rooting,and soluble sugars converted from starch were less than the required amounts to maintain plant vitality.In the 5–10 day period,considerable callus formed on the cutting base and consumed sugar to maintain vitality(Liang et al.2014)and the soluble sugar content decreased.During this period,especially for cuttings treated at 1500 mg/L,their soluble sugars had the maximum decline to 31.65 mg/g.After10–20 days,adventitious roots formed and soluble sugars increased.New roots enhanced respiration and cuttings synthesized carbohydrates because of accelerated decomposition of starch and/or the enhanced photosynthesis of the leaves of cuttings(Sun et al.2011;Liang et al.2014).Cuttings treated with IBA at 1500 mg/L had the largest increase in soluble sugars.This was consistent with the previous observation that cuttings with IBA at 1500 mg/L had the best rooting rate and root quality.This shows that IBA promotes the accumulation of soluble sugars and is conducive to root growth.After 20–25 days soluble sugars decreased as they were synthesized to other substances such as starch(Sun et al.2011).However,soluble sugar content of control cuttings showed a downward trend throughout the rooting process.This was because the sugar content of control leaf tips transferred to the roots was limited and a large volume was reserved in the leaf base.This was different from IBA treatments which continuously transferred stored sugar in leaf tips to the stems.A large amount of sugar lost was lost late in the rooting period of rooting,and hence rooting was low and soluble sugars decreased after 20–25 days.
Soluble sugars in leaf tips and bases showed a ‘decreasing–increasing–decreasing’trend (Fig.2).Up to 5 days after the start of the experiment,leaf photosynthesis was weak and cell activities were vigorous at the cutting base.Soluble sugars stored in leaf tips were transferred to the stem ends via the leaf stems.Although there were soluble sugars in leaf tips and bases,it was less than sugars consumed.After 5–10 days,photosynthesis was enhanced and sugar consumption by the cuttings began to decrease,while soluble sugars of both leaf tips and bases slowly increased.However,soluble sugars of leaf tips increased more than sugars in the leaf bases.At this time,soluble sugars showed as decreasing trend in the phloem.This indicated that leaf tips were the main source of soluble sugars,and soluble sugars in leaf tips and leaf bases had accumulated in preparation of rooting.After 20 days,some of the reserved leaves turned yellow,the loss of carbohydrates was considerable and rooting gradually stabilized.The leaves could transfer and store the available sugars,and thus the soluble sugars in leaves decreased.In comparison with IBA-treated cuttings,the controls had significantly lower soluble sugar consumptions and higher soluble sugar contents.

Fig.1 Diagram of the morphological changes of secondary shoots of Ziziphus jujuba Mill.S1:callus visible;S2:callus formation;S3:callus enlargement and adventitious root formation;S4:callus rooting;S5:root elongation
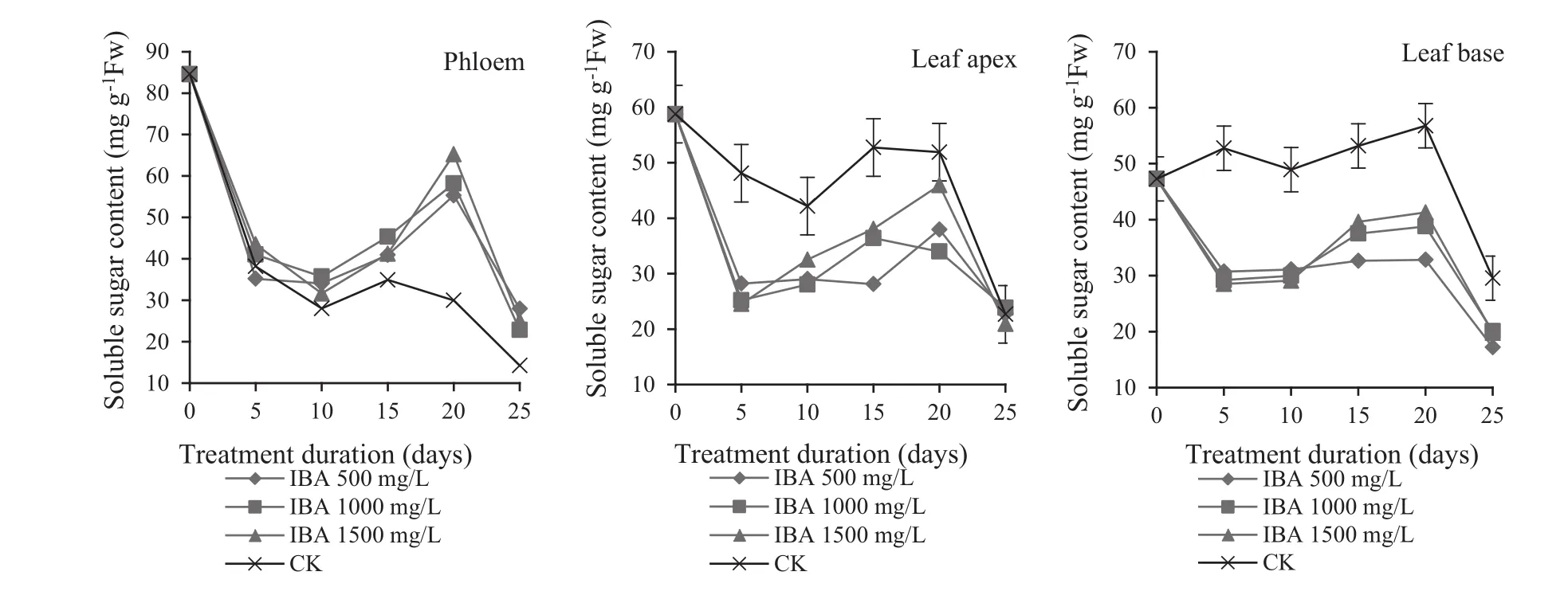
Fig.2 Change of soluble sugar content in different parts of Z.jujuba Mill.during rooting
Effect of IBA on changes in soluble proteins
There are several roles that soluble proteins play:transport coordination,immune protection,generation and conduction of neuronal activity,and control of growth and differentiation(Wang et al.2009).For IBA-treated cuttings,soluble protein content in the phloem decreased,then increased and finally stabilized over the entire rooting process(Fig.3).Soluble protein declined over the first 10 days because a large volume of protein broke into amino acids to create the conditions for callus formation and the induction of root primordia.Protein decreased to 8.38 mg/g when IBA concentration was 1500 mg/L.Moreover,with an increase of IBA,soluble protein consumption increased.This indicates that IBA promotes the breakdown of protein,and the rates increase as the IBA concentration increases.The soluble proteins in each part of the cuttings started to rise in the 10–20 day period because consumption gradually decreased after adventitious roots formed.The rate of increase in proteins was greatest when IBA concentration was 1500 mg/L.This proved to be the best rooting rate and produce the best root quality as previously mentioned.Soluble protein content gradually stabilized or even declined by 20 days after start because it had less involvement with the formation and differentiation of root primordia after numerous adventitious roots formed(Liu et al.2003).The controls essentially displayed the same trend in the rooting process.However,by 15 days,the root primordia of the controls broke through the callus to form new roots.Soluble proteins then significantly decreased,verified by the low rooting rate of the controls as previously noted.
Soluble protein contents in leaf tips treated with IBA showed an ‘increasing–decreasing–increasing’ trend(Fig.3).Soluble proteins 0–5 days increased because the tips accumulated large amounts in early callus formation.This provided the basis for the formation of callus and the induction of adventitious roots.Contents increased to 11.83 mg/g at 1500 mg/L IBA,indicating high IBA concentrations could speed up the synthesis of soluble proteins.Next,soluble proteins decreased in the 5–10 days period because large amounts were transferred to form callus and induce adventitious roots.Later,after 10–15 days,soluble protein contents increased due to the root primordia breakthrough from callus and the growth of adventitious roots.
Unlike leaf tips,leaf bases showed a ‘decreasing–increasing’change in soluble proteins(Fig.3),indicating that there was a relationship between leaf tips,leaf bases and phloem.Soluble proteins slightly decreased in 0–5 days as all functional parts of the cuttings were in a weak state in the initial stage of rooting.Although soluble proteins were mainly produced in leaf tips,they could not be transported to new roots on time and thus decreased.However,after 5–15 days,protein contents began to increase and achieved stability as cuttings recovered and the proteins were transported to the new roots to provide energy for the formation of callus and the induction of root primordia.
Soluble proteins in the leaf tips of the controls showed a downward trend 5 days after initiation,while leaf bases maintained relatively stable levels.At the same time,it was observed that the peak of soluble protein contents in leaf tips and leaf bases appeared much later than that in IBA treatments.This indicates that IBA treatment can significantly improve soluble protein activities,ensure that a steady stream of soluble protein is transported to new roots,and provide energy to form callus and induce root primordia.
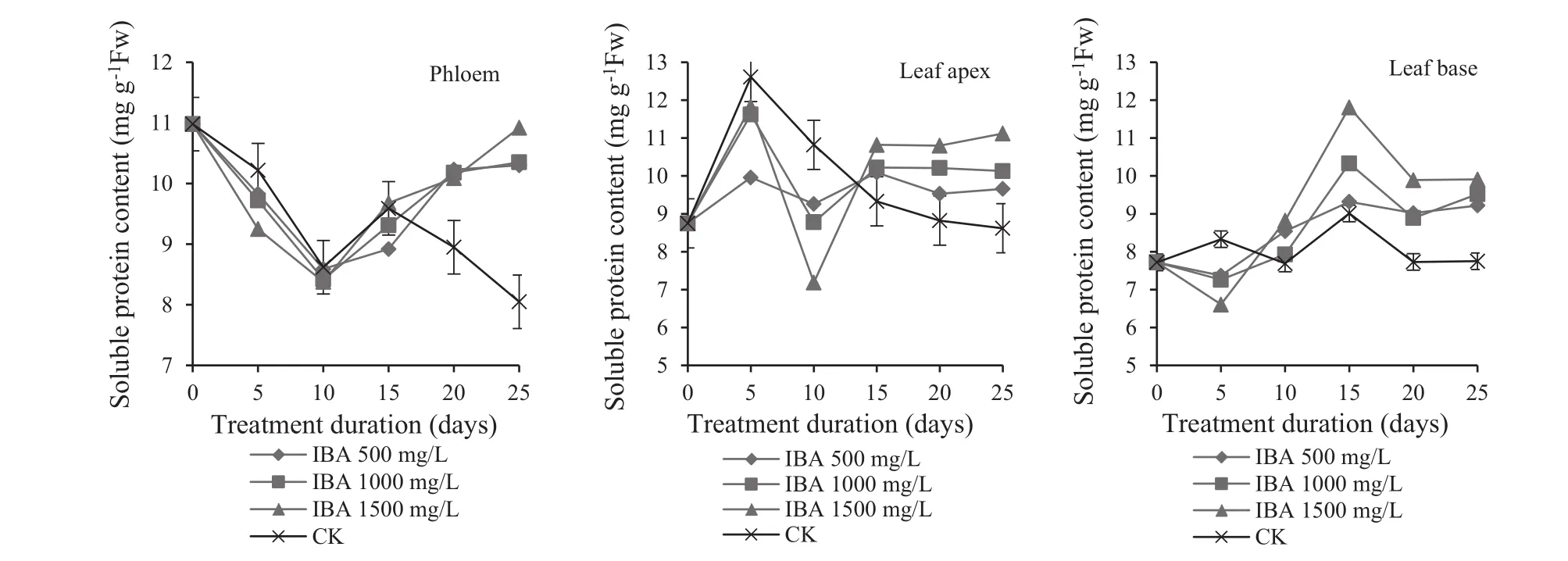
Fig.3 Changes in soluble proteins content in different parts of Z.jujuba Mill.
Effect of IBA treatment on IAA(indole-3-acetic acid)content
The degree of difficulty in rooting is determined by IAA levels within cuttings(Xu and Chen 1989).When IAA content is low,only callus tissues are formed;when high,both callus and root primordia are formed.Throughout the rooting process,IAA content in the phloem of cuttings treated with IBA showed—a ‘increasing–decreasing–increasing–decreasing’trend,and at all concentrations,was higher than the controls,declining with IBA concentration decreasing(Fig.4).This suggests that the synthesis of endogenous IAA could be promoted by IBA.In the initial 5 days,early callus tissues were visible,and the IAA content significantly increased.For cuttings treated with IBA at 1500 mg/L,IAA content increased to 59.93 ng/g(nanogram/gram).This may be because an amount of IAA possibly accumulated before callus formed,which could accelerate cell differentiation in the rooting zone to promote rooting(Yu et al.2007).After 5–10 days,the IAA content decreased.With the accumulation of IAA,stored starch was converted into soluble sugars,and cell osmotic pressure improved.Cells absorbed more water,increasing their volumes,enzyme systems were active,respiration and metabolism were enhanced,and therefore cells consumed significant amounts of IAA to form callus and adventitious root primordia(Wang 2008).After 10–20 days,root primordia broke through the callus tissue to form new roots,and large amounts of IAA were synthesized.Up until day 20,new roots elongated and IAA synthesis weakened in order to maintain normal growth.
IAA contents in the phloem of control cuttings showed an ‘increasing–decreasing’trend.Content rose in the first 10 days because IAA consumption within the cuttings was less than produced.Callus formation was slow and adventitious root primordia differentiation became less.IAA content decreased 10 days later as root primordia grew and differentiated.
Leaves are the source of IAA in the rooting process(Reuveni and Raviu 1980).After the cuttings were treated with IBA,the IAA changes in leaf tips and leaf bases were substantially the same as in phloem,showing ‘increasing–decreasing–increasing—slightly decreasing then becoming stable’(Fig.4).IAA contents in leaves were higher than in the phloem.Moreover,the increase in leaf tips was higher than in leaf bases as leaf tips are the main source of IAA.After 0–5 days,leaf tips and leaf bases accumulated large amounts of IAA for callus formation.After 5–10 days,IAA content of leaf tips and leaf bases decreased as a large volume was continuously transported to form callus and induce root primordia.By 10–20 days,with the formation of adventitious roots,IAA content began to rise and then stabilized.After 20 days,IAA synthesis decreased and growth remained stable.In the controls,IAA content in leaf tips and leaf bases showed a slow downward trend because most of leaf of CK cuttings died.
Effect of IBA on ABA(abscisic acid)changes
ABA is a hormone that inhibits rooting(Liang and Long 1993).For cuttings treated with IBA,ABA content in the phloem was basically a ‘decreasing–increasing–decreasing’trend during the rooting process(Fig.5).Compared with controls,IBA-treated cuttings had lower ABA contents,reaching the lowest level with IBA at 1500 mg/L.In addition,the lower the IBA concentration,the higher the ABA levels were.This suggests that high concentrations of IBA could reduce the ABA synthesis in cuttings.ABA content decreased in 0–5 days,with the lowest content 50.21 ng/g in cuttings treated with 1500 mg/L IBA.Low ABA levels were beneficial callus formation and root primordia induction(Zhang et al.2012),providing favorable conditions in the initial rooting stage.ABA content rose after 5–15 days as considerable IAA was consumed in callus formation and root induction.ABA content increased with a decrease of phloem IAA.It decreased by day 15 because of root elongation.
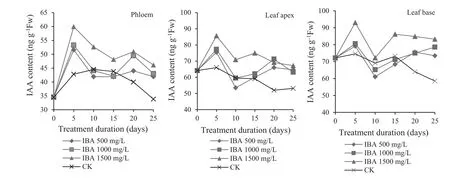
Fig.4 Change of IAA content in different parts of Z.jujuba Mill.
After cuttings were treated with IBA,ABA content in leaf tips and leaf bases increased and was relatively higher than in the phloem(Fig.5).High ABA levels in leaves inhibit the growth of stems and leaves,thus promoting root growth(Wang et al.2009).In comparison with IBA-treated cuttings,the controls had significantly more ABA.This indicates that ABA contents in leaf tips and leaf bases affect rooting;IBA reduces the accumulation of ABA in these plant parts.
Effect of IBA on change of IAA/ABA ratios
IAA/ABA ratios are often used to indicate the level of difficulty of rooting cuttings(Shi et al.2006).In general,the higher IAA/ABA ratio,the more favorable for root formation(Ao et al.2002).Throughout the rooting process of Chinese jujube cuttings with IBA treatment,IAA/ABA ratios in the phloem basically followed the same ‘increasing–decreasing–increasing’trend(Fig.6).IAA/ABA ratios in IBA-treated cuttings were significantly greater than in controls,and ratios increased as concentrations increased.With IBA at 1500 mg/L,cuttings had the highest IAA/ABA ratios.Higher IAA/ABA ratios are beneficial to adventitious root formation and would be dramatically improved with high concentrations of IBA.IAA/ABA ratios rose to their highest value within 5 days after the start of the experiment when callus tissue formed,then decreased within 5–10 days,and significantly increased after day 15.However,the controls had no change trend and low IAA/ABA ratios.
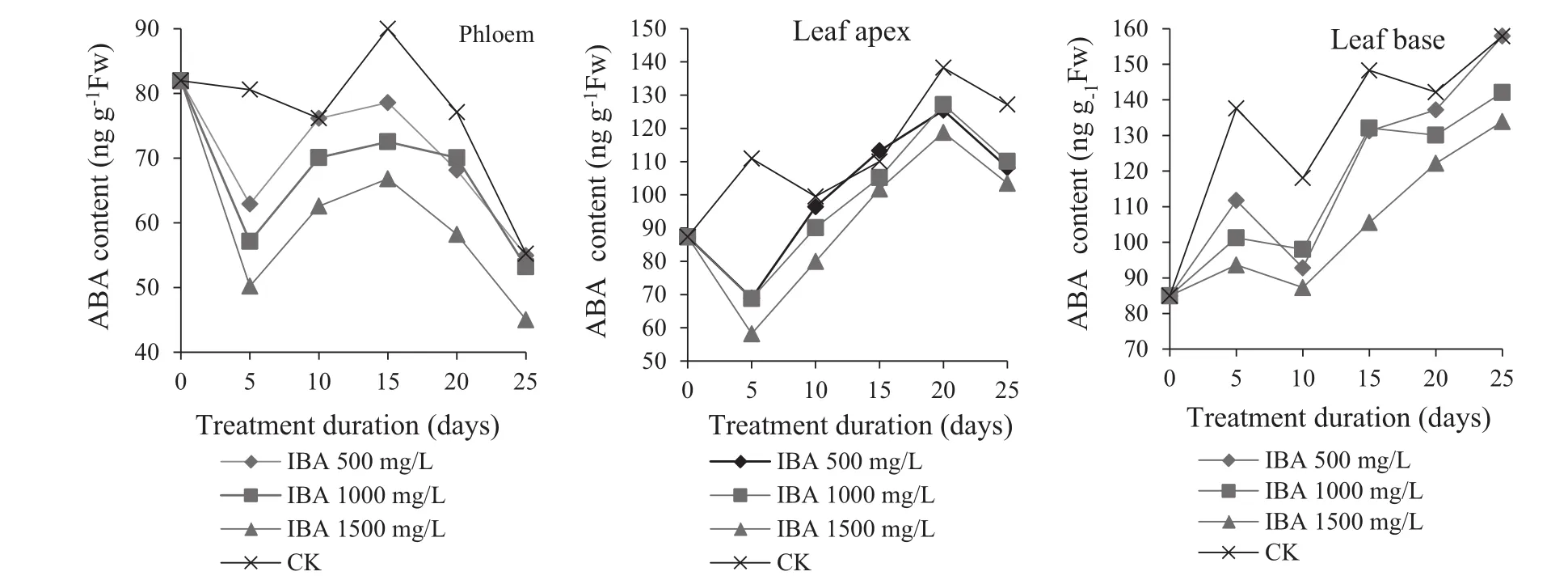
Fig.5 Changes of ABA contents in different parts of Z.jujuba Mill.cuttings
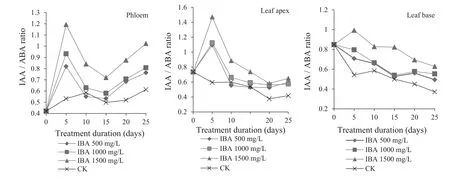
Fig.6 Change of IAA/ABA ratios in different parts of Z.jujuba Mill.during rooting
IAA/ABA ratios in leaf tips showed a trend as the phloem, ‘increasing–decreasing and then maintaining a balance’,while leaf bases showed a relatively flat trend(Fig.6).IAA/ABA values were:leaf tips>phloem>leaf bases,indicating a close relationship between leaf tip endogenous hormones and rooting compared with those of the phloem and leaf bases.Moreover,compared with controls,leaf tips and leaf bases of IBA-treated cuttings had higher IAA/ABA ratios,attaining the highest at 1500 mg/L IBA.This indicates that high concentrations of IBA would significantly improve the IAA/ABA ratios in leaves and promote rooting.IAA/ABA ratios in leaf tips rose in the first 5 days,then decreased,and stabilized by day 5.
Effect of IBA treatment on the changes in ZR(zeatin riboside)content
During root development of the IBA-treated cuttings,endogenous ZR in the phloem showed a ‘increasing–decreasing–increasing–decreasing’trend (Fig.7).In the 0–5 day period,ZR phloem content increased during early callus formation,and was lowest(9.96 ng/g)at an IBA concentration of 1500 mg/L.Higher ZR content maybe required for callus formation.By 5–10 days,ZR content decreased,and although it was 8.94 ng/g when IBA was 1500 mg/L,rooting was highest because low ZR levels were beneficial to root induction(Song 2012).With a decrease in IBA,ZR phloem levels rose,indicating that high concentrations of IBA could reduce ZR synthesis.In the 10–15 day period,when adventitious roots broke through the callus tissue,ZR levels slowly increased.At 1500 mg/L IBA,ZR levels peaked and were consistent with the best rooting rate.Cuttings required high ZR concentrations to promote root growth after the formation of adventitious roots(Zhao et al.2013).After 15–25 days,ZR levels stabilized when a large number of roots developed and elongated.In the controls,ZR was relatively high in 1–15 days after the start of the experiment and inhibited the induction of root primordia.ZR then decreased by day 15 and inhibited root growth.
After the cuttings were treated with IBA,ZR levels in leaf tips maintained the same as ZR levels in the leaf bases,but ZR volatility in leaf tips was more obvious than in leaf bases(Fig.7).This indicates that ZR in leaves had effects on the rooting of cuttings during root primordia induction and growth,and that the main activities were in leaf tips.Five days after the start of the experiment,ZR contents peaked at a minimum when IBA was 1500 mg/L,but later reach a peak value by 15 day.This demonstrated that relatively low ZR levels in leaf tips could favor root formation and a high ZR levels could root growth.Both 5 and 15 days ZR contents in leaf bases reached peak value but there were no obvious difference between these two values.ZR levels in leaf bases were relatively stable compared with levels in leaf tips.However,for controls,ZR content in leaf tips and leaf bases significantly decreased after 15 days.
Effect of IBA on GA(gibberellic acid)content
During the rooting process,GA content in the phloem of cuttings treated with IBA showed the ‘increasing–decreasing’trend(Fig.8)and also was lower than the controls.GA was lowest when IBA was 1500 mg/L.With a decrease in IBA,GA content increased,indicating that GA synthesis could be reduced by IBA with an increase of IBA.In 0–5 days,GA rose significantly during callus formation because it is closely related with callus formation and root primordia differentiation(Liu et al.2001).Large amounts of GA were required when callus formed at cut-surfaces(Pan and Dong 1995).After 5–10 days,the GA contents lowly increased,after10–15 days,it decreased during root formation and fell to 5.33 ng/g when IBA was 1500 mg/L.This demonstrates that a decrease of GA stimulated root primordia and promoted the formation and growth of roots.After 15–20 days,the GA content increased;after 20 days,it declined,which promoted root growth.
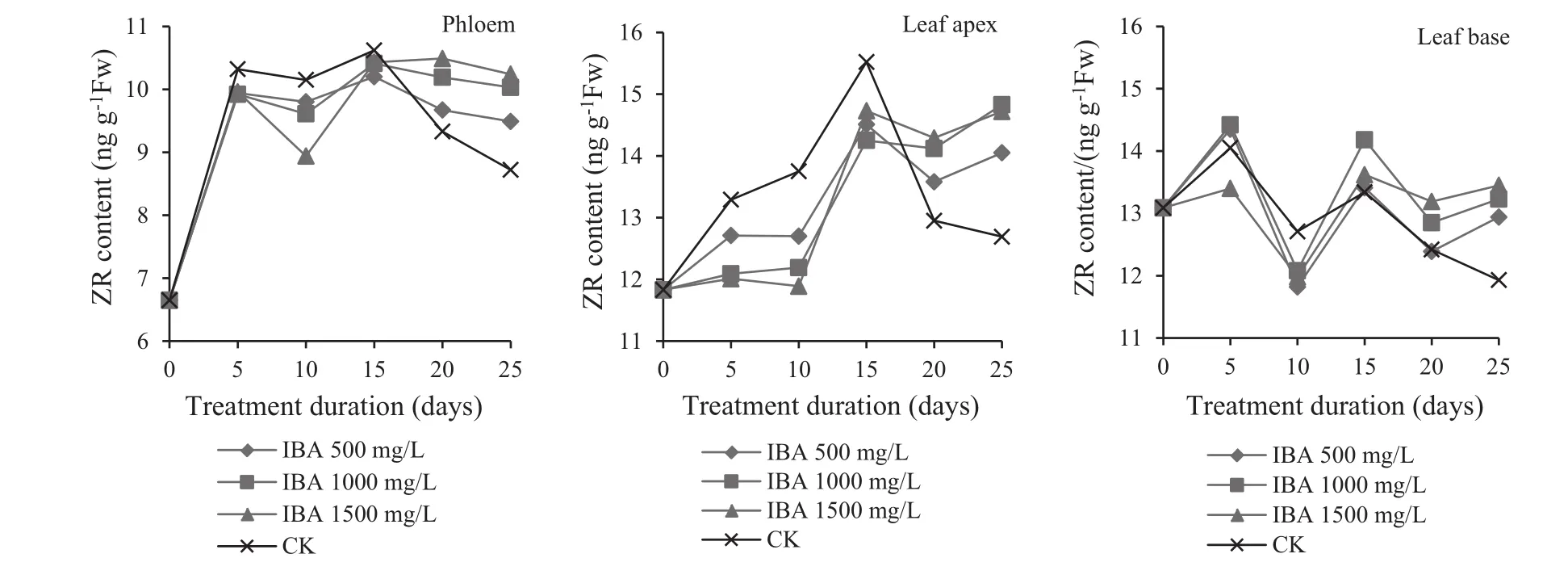
Fig.7 Change in zeatin riboside levels in different parts of Z.jujubea Mill.during rooting
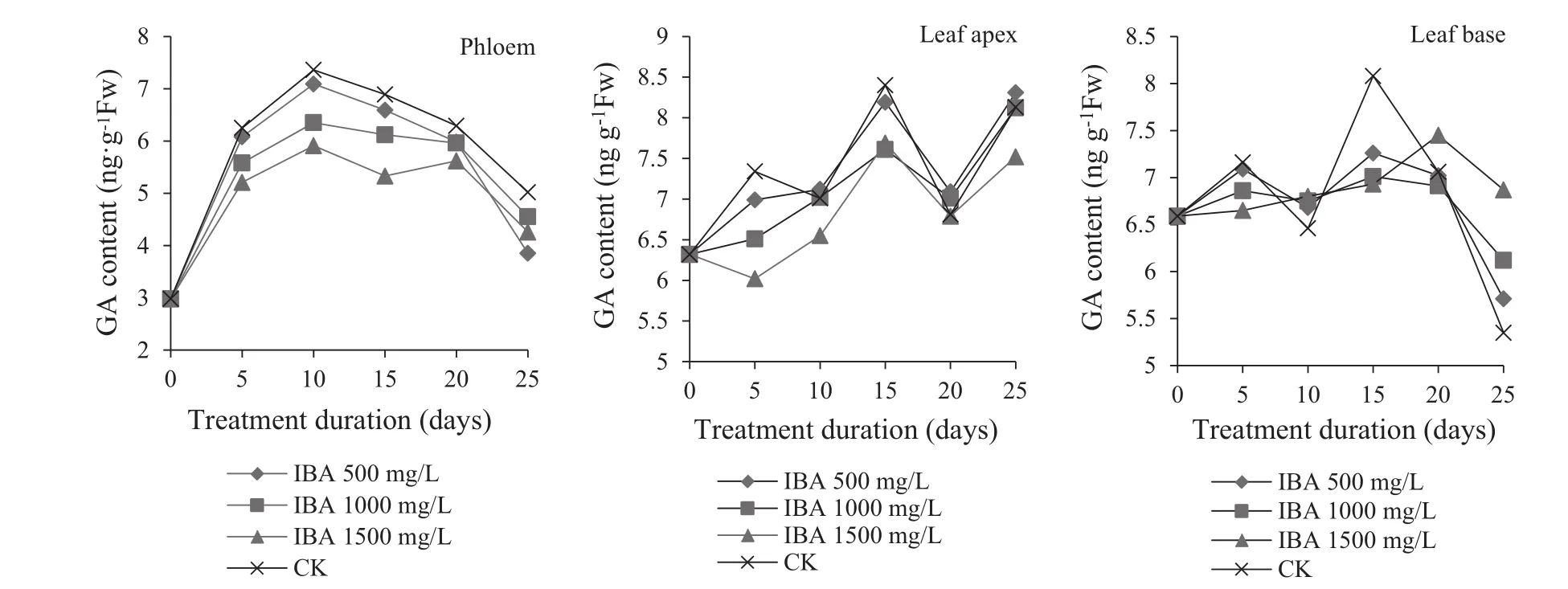
Fig.8 Change of gibberellic acid content in different parts of Z.jujuba Mill.during rooting
IBA treatment made the GA content in leaf apices have an upward trend.When IBA was 1500 mg/L,GA was lowest.GA content in leaf bases was relatively stable with a range of 6–7 ng/g.This indicated that leaf tips were the main source of GA in leaves and played a main role in rooting.
Conclusions
IBA treatment promotes rooting of Chinese jujube cuttings
Control cuttings had low rooting rate and poor root quality.However,IBA treatments significantly improved the quality and rate of rooting.IBA at 1500 mg/L was the optimal concentration for promoting rooting;rooting rate was 93.3%,the number of roots was 5.2,root length reached 35.4 mm,and root diameter was 1.2 mm.
IBA was beneficial to the consumption and accumulation of nutrients
The results of this research demonstrate that using IBA to treat Chinese jujube cuttings promoted the metabolism of soluble sugars and proteins in the phloem,improve rooting rate and ensuring root quality.
During the period of callus formation,rooting had a positive correlation with phloem soluble sugar consumption,which was highest when IBA was 1500 mg/L.During root formation,there was a positive relation between rooting rate and the soluble sugar accumulation.At 1500 mg/L concentration,soluble sugars were highest and root quality was best.This is consistent with other research that soluble sugars decrease during callus formation and increase after the formation of roots(Sun et al.2011;Xu et al.2012;Liang et al.2014).Rooting also was influenced by soluble sugars in leaf tips and leaf bases,especially by leaf tip activities.The leaf tip was the main source of soluble sugars to promote the formation of callus and adventitious roots.
For cuttings treated with IBA,changes of soluble proteins followed a V-shaped curve.During callus formation,the rooting rate was positively correlated the decomposition of soluble protein the best root quality was 1500 mg/L IBA.The soluble proteins were used in the formation of callus tissue and induction of root primordia.During root formation,there was a positive correlation between rooting rate and soluble protein accumulation,in agreement with Tian(2002),Liu et al.(2003)and Liang et al.(2014).Soluble protein content was high when root cambium activity was relatively weak and vice versa.During root elongation,soluble protein content was stable or slightly lower,which is consistent with research results that the features of soluble protein that regulate cell growth and differentiation gradually disappear(Liu et al.2003).Rooting also was affected by soluble protein content in leaf tips and leaf bases,which showed opposite change trends.Together with the phloem,both regulate the formation and growth of adventitious roots,especially activities of leaf tips,the main source of soluble protein.IBA treatment enhanced the activities of leaf tips and leaf bases and thus promoted the formation of callus and adventitious roots.
IBA treatment balances endogenous hormones
IBA had an impact on the endogenous hormones in the phloem of cuttings,and the mechanism at different concentrations was the same.Different endogenous hormones had different influences on rooting.There was a positive relation between rooting rate and IAA(indole-3-acetic acid)in the phloem;IAA increased during early callus formation,then declined,and finally increased.This is consistent with research that showed that some IAA was consumed when callus formed and IAA influence disappeared in the rooting stage(Zeng et al.2006;He et al.2011).IAA content was always highest at 1500 mg/L IBA,and indicates that high IAA content was beneficial to the formation of adventitious roots(Yu et al.2007;Wang et al.2009).
Rooting was negatively influenced by ABA(abscisic acid)levels and decreased in early tissue injury,increased in callus formation,and declined during root formation and elongation.This shows that there was a relationship between ABA and IAA.In studies by Wang et al.(2009);Ao et al.(2002)and Sun et al.(2009),IBA inhibited and significantly reduced ABA synthesis and promoted rooting.The ABA content was always low with 1500 mg/L IBA,leading to the best rooting rate.This indicates that a high ABA content inhibited rooting(Shi et al.2006).Rooting rate and IAA/ABA ratios in phloem were positively correlated.The higher the IAA/ABA ratio,the better the callus formation and adventitious root growth were.This is consistent with the results of Ford et al.(2002),Shi et al.(2006)and Zhang et al.(2004).Rooting was best at 1500 mg/L IBA concentration and resulted in the highest IAA/ABA ratio in early callus and root formation.
ZR(zeatin riboside)content increased during callus formation,decreased and then increased during root formation.Low levels of ZR promoted adventitious root induction and high levels promoted root growth(Zhao et al.2013).This was most obvious at 1500 mg/L IBA.
Rooting was negatively correlated with GA(gibberellic acid)in the phloem.GA levels rose in the early callus formation and then declined in later callus formation and root induction.The GA content was always lowest at 1500 mg/L IBA,leading to the best rooting rate.This is consistent with the research results that show a low GA content favors rooting(Pagnussat et al.2003;Wang et al.2009;Zhao et al.2013).
This research on the changes in endogenous hormones in rooting of Chinese jujube cuttings treated with IBA at different concentrations shows that IBA had an impact on hormone levels in leaf tips and leaf bases.Its mechanism at different concentrations was basically the same,with the main activities in leaf tips.To a certain extent,together with phloem and leaf bases,leaf apices regulated root formation,and were the major source of the production and consumption of IAA.IAA contents in leaf apices and leaf bases peaked in early callus formation and rooting when its metabolism in leaf apices was more active.ABA levels in leaf tips and leaf bases increased during rooting and inhibited the growth of stems and leaves.This provided a favorable environment for the formation and growth of adventitious roots.During rooting,the IAA/ABA relationship between phloem,leaf tips and leaf bases was:leaf tips>phloem>leaf bases,which showed that endogenous hormones in leaf tips played a significant role on rooting with leaf bases and the phloem.ZR levels in leaf tips greatly influenced callus formation and rooting.Leaf tips were the main source of GA.Leaf tips and leaf bases consumed part of the GA to promote root elongation after the formation of adventitious roots,and the main activities were in leaf tips.
AcknowledgementsThis work was financially supported by the national 948 subject‘Introduction of fresh-eating jujube cultivar and new cultivating technology from Israel(2012-4-61)’.
 Journal of Forestry Research2018年6期
Journal of Forestry Research2018年6期
- Journal of Forestry Research的其它文章
- Black locust(Robinia pseudoacacia L.)as a multi-purpose tree species in Hungary and Romania:a review
- The impact of the environmental factors on the photosynthetic activity of common pine(Pinus sylvestris)in spring and in autumn in the region of Eastern Siberia
- Osmoregulators in Hymenaea courbaril and Hymenaea stigonocarpa under water stress and rehydration
- Effect of nitrogen levels on photosynthetic parameters,morphological and chemical characters of saplings and trees in a temperate forest
- Free amino acid content in trunk,branches and branchlets of Araucaria angustifolia(Araucariaceae)
- Exogenous application of succinic acid enhances tolerance of Larix olgensis seedling to lead stress
